Introduction
Xerostomia caused by gland radiation injury is a
common complication of radiotherapy (RT) in patients with
nasopharyngeal carcinoma (NPC) that adversely affects long-term
quality of life. Studies have confirmed that the degree of
glandular functional impairment is highly correlated with radiation
dose (1,2). Therefore, modern RT techniques, such
as conformal RT, intensity modulated RT (IMRT) and salivary gland
preservation, are used to minimize radiation-mediated damage to the
parotid gland. In addition, specific drugs are also used to protect
the microstructure of the parotid gland, to minimize functional
damage, thereby reducing the symptoms of dry mouth (3). However, treatment remains inadequate,
and further investigations into alternative strategies are required
to improve the protection of the parotid tissue. In addition,
further understanding of the mechanisms and evolution of radiation
injury of the parotid gland during the whole course of RT is
required, particularly in the acute phase. Notably, a decline in
gland function due to radiation damage is reversible during the
acute phase (4). Results of
previous studies demonstrated that inflammation, edema,
degeneration and necrosis of acinus cells, vascular injury and
organ atrophy occurred in the glandular tissues of rats, pigs and
rhesus monkeys following radiation exposure (4,5). Due
to the invasive nature of parotid tissue biopsies and the lack of
comprehensive access to the complete glandular tissue damage,
similar studies in human subjects are not possible. Therefore, it
is of great clinical value to develop in vivo techniques for
monitoring radiation damage of the parotid gland at both cellular
and vascular levels.
Quantitative functional magnetic resonance imaging
(MRI) is used for accurately and non-invasively determining
microscopic changes in radiation damage in glands. For example,
diffusion and perfusion MRI technology accurately provide multiple
quantitative indicators associated with tissue biology, such as
cell density and vascular perfusion (6,7).
Dynamic contrast-enhanced (DCE) MRI (8) is a well-established technique for
evaluating hemodynamic characteristics of various tissues, such as
vascular leakage and permeability. However, DCE MRI is limited, as
the injection of contrast media may cause renal impairment and
renal fibrosis. Three-dimensional pulsed continuous arterial spin
labeling (3D-pCASL) technology safely and non-invasively evaluates
tissue perfusion without the use of a contrast agent. Instead,
freely diffusing water molecules are used as endogenous contrast
agents (9). Diffusion-weighted
imaging (DWI) provides insights into the cellular structure and is
sensitive to the heat-driven motion of water molecules in tissues
(6). Therefore, DWI accurately
reflects the state of radiation-induced cell inactivation. Intrixel
incoherent motion imaging (IVIM) is a further development of DWI,
capable of delivering both diffusion and perfusion imaging results
without the need for contrast injection, with the advantage of
being completely non-invasive (9,10).
IVIM has been widely used in hepatic fibrosis evaluation (11), staging (9,10),
efficacy evaluation (12), the
differential diagnosis of fibrosis, and monitoring of NPC
recurrence following RT (13).
In January 2019, a prospective study of IVIM and
3D-pCASL was established to assess the tumor response to IMRT in
patients with NPC. As part of this ongoing study, the effects of
radiation damage to the parotid gland were investigated following
treatment. The present study aimed to investigate the effects of
radiation damage to the parotid gland in a total of 18
patients.
Materials and methods
Patients
In total, 22 patients with NPC who were diagnosed
using nasopharyngeal endoscopy and pathology in The First People's
Hospital of Foshan(Guangdong, China) from January 2019 to July 2019
were included in the present study, and the corresponding data were
collected. The inclusion criteria were as follows: i) All patients
were diagnosed for the first time and had not received any
treatment (chemotherapy or chemoradiotherapy); ii) patients had no
history of other parotid diseases; and iii) there were no MR
examination contraindications. Patients were excluded from the
present study according to the following criteria: i) The presence
of other head and neck tumors; ii) a history of factors affecting
the function of the parotid gland, including mumps, rheumatic
immune disease, a history of tumor chemotherapy or secretory
disease; iii) poor image quality; and iv) poor compliance
(Including the intake of acidic food within 1 h of MR examination).
In total, 4 patients were excluded from the present study. Notably,
2 cases exhibited artifacts on imaging, 1 case failed to be
re-examined within the specified time period, and 1 patient
consumed a carbonated drink 15 min prior to examination. Thus, 18
patients (14 men and 14 women; age range, 34–56 years; median age,
50 years) with NPC were included in the present study, leading to
data being available for a total of 36 parotid glands. The detailed
clinical information of all patients is displayed in Tables I and SI. Tumor staging was carried out
according to the staging standard of the eighth edition of the
American Joint Committee on Cancer guidelines (14).
 | Table I.Selected patient and tumor
characteristics. |
Table I.
Selected patient and tumor
characteristics.
| Characteristic | Value |
|---|
| Patients (parotid
glands), n | 18 (36) |
| Sex (male/female),
n | 14/4 |
| Median age (IQR),
years | 49.50 (12) |
| T stage, n (%) |
|
| T1 | 0 (0.00) |
| T2 | 9 (50.00) |
| T3 | 3 (16.67) |
| T4 | 6 (33.33) |
| N stage, n (%) |
|
| N0 | 1 (5.56) |
| N1 | 7 (38.89) |
| N2 | 7 (38.89) |
| N3 | 3 (16.67) |
| M stage |
|
| M0 | 18 (100.00) |
| Clinical stage |
|
|
II–III | 11 (61.11) |
| IV | 7 (38.89) |
| Radiation dose,
mGy |
|
| Median
(IQR) | 3715.65
(477.02) |
|
Range |
3076.50–6897.50 |
This prospective study was carried out in accordance
with the latest version of the Declaration of Helsinki and was
approved by the Ethics Committee of The First People's Hospital of
Foshan [approval no. 2018 (18th)]. Written informed consent was
obtained at the time of the first MR examination from each
patient.
Chemoradiotherapy regimen
According to the China Nasopharyngeal Cancer Staging
2017 Edition (15), all enrolled
patients exhibited disease stages II–IVA. Three stages of induction
chemotherapy were performed in all patients, followed by radical
concurrent chemoradiotherapy plus targeted therapy, in which RT was
performed with head and neck IMRT. The total gross dose of the
tumor was 70 mGy, the segmentation dose was 2.12 mGy and the number
of segmentations was 33. Chemotherapeutic agents were administered
prior to RT (docetaxel, 60 mg/m2, intravenous drip;
Nedaplatin (SinopOD: H20064294, specification: 10 mg/branch) 60
mg/m2, intravenous drip; 21 days as a course of treatment,
continuous treatment for 2 courses.
Radiation dosage data acquisition
The RT regimen for all patients was based on CT
positioning images. Patients wore a thermoplastic body membrane of
the head, neck and shoulder in a supine position, and were
positioned in the Philips 16-row large-aperture spiral CT
simulation machine (Philips Healthcare). The scanning range spanned
from the top of the head to ~2 cm below the edge of the clavicle,
and the thickness of the image layer was ~3 mm. Localized CT images
and 3D-T1 Bravo images obtained during pre-treatment scans of
patients were transferred to the treatment planning system (Varian
Eclipse version 10.0; Varian Medical Systems, Inc.). CT location
images were fused with the MR 3D-T1 Bravo images using the
automatic matching function. Two nasopharyngeal radiotherapists
delineated the target area on the fusion image. Mean and maximum
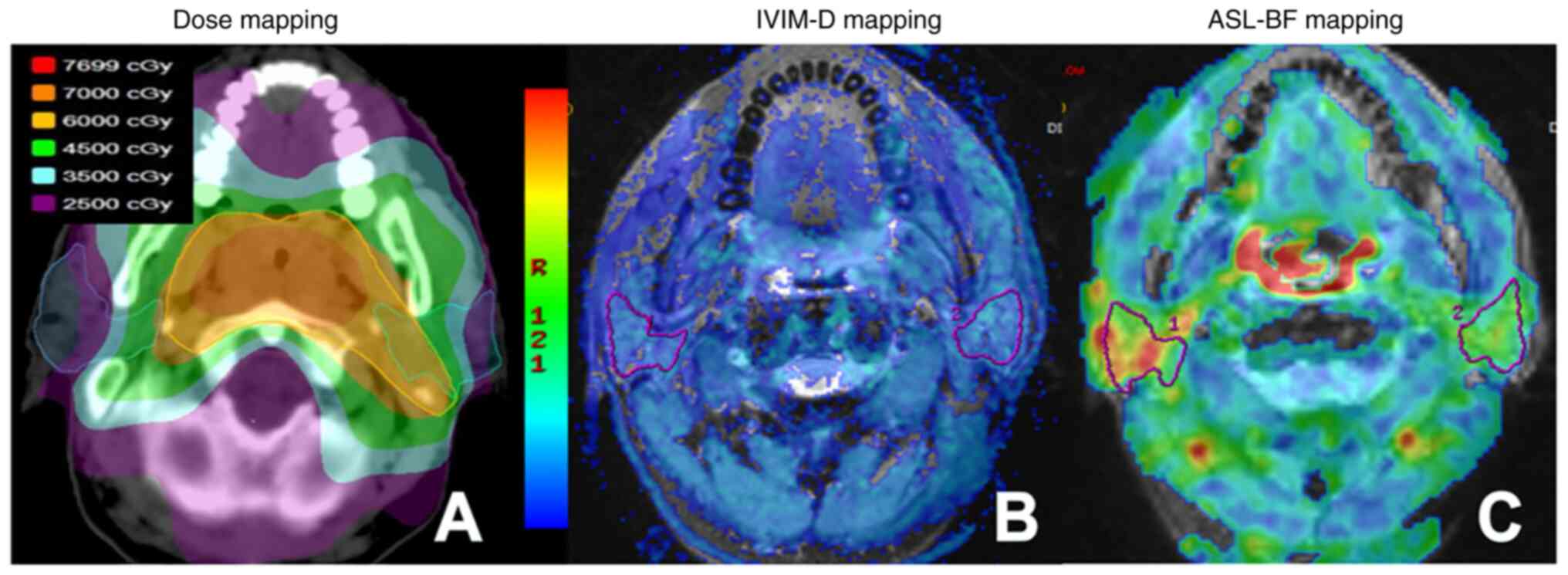
doses were obtained (Fig. 1A).
MRI protocols
Examinations were performed using a 3.0T MR scanner
(Discovery 750w 3.0T MR system; Cytiva) and an 8-channel
head-and-neck combined coil. The conventional MR examination
included the T1-weighted imaging fluid-attenuated inversion
recovery sequence as follows: Time of repetition (TR), 657 msec;
time of echo (TE), 24 msec; time of inversion, 780 msec; a
fat-saturated T2WI fast spin echo (FSE)-based sequence; TR/TE,
5,114/110 msec; number of excitations (NEX), 2.0; field of view
(FOV), 240×240 mm; matrix, 288×224; and section thickness/gap, 5/1
mm.
IVIM-DWI was performed using 13 b-values (0, 10, 20,
40, 60, 100, 120, 160, 200, 400, 600, 800 and 1,000
sec/mm2), and applied using the following single-shot
spin echo echoplanar imaging sequence: TR, 3,685 msec; TE, 43 msec;
NEX, 2.0; FOV, 240×240 mm; matrix, 160×160; and section
thickness/gap, 5/0 mm. For 3D-pCASL, an ASL sequence with a 3D FSE
spiral acquisition was performed using the following parameters:
TR, 4,295 msec; TE, 11.5 msec; NEX, 3.0; section thickness/gap, 3/0
mm; layer number, 30; FOV, 220×220 mm; matrix, 288×192; post Label
Delay (PLD) time, 1,025 msec; and scanning time, 4 min 10 sec.
Axial T2WI-FSE was performed using a scanning range and positioning
line consistent with those of ASL, and the following additional
parameters: TR/TE, 3,000/68 msec. All patients underwent
conventional MRI, IVIM and ASL imaging of the bilateral parotid
glands within 2 weeks prior to RT and 1 week and 3 months following
RT.
The consumption of food or drink was not permitted
for at least 1 h prior to MR examination.
MRI data analysis
The IVIM and ASL raw data obtained from scans were
transferred to GE-AW 4.6 workstation software (GE Healthcare) for
post-processing. Three parameters, namely, pure diffusion
coefficient (D), pseudo-diffusion coefficient (D*) and perfusion
fraction (F), were automatically derived and calculated from IVIM,
by fitting the MR signal acquired at 13 b-values to a
bi-exponential model. Blood flow (BF) was automatically derived and
calculated from ASL data. The regions of interest (ROIs) were
determined within the largest section of the parotid gland on the
fusion diagram, using parameters derived from IVIM or ASL and T2WI.
The ROI in the D map included the maximal potential amount of
parotid parenchyma, excluding visible parotid ducts and
retromandibular veins (Fig. 1B).
Additional ROIs were automatically matched on D* and F maps.
Selection of the ROI in ASL-BF images was consistent with the
parameter images of IVIM (Fig. 1C).
To improve the accuracy of the ROI outline, all images were
enlarged 2–3 times. The change rates of ASL-BF and IVIM (D, D* and
F) parameters were calculated using the following equation:
ΔR(T)%=(Rpost-RT-Rpre-RT)/Rpre-RT ×100, where R represents BF, D,
D* and F; and T represents time.
Measurements of all MR parameters were performed by
two radiologists with 8 and 12 years of experience in head and neck
radiology, respectively. Both radiologists were blinded to the
clinical data of the patients and were not aware of the aims of the
present study. In total, two doctors reviewed the images and
measured the data, and all image analyses and parameter
measurements were consistent.
Statistical analysis
Statistical analysis was performed using SPSS
software (version, 24.0; IBM Corp.). Descriptive statistics were
used to analyze the demographic data and clinical characteristics
of all patients. Numerical data are presented as the mean ±
standard deviation when normally distributed. All other data are
presented as the median (IQR). Kolmogorov-Smirnov's test was used
to determine whether the parameters were normally distributed. The
difference in percentage changes of all parameters derived from
IVIM and ASL over time were assessed using a repeated measures
Kruskal-Wallis test, and the Wilcoxon rank-sum test was then used
as the post hoc test, with Bonferroni's correction used to correct
P-values. Potential correlations between MR parameters and dose
were analyzed using Spearman's correlation coefficient. P<0.05
was considered to indicate a statistically significant
difference.
Results
Basic information of patients
Among the 18 included patients, all patients
successfully underwent the planned therapeutic regimens and
follow-up MR examinations. Detailed clinical data of each patient
were available in tale S1. No
measurements were excluded due to insufficient quality. ASL-BF and
multi-parameters of IVIM (D, D* and F) images of bilateral parotid
glands of one representative subject pre- and post-RT are displayed
in Fig. 2. All patients exhibited
different degrees of dry mouth without quantitative grading.
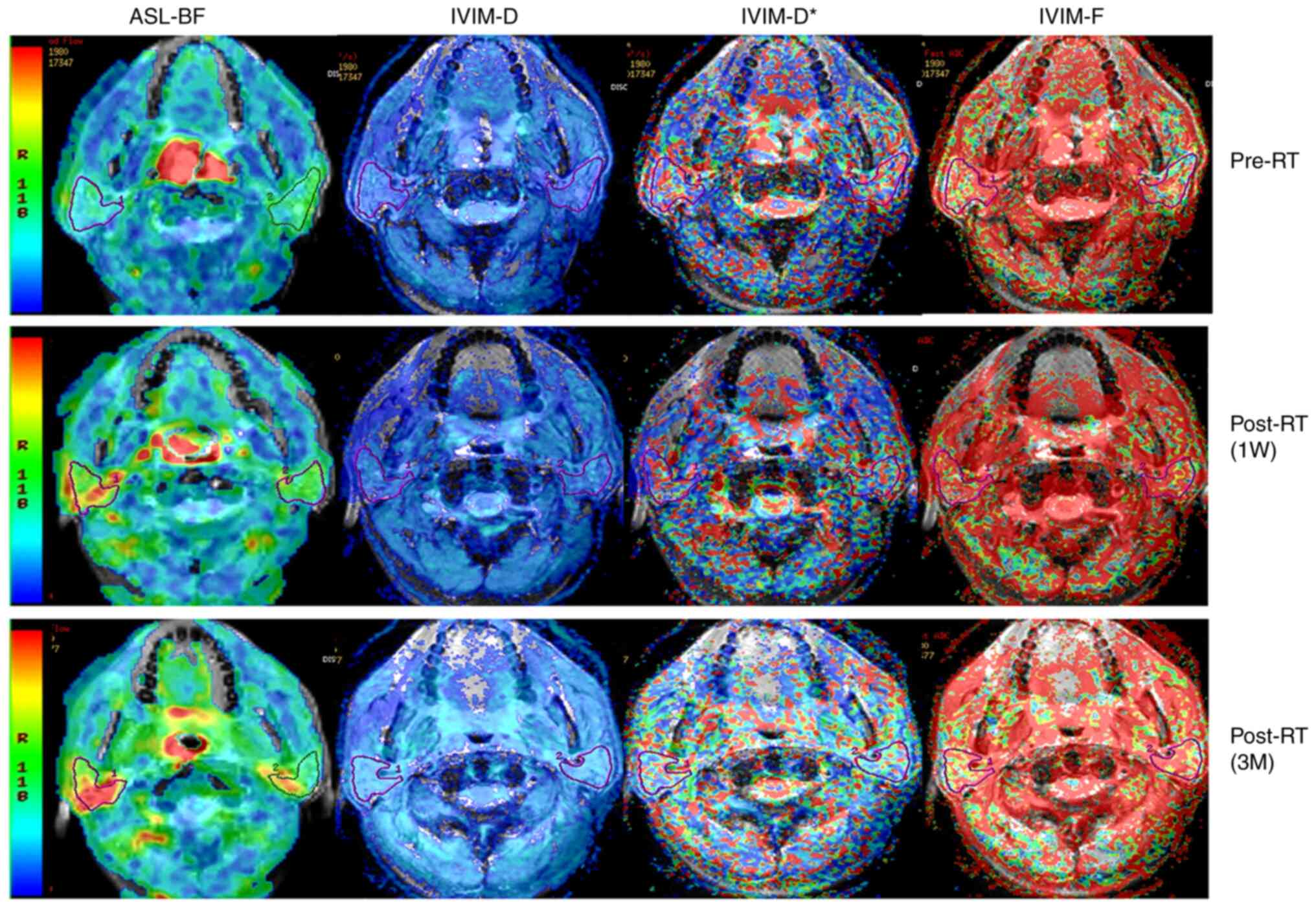 | Figure 2.Representative 3D-ASL and IVIM images
of bilateral parotid glands of a 58-year-old male patient with
nasopharyngeal carcinoma at different time-points pre- and post-RT.
For the parotid glands, BF images and D images both illustrated a
significantly higher signal at 1W post-RT and 3M post-RT compared
to that pre-RT. D* images illustrated a gradually decreasing signal
at 1W post-RT and then 3M post-RT compared with that pre-RT. F
images illustrated a significantly higher signal at 1W post-RT
compared with that pre-RT, and with a similar signal at 3M post-RT.
IVIM, intravoxel incoherent motion imaging; D, pure diffusion
coefficient; D*, pseudo-diffusion coefficient; F, perfusion
fraction; BF, blood flow; ASL, arterial spin labeling; RT,
radiotherapy; 1W, 1 week; 3M, 3 months. |
Dynamic changes in D, D*, F and BF at
different follow-up time-points
Dynamic changes in D, D*, F and BF values are
displayed in Table II and Fig. 3. Results of the present study
demonstrated that D and BF values both increased significantly
pre-RT to 1 week (1W) post-RT [median change rate: Median (IQR),
ΔD1W%: 39.28% (38.23%) and ΔBF1W%: 60.84%
(54.88%)], and these continued to increase from 1W post-RT to 3
months (3M) post-RT [change rate: Median (IQR), ΔD%: 55.44%
(40.56%) and ΔBF%: 120.39% (128.74%)]. The results of the present
study also demonstrated that the parotid F value was significantly
increased from pre-RT to 1W post-RT [change rate: Median (IQR),
ΔF1W%: 28.13% (44.66%)], and this was significantly
decreased from 1W to 3M post-RT. Notably, there were no significant
differences in the change rates between pre-RT and 3M post-RT
[change rate: Median (IQR), ΔF3M%: 3.30% (40.43%)]. From
pre-RT to 1W post-RT and 3 month (3M) post-RT, the parotid D* value
was significantly decreased [change rate: Median (IQR),
ΔD*1w%: −41.86% (51.71%) and ΔD*3M: −29.11%
(42.67%)]. However, no significant differences were observed
between different post-RT time intervals.
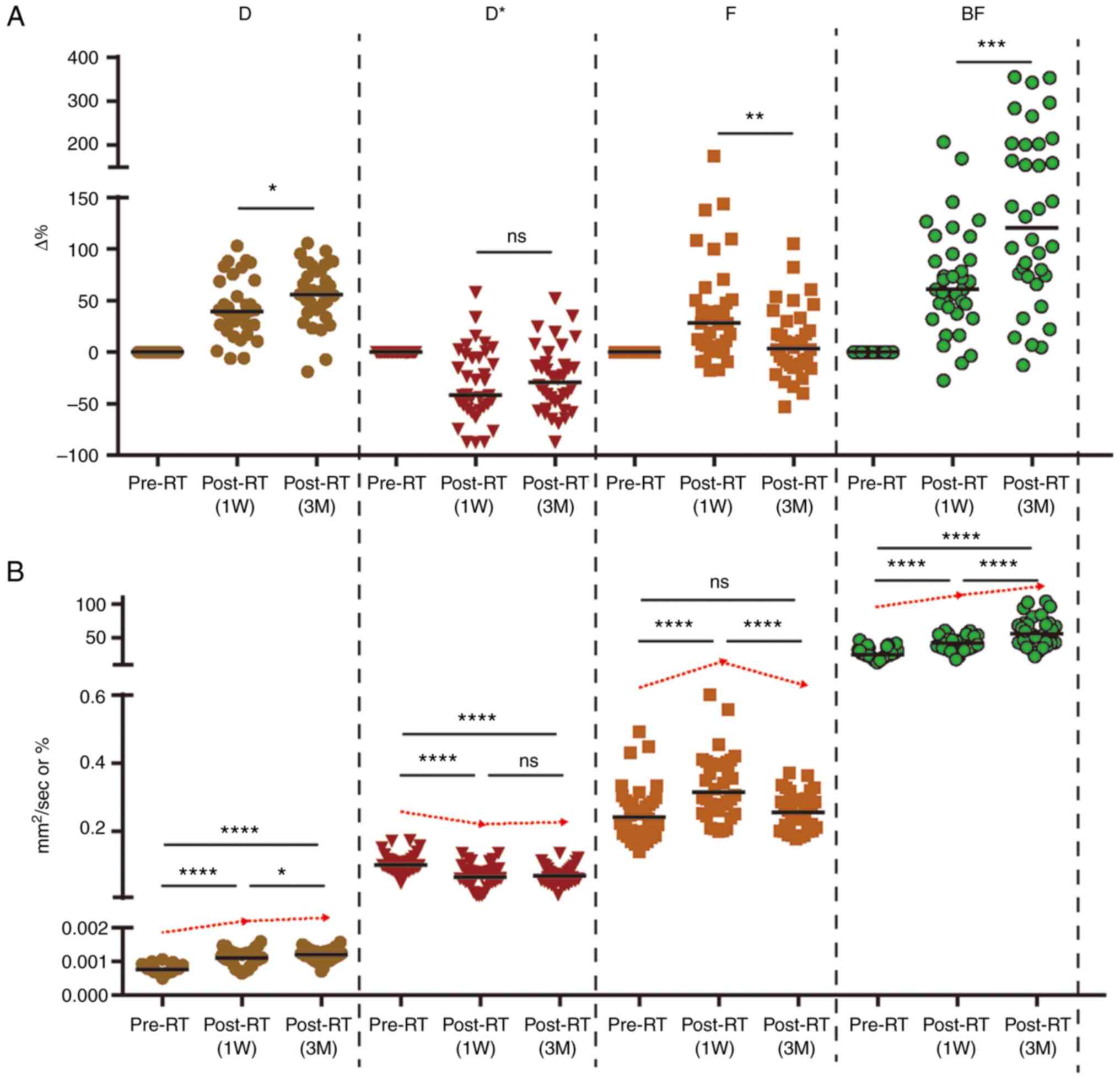 | Figure 3.Dynamic changes of
multiple-parameter (D, D*, F and BF) values. (A) ΔD%, ΔF% and
ΔBF% showed significant differences between 1W post-RT and 3M
post-RT. (B) Values of D and BF increased significantly after RT at
different follow-up time-points. F value increased first and then
decreased, while the D* value was reversed. IVIM, intravoxel
incoherent motion imaging; D, pure diffusion coefficient; D*,
pseudo-diffusion coefficient; F, perfusion fraction; BF, blood
flow; ASL, arterial spin labeling; RT, radiotherapy; ns,
non-significant; 1W, 1 week; 3M, 3 months. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. |
 | Table II.Dynamic changes of D, D*, F and BF at
different follow-up time-points. |
Table II.
Dynamic changes of D, D*, F and BF at
different follow-up time-points.
| Parameters | Pre-RT | 1W post-RT | 3M post-RT |
|---|
| D, ×10−3
sec/mm2 | 0.77 (0.19) | 1.11 (0.39) | 1.21 (0.28) |
| D*,
×10−3 sec/mm2 | 100.05 (28.85) | 65.65 (35.08) | 69.80 (35.70) |
| F, % | 0.241 (0.10) | 0.315 (0.14) | 0.256 (0.09) |
| BF, ml/100
g/min | 24.64 (10.46) | 42.00 (16.24) | 55.75 (26.92) |
| ΔD, % | - | 39.28 (38.23) | 55.44 (40.56) |
| ΔD*, % | - | −41.86 (51.71) | −29.11 (42.67) |
| ΔF, % | - | 28.13 (44.66) | 3.30 (40.43) |
| ΔBF, % | - | 60.84 (54.88) | 120.39
(128.74) |
Correlation between ΔD, ΔD* ΔF and ΔBF
and RT dose
Results of the present study demonstrated that there
was a significant positive correlation between percentage change in
ΔBF1W and radiation dose (ρ=0.548; P=0.001). Notably, a
higher radiation dose was correlated with a larger percentage
change in ΔBF1W. However, the correlation was
insignificant between ΔBF3M and radiation dose
(ρ=0.095). No significant correlations were observed between ΔD,
ΔD* and ΔF and radiation dose at the different follow-up
time-points (Table III).
 | Table III.Correlation of radiation dose with
change in intravoxel incoherent motion imaging and arterial spin
labeling parameters at different follow-up time-points. |
Table III.
Correlation of radiation dose with
change in intravoxel incoherent motion imaging and arterial spin
labeling parameters at different follow-up time-points.
| Statistic | RT dose |
ΔD1W |
ΔD3M |
ΔD*1W |
ΔD*3M |
ΔF1W |
ΔF3M |
ΔBF1W |
ΔBF3M |
|---|
| ρ | 1.00 | −0.092 | −0.070 | −0.233 | −0.243 | 0.242 | 0.042 | 0.549 | 0.283 |
| P-value |
| 0.592 | 0.685 | 0.171 | 0.153 | 0.156 | 0.807 | 0.001 | 0.095 |
Discussion
Salivary gland cells are sensitive to radiation;
therefore, a lack of saliva secretion in patients with head and
neck tumors following RT may cause a series of complications, such
as dry mouth, dysphagia, loss of taste and oral ulcers (16). These symptoms may also affect a
patients' quality of life. Results of a previous study revealed
that patients with head and neck tumors who received RT experienced
a significant reduction in the size of the parotid glands in the
early stages following RT, which may be associated with a decrease
in the number of gland cells and acinar atrophy (6,7,17,18).
In addition to gland cells, vascular endothelial cells have
remained the focus of research surrounding radiation-mediated
injury, and the results of previous animal experiments demonstrated
that radiation reduces the microvessel density of the parotid gland
and local blood flow (5,19). The reduction of local tissue
perfusion may affect the function of the excretory duct in the
parotid gland, thereby affecting the secretion and excretion of
saliva. Non-invasive imaging technology is used to accurately
determine potential changes in the morphology and function of the
salivary glands damaged by radiation, and to aid in determining the
pathophysiological mechanisms underlying radiation damage. As the
function of the salivary glands and the degree of radiation damage
differ between individuals, imaging techniques sensitive to tissue
hemodynamics and cell characteristics are used to clarify the
different mechanisms underlying radiation-induced blood vessel and
cell damage in healthy tissues. The evaluation and dynamic
monitoring of changes in the functional structure of the salivary
glands are invaluable during radiation injury. Therefore, the
present study used ASL and IVIM technologies to determine the
pathological and physiological changes in parotid gland tissue
following RT.
Compared with values at baseline pre-RT, the BF
value demonstrated a continuous upward trend at 1W, 1M and 3M
post-RT. We hypothesized that this trend may be associated with the
expansion of microvascular lumen and an increased local blood flow.
In the early stages of RT, the number of vascular endothelial cells
are significantly decreased, leading to damage to the structure and
function of microvessels. This damage may be manifested as abnormal
expansion and rupture of the lumen, and other pathological changes
(20). The apoptosis of vascular
endothelial cells may lead to an inflammatory response in blood
vessels (21), and the expression
of tumor necrosis factor α and interleukin 1, and other
inflammatory factors. Upregulation leads to reactive dilation of
microvessels and increased local blood perfusion (20,21).
In another study using DCE technology, similar results were
obtained. The volume transfer constant (Ktrans)
continued to rise following RT, which may be associated with the
increase in vascular permeability following early RT (7,22).
Compared with traditional DWI, the IVIM uses a
double exponential model to distinguish the signal generated by the
diffusion motion of water molecules from the microvascular
perfusion signal, to determine an accurate reading of the expansion
speed of water molecules in the tissue (23). Results of the present study
demonstrated that at 1W and 3M post-RT, the coefficient D value of
the parotid gland continued to increase. These results are
indicative of the continuous increase in the diffusion rate of
water molecules in the parotid gland tissue following RT,
supporting the results of previous studies (6,24).
Trends observed in the D value are indicative of the gradual
shrinkage and degeneration of serous acinar cells of the parotid
gland, and these may decrease in number with an increase in the
cumulative dose (6,18,22,24). A
decrease in the density of glandular cells leads to an increase in
the diffusion in tissues. During treatment, internal D gradually
increased in the present study. In previous studies, the
observation time varied from a few weeks to a few months following
RT; however, an increase in the observed D value may be associated
with the presence of necrosis and fibrosis (18,24).
Moreover, previous studies on DCE MRI demonstrated that an increase
in the extracellular space (Ve) and plasma volume (Vp) of the
parotid gland tissue following RT may also lead to a significant
increase in the free diffusion of water molecules (7,8,22). In
the present study, compared with the value observed at baseline,
the D* value reflecting the microvascular perfusion component
continued to decrease at 1W and 3M post-RT, while the F-value
reflecting the microvessel density increased at 1W post-RT and
decreased at 3M post-RT. However, these results were not
statistically significant. Notably, these results differed from the
change trend of the BF value observed using ASL. We hypothesized
that the type of imaging technology may lead to differences, and
the histology and cytology of the response also differ. Notably,
the diameter and number of capillaries will impact the local BF
value. D* and F values are indicative of the number and density of
microvessels, and are therefore used more frequently in tumor
grading, and in the differentiation between benign and malignant
tumors (23,25). Further investigations into the
stability of the D* value are required, as this is also affected by
vascular osmotic pressure (26).
Based on the results of the present study, we hypothesized that the
decrease in the number of microvessels in the early stage of RT is
not significant, and although there is a transient decrease in the
number of microvessels at 3M post-RT, this number remains within a
recoverable range.
Results of the present study demonstrated no
significant correlation between changes in the parameters of IVIM
(ΔD, ΔD* and ΔF) and the average dose received by the parotid
glands. These results were similar to those obtained by Marzi et
al (24). Protecting the
parotid gland tissue results in a limited range of dose received by
some parotid glands. In addition, differences in parotid gland
tissue type, such as fat content, may also affect the results. van
Dijk et al (27)
demonstrated that the ratio of parotid gland fat content to
functional parotid gland tissue exerted a positive effect on
radiation damage. Previous studies (7,22) also
reported the association between average dose and perfusion index
(Ve and Vp); however, some studies revealed that the significant
correlation between D value and mean dose (Dmean) is closely
associated with tissue cell density, which is indicative of a
dose-dependent loss of acinar cells (22,24,28).
ΔBF is positively correlated with Dmean, and ΔBF1W
exhibits a significant correlation with Dmean (24,26,28).
Notably, results of the present study demonstrated that there was a
trend for BF to increase at 1W and 3M post-RT; however, ΔBF
gradually decreased. We hypothesized that some glands may have
received higher doses. In the later stage of injury, the effects of
inflammation and endothelial destruction are offset by the effects
of vascular injury, leading to a decrease in permeability and blood
vessel density, which ultimately leads to a decrease in BF. These
results are consistent with those described by Lee et al
(22), and results of the DCE MR
study revealed that changes in Ktrans were
consistent.
The present study adopted a prospective research
approach to evaluate the values of various functional parameters
obtained by MRI, IVIM and ASL in patients with NPC prior to RT, and
at 1W and 3M post-RT. Investigating the dynamic changes in various
parameters before and after RT at each stage may aid in determining
the microscopic changes in parotid gland tissue, including
transient pathological changes. Results of the present study may
provide novel insights into the mechanisms underlying radiation
injury in parotid gland tissue.
However, the present study exhibits numerous
limitations. The clinical data of xerostomia (grading of dry mouth
and salivary gland secretion) were not included in the present
analysis. Therefore, clinical data analysis of parotid gland
function was not available in the present study. In addition, the
duration of follow-up was limited to 3M post-RT, while salivary
gland function may recover up to 5 years after RT (29). The sample size of the present study
was small, and factors that may affect parotid gland tissue damage,
such as dosage and dose distribution, and differences in parotid
gland tissue composition were not classified. Therefore, the
results of the present study require further validation in future
studies. Moreover, IVIM-DWI and ASL were performed in the present
study using parotid gland tissue in the resting state, and
investigations were not conducted during the functional state.
Thus, further olfactory and taste stimulation studies are required.
A combination with ASL technology may lead to improved results.
In conclusion, IVIM-DWI and ASL may aid in
determining the mechanisms underlying radiation damage to the
parotid gland, through the acquisition and recording of water
molecular diffusion, microcirculation and perfusion parameters.
Further investigations into the changing trends of D, F and BF
values, and the change rate at various follow-up time points may
improve the prediction of microstructural changes to parotid gland
tissue.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Innovative
Project of Foshan (grant no. FSOAA-KJ218-1301-0021) and The 14th
Five-Year Plan Key Discipline Foundation Of Foshan (grant no.
SGSP145036).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZX was responsible for study design, interpretation
of data and drafting the manuscript. XZ was responsible for the
interpretation of data and drafting the manuscript. YJ, LH, WW, and
MG were responsible for the analysis and interpretation of data.
XZ, ZX, YJ, LH, WW and MG confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present prospective study was carried out in
accordance with the latest version of the Declaration of Helsinki
and was approved by the Ethics Committee of The First People's
Hospital of Foshan (Foshan, China). All patients provided informed
consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
RT
|
radiotherapy
|
|
IMRT
|
intensity modulated RT
|
|
MRI
|
magnetic resonance imaging
|
|
3D-pCASL
|
three-dimensional pulsed continuous
arterial spin labeling
|
|
DWI
|
diffusion-weighted imaging
|
|
IVIM
|
intravoxel incoherent motion
imaging
|
|
ASL
|
arterial spin labeling
|
|
BF
|
blood flow
|
|
ROIs
|
regions of interest
|
References
|
1
|
Guo Y, Jiang W, Lakshminarayanan P, Han P,
Cheng Z, Bowers M, Hui X, Shpitser I, Siddiqui S, Taylor RH, et al:
Spatial radiation dose influence on xerostomia recovery and its
comparison to acute incidence in patients with head and neck
cancer. Adv Radiat Oncol. 5:221–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang K, Pearlstein KA, Moon DH, Mahbooba
ZM, Deal AM, Wang Y, Sutton SR, Motley BB, Judy GD, Holmes JA, et
al: Assessment of risk of xerostomia after whole-brain radiation
therapy and association with parotid dose. JAMA Oncol. 5:221–228.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang T, Liu C, Ma S, Gao Y and Wang R:
Protective effect and mechanism of action of rosmarinic acid on
radiation-induced parotid gland injury in rats. Dose Response.
18:15593258209077822020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng X, Wu Z, Xu J, Xu Y, Zhao B, Pang B,
Qu X, Hu L, Hu L, Fan Z, et al: Dietary nitrate supplementation
prevents radiotherapy-induced xerostomia. Elife. 10:e707102021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Yan X, Gao R, Mao L, Cotrim AP,
Zheng C, Zhang C, Baum BJ and Wang S: Effect of irradiation on
microvascular endothelial cells of parotid glands in the miniature
pig. Int J Radiat Oncol Biol Phys. 78:897–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan WJ, Teng F, Luo YR, Yu W, Zhang Q, Lu
YP and Ma L: Diffusion-weighted imaging as a follow-up modality for
evaluation of major salivary gland function in nasopharyngeal
carcinoma patients: A preliminary study. Strahlenther Onkol.
196:530–541. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juan CJ, Chen CY, Jen YM, Liu HS, Liu YJ,
Hsueh CJ, Wang CY, Chou YC, Chai YT, Huang GS and Chung HW:
Perfusion characteristics of late radiation injury of parotid
glands: Quantitative evaluation with dynamic contrast-enhanced MRI.
Eur Radiol. 19:94–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Z, Zheng S, Pan A, Cheng X and Gao M: A
multiparametric analysis based on DCE-MRI to improve the accuracy
of parotid tumor discrimination. Eur J Nucl Med Mol Imaging.
46:2228–2234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu W, Jiang G, Xu Z, Wang R, Pan A, Gao M,
Yu T, Huang L, Quan Q and Li J: Three-dimensional pulsed continuous
arterial spin labeling and intravoxel incoherent motion imaging of
nasopharyngeal carcinoma: Correlations with Ki-67 proliferation
status. Quant Imaging Med Surg. 11:1394–1405. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Lin CY, Qi YF, Wang X, Chen B, Zhou
HL, Ren J, Yang JJ, Xiang Y, He YL, et al: Three-dimensional
turbo-spin-echo amide proton transfer-weighted and intravoxel
incoherent motion MR imaging for type I endometrial carcinoma:
Correlation with Ki-67 proliferation status. Magn Reson Imaging.
78:18–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu YM, Wang W, Wen J, Zhang Y, Lu GM and
Zhang LJ: Detection of renal allograft fibrosis with MRI: Arterial
spin labeling outperforms reduced field-of-view IVIM. Eur Radiol.
31:6696–6707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poynton CB, Lee MM, Li Y, Laszik Z,
Worters PW, Mackenzie JD and Courtier J: Intravoxel incoherent
motion analysis of renal allograft diffusion with clinical and
histopathological correlation in pediatric kidney transplant
patients: A preliminary cross-sectional observational study.
Pediatr Transplant. 21:e129962017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia QJ, Zhang SX, Chen WB, Liang L, Zhou
ZG, Qiu QH, Liu ZY, Zeng QX and Liang CH: Initial experience of
correlating parameters of intravoxel incoherent motion and dynamic
contrast-enhanced magnetic resonance imaging at 3.0 T in
nasopharyngeal carcinoma. Eur Radiol. 24:3076–3087. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang M, Zhou P, Li G, Yan H and Wang R:
Validation of the 8th edition of the UICC/AJCC staging system for
nasopharyngeal carcinoma treated with intensity-modulated
radiotherapy. Oncotarget. 8:70586–70594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinese Committee for Staging of
Nasopharyngeal Carcinoma, . The 2017 edition for staging of
nasopharyngeal carcinoma in China (The Chinese 2008 expert
consensus on staging revision of nasopharyngeal carcinoma). Chinese
J Radiation Oncology. 26:1119–1124. 2017.(In Chinese).
|
|
16
|
Westgaard KL, Hynne H, Amdal CD, Young A,
Singh PB, Chen X, Rykke M, Hove LH, Aqrawi LA, Utheim TP, et al:
Oral and ocular late effects in head and neck cancer patients
treated with radiotherapy. Sci Rep. 11:40262021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Acauan MD, Figueiredo MA, Cherubini K,
Gomes AP and Salum FG: Radiotherapy-induced salivary dysfunction:
Structural changes, pathogenetic mechanisms and therapies. Arch
Oral Biol. 60:1802–1810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu VWC, Ying MT, Kwong DL, Khong PL, Wong
GK and Tam SY: A longitudinal study on parotid and submandibular
gland changes assessed by magnetic resonance imaging and
ultrasonography in post-radiotherapy nasopharyngeal cancer
patients. BJR Open. 2:202000032020.PubMed/NCBI
|
|
19
|
Hu S, Gao Y, Zhou H, Kong F, Xiao F, Zhou
P and Chen Y: New insight into mitochondrial changes in vascular
endothelial cells irradiated by gamma ray. Int J Radiat Biol.
93:470–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fajardo LF: The pathology of ionizing
radiation as defined by morphologic patterns. Acta Oncol. 44:13–22.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boström M, Kalm M, Eriksson Y, Bull C,
Ståhlberg A, Björk-Eriksson T, Hellström Erkenstam N and Blomgren
K: A role for endothelial cells in radiation-induced inflammation.
Int J Radiat Biol. 94:259–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee FK, King AD, Kam MK, Ma BB and Yeung
DK: Radiation injury of the parotid glands during treatment for
head and neck cancer: Assessment using dynamic contrast-enhanced MR
imaging. Radiat Res. 175:291–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bisdas S, Koh TS, Roder C, Braun C,
Schittenhelm J, Ernemann U and Klose U: Intravoxel incoherent
motion diffusion-weighted MR imaging of gliomas: Feasibility of the
method and initial results. Neuroradiology. 55:1189–1196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marzi S, Forina C, Marucci L, Giovinazzo
G, Giordano C, Piludu F, Landoni V, Spriano G and Vidiri A: Early
radiation-induced changes evaluated by intravoxel incoherent motion
in the major salivary glands. J Magn Reson Imaging. 41:974–982.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen N, Zhao L, Jiang J, Jiang R, Su C,
Zhang S, Tang X and Zhu W: Intravoxel incoherent motion
diffusion-weighted imaging analysis of diffusion and microperfusion
in grading gliomas and comparison with arterial spin labeling for
evaluation of tumor perfusion. J Magn Reson Imaging. 44:620–632.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dolgorsuren EA, Harada M, Kanazawa Y, Abe
T, Otomo M, Matsumoto Y, Mizobuchi Y and Nakajima K: Correlation
and characteristics of intravoxel incoherent motion and arterial
spin labeling techniques versus multiple parameters obtained on
dynamic susceptibility contrast perfusion MRI for brain tumors. J
Med Invest. 66:308–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Dijk LV, Thor M, Steenbakkers RJHM,
Apte A, Zhai TT, Borra R, Noordzij W, Estilo C, Lee N, Langendijk
JA, et al: Parotid gland fat related Magnetic Resonance image
biomarkers improve prediction of late radiation-induced xerostomia.
Radiother Oncol. 128:459–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Murata Y, Ishida R, Ohashi I,
Yoshimura R and Shibuya H: Functional evaluation with intravoxel
incoherent motion echo-planar MRI in irradiated salivary glands: A
correlative study with salivary gland scintigraphy. J Magn Reson
Imaging. 14:223–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hey J, Setz J, Gerlach R, Janich M,
Hildebrandt G, Vordermark D, Gernhardt CR and Kuhnt T: Parotid
gland-recovery after radiotherapy in the head and neck region-36
months follow-up of a prospective clinical study. Radiat Oncol.
6:1252011. View Article : Google Scholar : PubMed/NCBI
|