Introduction
Eosinophilic adenomas are composed of eosinophils,
which are present in numerous parts of the body, including the
kidney, salivary, thyroid and pituitary gland, eyelid, thymus and
spinal cord. Adrenal cortical eosinophilic adenoma is a rare
adrenal tumor. Eosinophilic adenoma of adrenal cortex is often
described as nonfunctional adrenal tumor, which may lead to
Cushing's syndrome. Adrenal cortical eosinophilic adenoma is a rare
pathological type of adrenal tumor (1). Studies have shown that the disease is
most common in the left adrenal gland, with a ratio of 2: 1 in the
left and 2: 1 in the right, and most common in female patients,
with a ratio of 2: 1 in the female:male, with an average onset age
of 44 years (1,2). The first adrenocortical oncocytoma was
reported by Kakimoto et al in 1986 (3). To date, ~200 cases have been reported
(4). Adrenal cortical oncocytoma is
usually described as non-functional adrenal tumor and may lead to
the development of Cushing's syndrome and complex clinical
diagnosis. In recent years, with the progress of hospital
pathological detection technology, the reporting of this rare
disease is on the rise due to the decrease in missed detection and
false detection rate (4,5).
Case report
A 30-year-old female patient was admitted to Wuchuan
People's Hospital (Zunyi, China) in November 2022 due to increased
body hair, weight gain and elevated blood pressure for >1 year.
The blood pressure of the patient fluctuated between 150–190
systolic and 80–110 mmHg diastolic. A physical examination revealed
a sanguine appearance, centripetal obesity, hypertrophy of the neck
and back, small purple lines on the abdomen and armpits and a
reduced temperature (35.8°C) at the ends of limbs. Potassium (4.99
mmol/l; normal reference value, 3.50–5.50 mmol/l), parathormone
(35.00 pg/ml; normal reference value, 15.00–65.00 pg/ml), serum
creatinine (70.10 µmol/l; normal reference value, 44.00–115.00
µmol/l) and blood calcium (2.42 mmol/l; normal reference value,
2.10–3.00 mmol/l) levels were all within healthy range. In
addition, hemoglobin levels were 155 g/l, hematocrit (HCT) was
46.7% and Blood dopamine 67.3 pmol/l (normal value ≤195.7 pmpl/l),
blood epinephrine 95.3 pmol/l (normal value ≤605.4 pmpl/l) and
blood norepinephrine 590.3 pmol/l (normal value ≤414.0–4,435.5
pmpl/l); 24-h urine dopamine 1,108.28 nmol/24 h (normal value
750.00–2,088.00 nmol/24 h), 24-h urine adrenaline 20.00 nmol/24 h
(normal value 4.31–61.60 nmol/24 h), and 24-h urine norepinephrine
147.75 nmol/24 h (normal value 60.00–30). The levels of sex
hormones were assessed; results revealed high testosterone levels
(7.480 nmol/l, normal value 0.290–1.670 nmol/l) and cortisol levels
of 450.10 nmol/l (normal value 63.40–129.60 nmol/l), 473.80 nmol/l
(normal value 133.00–537.50 nmol/l) and 533.90 nmol/l (normal value
68.20–327.60 nmol/l) at 12:00 a.m., 8:00 a.m. and 4:00 p.m.,
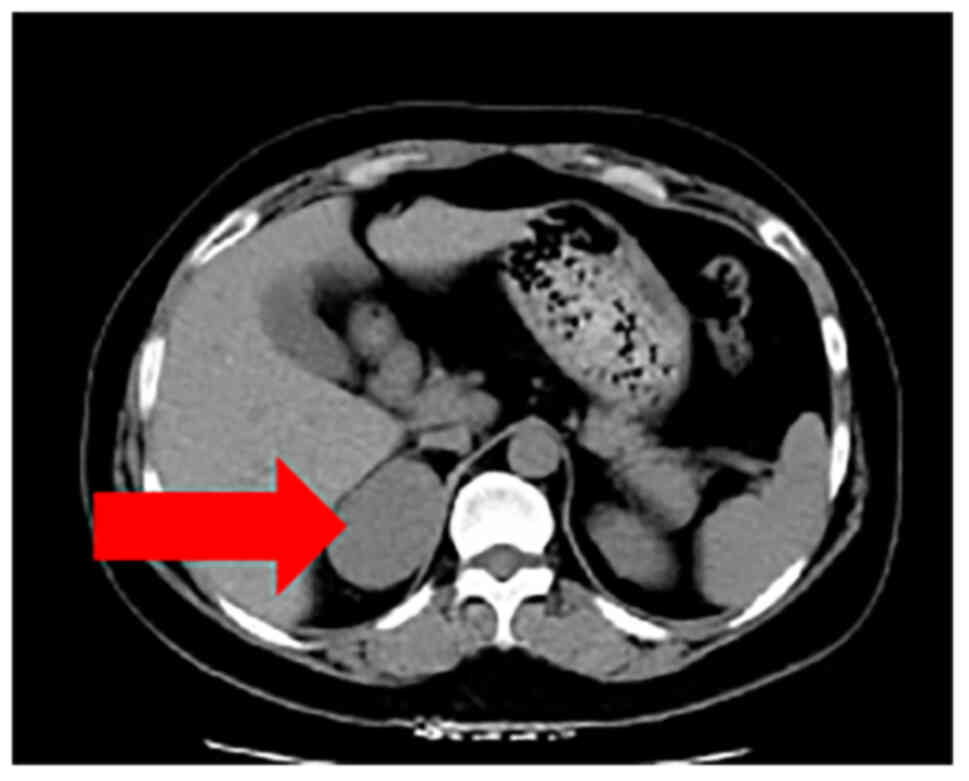
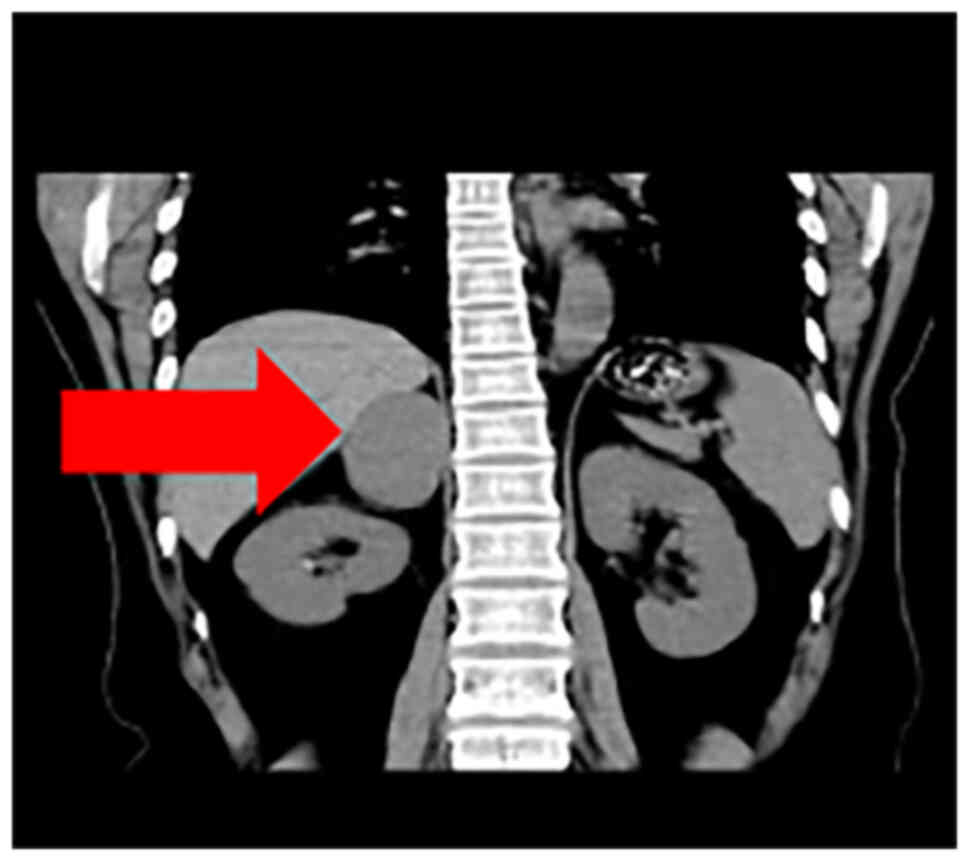
respectively. A computed tomography (CT, Philips CT, row 64) scan
of the adrenal gland revealed a space (45×40 mm) in the right
adrenal gland, indicative of pheochromocytoma or lymphoma (Figs. 1 and 2). Furthermore, there were no
abnormalities in renal (Fig. 3) and
cervical (Fig. 4) vascular,
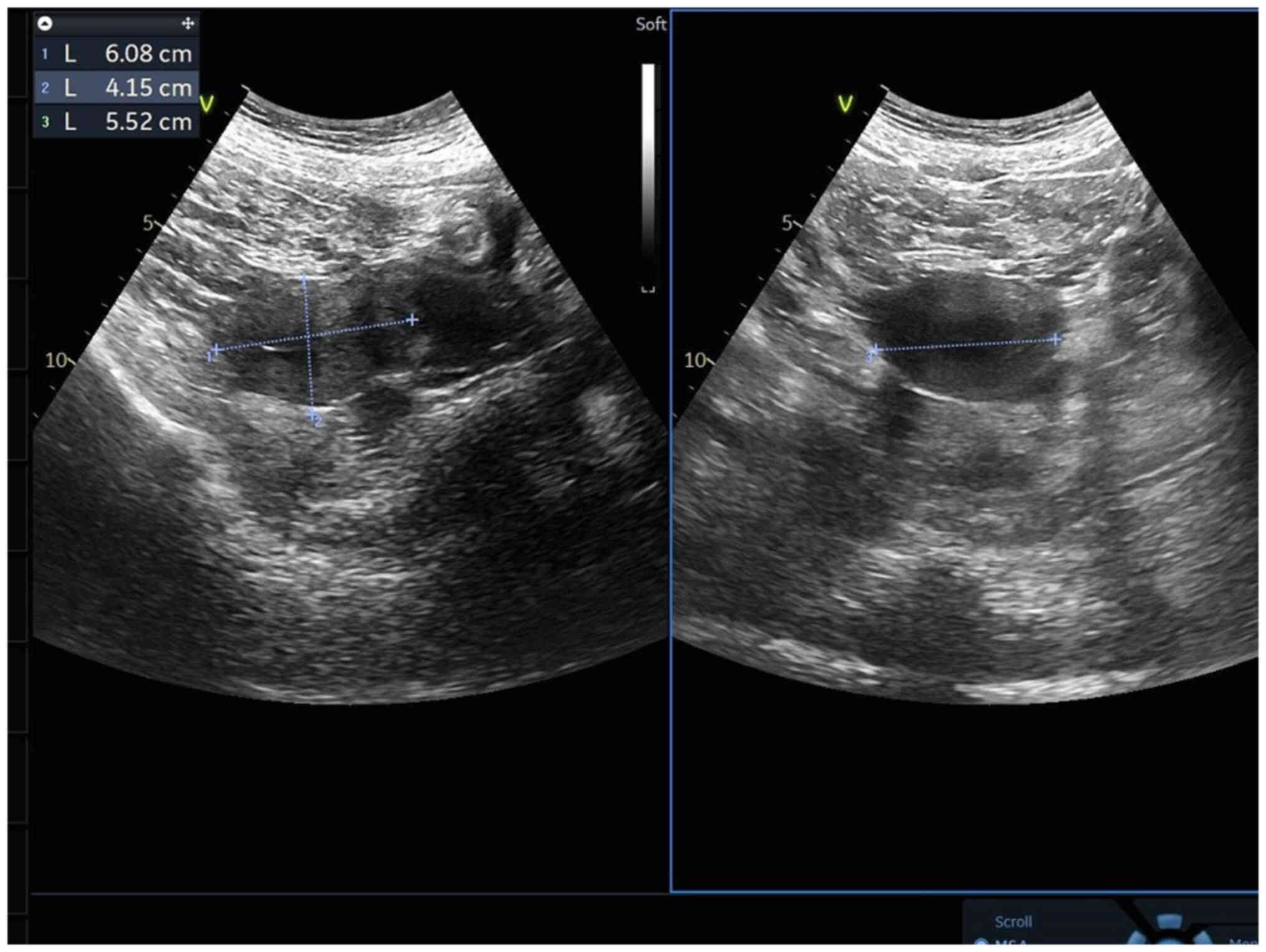
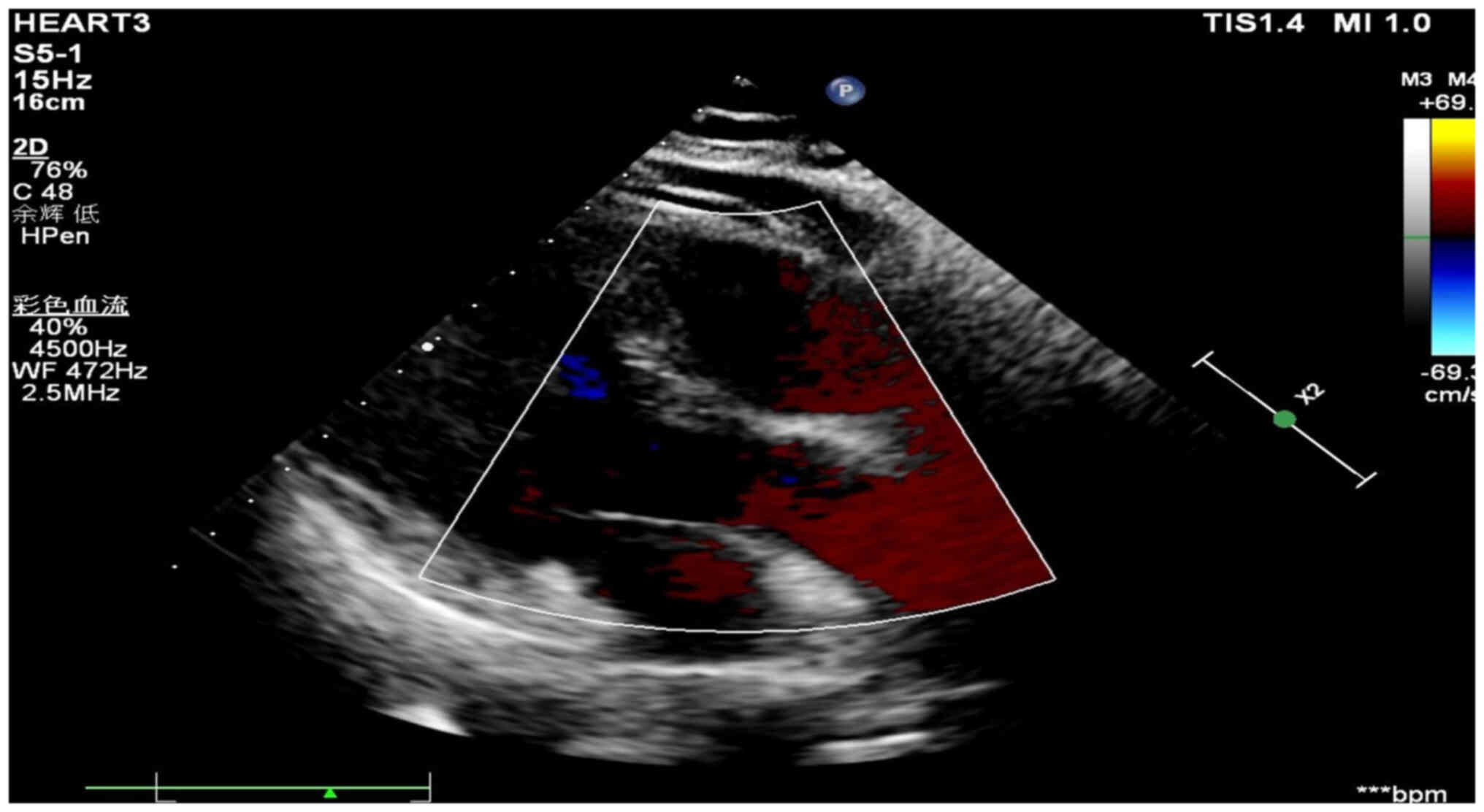
gynecological (Fig. 5) and cardiac
color Doppler ultrasound (Fig. 6)
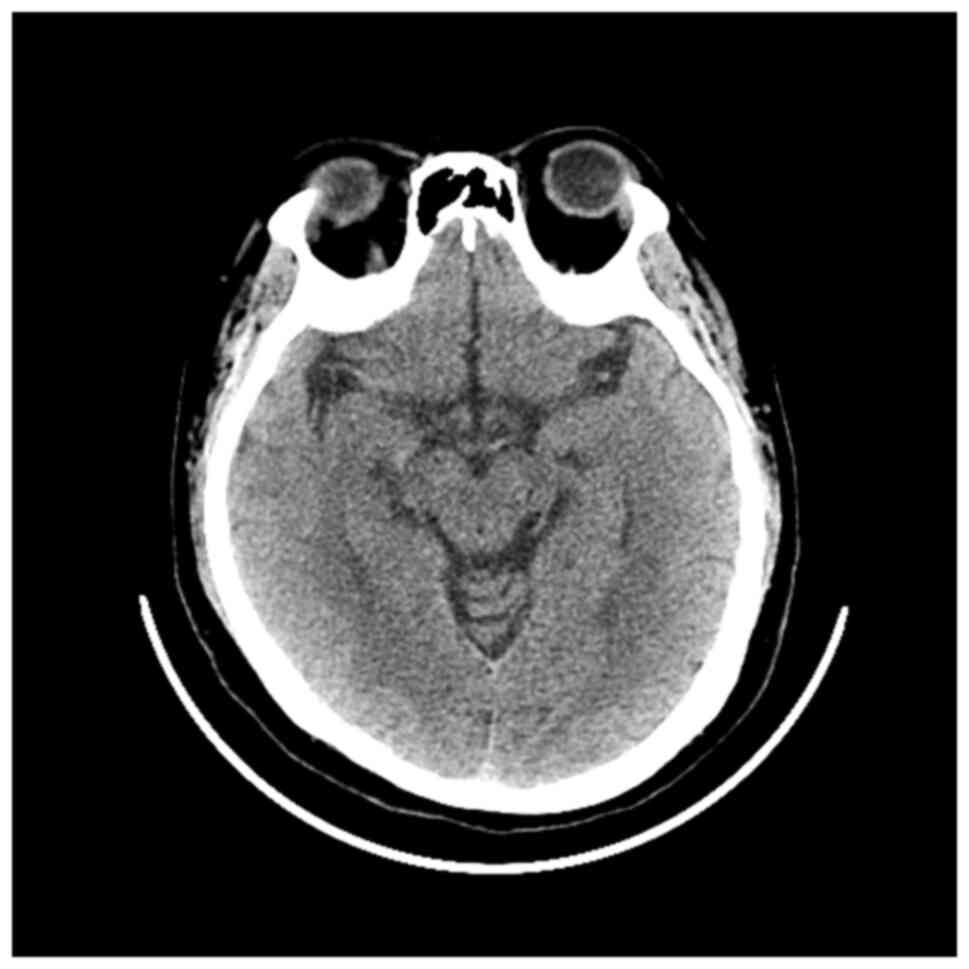
or CT scan of the skull (Fig. 7).
In the present hospital, bone mineral density screening is not a
routine preoperative preparation item for patients with adrenal
tumors, so the patient was not screened for bone mineral density
Following discussions with the department and an additional
multidisciplinary team, the following diagnoses were considered for
the right adrenal functional tumor: i) Cortical adenoma; ii)
pheochromocytoma; iii) cortical cancer; iv) Cushing's syndrome and
v) secondary hypertension.
According to the advice of a doctor in the
outpatient department of Guizhou Provincial People's Hospital
(Guiyang, China), the patient received oral terazosin (2 mg daily,
which was gradually adjusted to 2 mg, three times/day) and
metoprolol (23.75 mg, once/day) for 2 weeks. Notably, the blood
pressure of the patient was uncontrolled; thus, the cardiovascular
department was consulted and nifedipine gastrointestinal
therapeutic system (20 mg, twice/day) was administered for control.
Based on potential pheochromocytoma, preoperative volume expansion
preparation was performed. Following adequate blood pressure
control and the observation of indexes that reached the standard
levels (blood pressure ~120/80 mmHg, heart rate is less than 90
beats/min, and HCT <45%), laparoscopic right adrenal tumor
resection was performed under general anesthesia using an abdominal
approach. Surgery was performed according to the routine protocols
used in the treatment of adrenal tumors (1). Following anesthesia, 100 mg
hydrocortisone was administered intravenously and after the
completion of surgery, 100 mg hydrocortisone in 5% glucose solution
was continuously administered intravenously. During surgery, the
highest blood pressure measured was 179/115 mmHg. Following
surgery, the blood pressure was 128/90 mmHg. No blood transfusion
was performed during the operation.
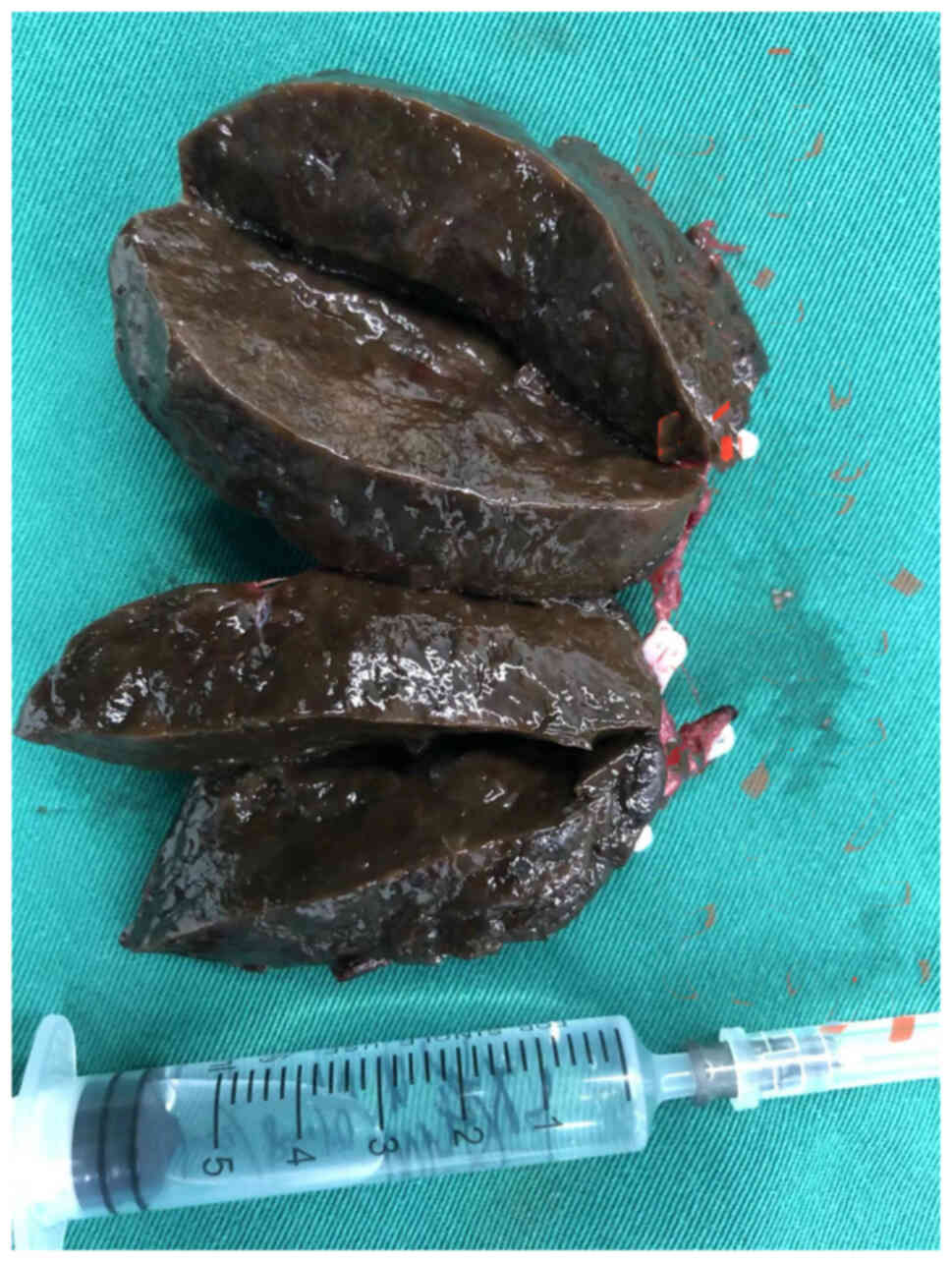
The tumor of the adrenal gland was 35×60×60
mm3 in size (Fig. 8),
dark brown in color, solid and soft. The inside of the tumor
remained dark brown. Pathological examination was performed as
follows: Specimen tissues were fixed in 4% paraformaldehyde for 24
h. Dehydration with gradient ethanol and xylene washes. Slice the
trimmed wax block on a paraffin slicer with a thickness of 4 µm.
Tissue was stained with Harris hematoxylin solution for 5min and
eosin solution (95% ethanol solution) for 20 sec. Use Lycra DM2500
microscope for microscopic examination and image collection and
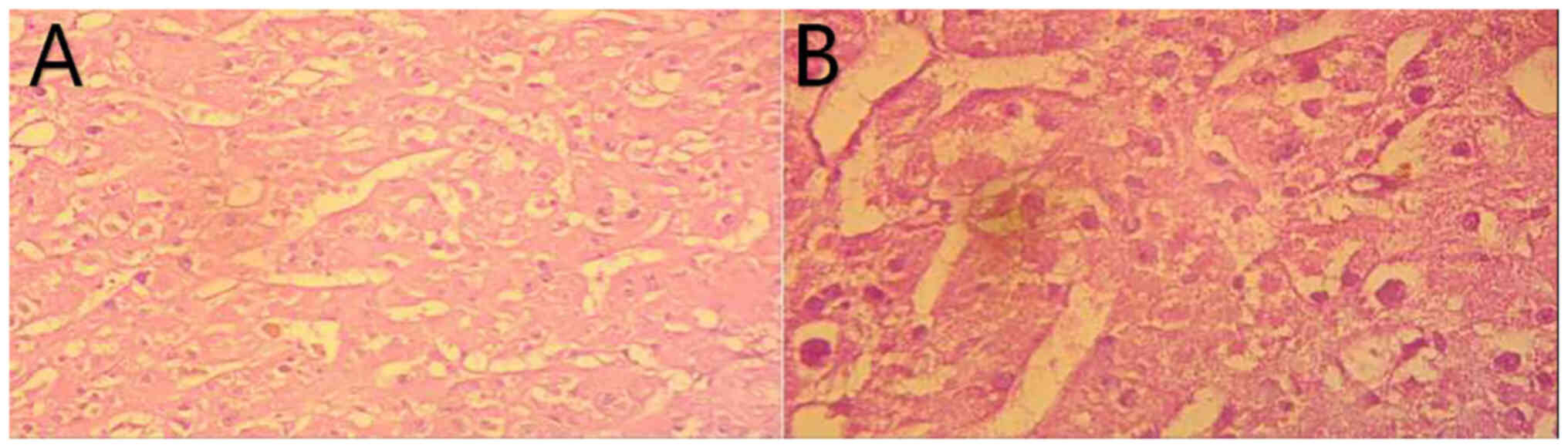
analysis. Paraffin-embedded tissue sections showed that the tumor
was mainly composed of diffuse eosinophilic epithelial cells. Only
acinar, tubular or fascicular structures were observed in the focus
area. The postoperative pathology was indicative of adrenocortical
eosinophilic adenoma (Fig. 9A and
B). The clinical manifestations were hair growth, weight gain,
disordered menstrual cycle, fluctuations in blood pressure,
centripetal obesity, neck and back hypertrophy, purple lines on the
abdomen and armpits and cortisol rhythm disorder. Thus, the patient
was diagnosed with Cushing's syndrome caused by adrenocortical
oncocytoma. On day 1 following surgery, cortisol levels of 25.04,
28.15 and 31.85 nmol/l were measured at 12:00 a.m., 8:00 a.m. and
4:00 p.m., respectively. The patient exhibited no signs of adrenal
failure and both blood pressure and heart rate were in normal
ranges at rest. The patient was administered hormone replacement
therapy for 1 month, which was gradually reduced. The post-surgery
hormone replacement therapeutic regimen was as follows: 200 mg
hydrocortisone administered intravenously on day 1; 100 mg
hydrocortisone administered intravenously on day 2 and 50 mg
hydrocortisone administered orally once/8 h on day 9. On days
10–16, 50 mg hydrocortisone was administered orally once/12 h. On
days 17–23, 30 mg hydrocortisone was administered orally every 12
h. On day 24, the dosage was changed to 20 mg and hydrocortisone
was administered orally once/12 h. The dosage was gradually reduced
to 5 mg/day until day 31. Subsequently, blood pressure and heart
rate of the patient fluctuated within healthy range (blood pressure
<130/80 mmHg, heart rate <90 beats/min.), and the levels
returned to those observed pre-disease. At 3 months post-surgery,
the patient conceived naturally.
Discussion
A previous study indicated that the incidence of
adrenocortical oncocytoma is twice as likely on the left side than
on the right side, and the incidence of adrenocortical oncocytoma
is twice as common in females than in males (2). Adrenocortical oncocytomas are often
described as non-functional adrenal tumors and these are discovered
via physical examination or assessment of other diseases. Thus,
adrenocortical oncocytomas are often misdiagnosed. Adrenal cortical
eosinophilic adenoma is mostly non-functional and usually found by
physical examination. Common manifestations include feminization of
male patients (lack of facial and body hair; chest and buttocks are
developed), masculinization of female patients (Breast atrophy,
shoulder and hip fat disappearance, menstruation reduction or
disappearance; Male characteristics such as beard and pubic hair
are male-like, muscular, acne on face and chest, enlarged Adam's
apple and deep voice) and Cushing's syndrome, accounting for 17–50%
(6). The present study reports the
case of a patient with adrenocortical oncocytoma with endocrine
abnormality. At present, the pathophysiological mechanisms of
adrenocortical oncocytoma are yet to be fully elucidated; however,
in vivo animal experiments have reported that
N-nitromorpholine is an important inducer of eosinophil
proliferation (7,8). Following N-nitromorpholine treatment,
excessive proliferation of mitochondria is observed as a
compensation mechanism, leading to formation of adrenocortical
oncocytoma (7,8). Duregon et al (9) demonstrated that development of
adrenocortical oncocytoma may be due to mutations in the
mitochondrial genome, leading to proteins being encoded in
eosinophils. In addition, Song et al (10) assessed the significance of base
mutations in the non-coding control region (D-loop region) of
mitochondrial (mt)DNA in oncocytoma, using thyroid and renal
oncocytoma and corresponding healthy tissue; D-loop region of
mtDNA, particularly hypervariable region I, was a mutation hotspot
of oncocytoma, and this mutation may have caused changes in the
replication rate of mtDNA, which is associated with the abnormal
function of mitochondria.
The presence of adrenocortical oncocytoma may not be
revealed using imaging; thus, diagnosis of adrenocortical
oncocytoma is complex. Although the observed tumor is often large
in size (median diameter, 80 mm), the majority of these tumors
possess a complete capsule. Thus, the tumor may continue to grow
non-invasively (11–13). In previous studies, imaging of
adrenocortical eosinophilia revealed uniform density, with a CT
value of 20–40 HU. Furthermore, results of enhanced CT scans
revealed an uneven enhancement (11–13).
Often, adrenocortical oncocytoma is misdiagnosed as large adrenal
pheochromocytoma; however, pheochromocytoma is more hemorrhagic and
necrotic than adrenocortical oncocytoma. In addition, the majority
of pheochromocytomas are accompanied by internal scars (11). Adrenal cortical oncocytoma also
demonstrates low signal intensities on T1 and T2 weighted images,
with uneven enhancement and vascular shadows (11). Results of previous studies have
reported that adrenal cortical oncocytomas are 20–200 mm in
diameter (median diameter is 80 mm), round, possess a complete
envelope, are brown or yellowish-brown in color and demonstrate
bleeding or necrosis (1,11,12).
Furthermore, immunohistochemical analysis has revealed positive
α-inhibin and melan-A and negative S100 staining (11,14).
However, immunohistochemical markers are limited in differentiating
between benign and malignant adrenocortical oncocytoma. According
to the criteria described by Wong et al (15), the following pathological
characteristics are indicative of adrenocortical oncocytoma: i)
>5/50 high power field atypical mitotic images and ii) venous
infiltration. The secondary criteria include the following
pathological characteristics: i) Tumor diameter >100 mm and/or
mass >200 g; ii) necrosis; iii) capsule infiltration and iv)
blood sinus infiltration. Notably, malignant adrenocortical
oncocytoma is diagnosed following the presence of any one of the
main evaluation criteria; benign adrenocortical oncocytomas do not
meet any one of the main or secondary evaluation criteria and
adrenocortical oncocytomas meet ≥1 of the secondary evaluation
criteria. In the present case, the size of the observed tumor was
35×60×60 mm3, and necrosis, capsule invasion or blood
sinus invasion were not observed. Thus, the secondary criteria were
not met. In addition, pre-operative imaging of the head, abdomen
and blood vessels did not reveal other tumors. Following the
observation of further postoperative pathological manifestations
(tumor cells are flaky, while the cytoplasm of tumor cells is
eosinophilic and fine granular), benign adrenal cortical
eosinophilia was determined.
The main treatment of adrenocortical oncocytoma is
surgical resection, and the most common surgical methods include
minimally invasive laparoscopy or traditional laparotomy (1,7,16,17).
A previous study revealed that open surgery is optimal when the
diameter of the tumor is >60 mm (16). Following development of minimally
invasive techniques, laparoscopic adrenalectomy is considered the
gold standard of adrenalectomy and laparoscopic surgical methods
have developed transabdominal, retroperitoneal and robot-assisted
approaches. However, it remains to be established whether
laparoscopic surgery or open surgery is optimal for tumors with a
diameter of >60 mm, considering the potential for malignancy
prior to surgery (7). In the
present case, the size of the adrenocortical oncocytoma was
35×60×60 mm3, and the right adrenal tumor was resected
using an abdominal approach. No complications occurred during or
after the operation. Therefore, the present study demonstrated that
adrenal surgery was both safe and effective, and a transabdominal
approach with improved vision should be used in treatment of
patients with a large tumor volume.
In conclusion, Cushing's syndrome caused by an
adrenocortical oncocytoma is a rare adrenal tumor disease. The
tumor observed in the patient described in the present case was
malignant and misdiagnosis occurred. An accurate diagnosis may be
achieved following both pathological examination and
immunohistochemical analysis. Laparoscopic adrenal tumor resection
exhibits numerous advantages, including low levels of associated
trauma, reduced recovery time and fewer complications. Thus, the
treatment used in the present study was both safe and feasible.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CG and ZG confirm the authenticity of all the raw
data. CG and ZG conceived and design of the work, analyzed and
interpreted data and wrote the manuscript. QJ, MT, JL and YZ
performed imaging and designed the experiments. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of The People's Hospital of Wuchuan (Zunyi, China).
The ethical batch number is WCXYYLL-2023-012.
Consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guangxu L and Liu T: Report of three cases
of adrenal eosinophilic adenoma. Chin J Endoscopic Urol.
15:163–165. 2021.
|
|
2
|
Kanitra JJ, Hardaway JC, Soleimani T,
Koehler TJ, McLeod MK and Kavuturu S: Adrenocortical oncocytic
neoplasm: A systematic review. Surgery. 164:1351–1359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakimoto S, Yushita Y, Sanefuji T, Kondo
A, Fujishima N, Kishikawa M and Matsumoto K: Non-hormonal
adrenocortical adenoma with oncocytoma-like appearances. Hinyokika
Kiyo. 32:757–763. 1986.PubMed/NCBI
|
|
4
|
Godin K, Bang N and Tolkach Y: Case
report: Heterotopic intrarenally located adrenocortical oncocytoma.
F1000Res. 3:732014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Narayanan N, Kamalanathan S, Sahoo J, Naik
D, Kumaravel S and Ganesh RN: Pediatric adrenocortical oncocytoma
presenting as Cushing's syndrome and peripheral precocious puberty:
A case report and review of literature. J ASEAN Fed Endocr Soc.
36:205–208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Shen L, Chen D, Chen Y, Li X and
Guo J: Analysis of CT and MRI features of adrenal eosinophilic
adenoma. Chin J Urol. 39:289–293. 2018.(In Chinese).
|
|
7
|
Liu J, Zheng D, Shang P, Qi P, Dai Y, Diao
L, Yue Z and Qu G: Laparoscopic adrenocortical eosinophilic adenoma
resection in 4 cases. China J Minimally Invasive Surg.
17:1144–1147. 2017.(In Chinese).
|
|
8
|
Krech R, Zerban H and Bannasch P:
Mitochondrial anomalies in renal oncocytes induced in rat by
N-nitrosomorpholine. Eur J Cell Biol. 25:331–339. 1981.PubMed/NCBI
|
|
9
|
Duregon E, Volante M, Cappia S, Cuccurullo
A, Bisceglia M, Wong DD, Spagnolo DV, Szpak-Ulczok S, Bollito E,
Daffara F, et al: Oncocytic adrenocortical tumors: Diagnostic
algorithm and mitochondrial DNA profile in 27 cases. Am J Surg
Pathol. 35:1882–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song WJ, Yan LM, Zhao XL, Liu ZH and Sun
BC: Analysis of mitochondrial DNA D-loop region mutation and its
significance in human oncocytoma. Zhonghua Zhong Liu Za Zhi.
32:767–770. 2010.(In Chinese). PubMed/NCBI
|
|
11
|
Li Q, Wang L, Dai Z and Wan H: An
eosinophilic adenoma of adrenal cortex. J Dalian Med Univ.
41:476–478. 2019.(In Chinese).
|
|
12
|
Shah RK, Oto A, Ozkan OS, Ernst RD,
Hernandez JA, Chaudhary HB and Koroglu M: Adrenal oncocytoma: US
and CT findings. JBR-BTR. 87:180–182. 2003.
|
|
13
|
Yordanova G, Iotova V, Kalchev K, Ivanov
K, Balev B, Kolev N, Tonev A and Oosterhuis W: Virilizing adrehal
oncocytoma in a 9-year-old girl: Rare neoplasm with an intriguing
postoperative course. J Pedliatr Endocrinol Metab. 28:685–690.
2015.PubMed/NCBI
|
|
14
|
Chisté M, Poppiti RJ and Bianeo FJ:
Oncoeytoma of the adrenal gland Medulla. Ann Diagn Pathol.
17:123–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong DD, Spagnolo DV, Bisceglia M, Havlat
M, McCallum D and Platten MA: Oncocytic adrenocortical neoplasms-a
clinicopathologic study of 13 new cases emphasizing the importance
of their recognition. Hum Pathol. 42:489–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu S, Jiang D, Zhang X, Gao F and Hou Y:
Clinicopathological features of 9 cases of adrenocortical
eosinophilic tumor. J Clin Exp Pathol. 37:555–558. 2021.(In
Chinese).
|
|
17
|
He H, Zhu Y, He W, Xie X, Dai J, Wang X,
Wang H, Rui W and Sun F: Clinical investigation on minimal invasive
surgery for Cushing syndrome caused by adrenocortical adenoma:
Experience of 121 cases in a single center. Chin J Urol.
38:244–247. 2017.(In Chinese).
|