Introduction
Esophageal cancer ranks as the ninth most common
cancer (1), with 54% of the cases
occurring in China in 2018 (2).
Esophageal squamous cell carcinoma (ESCC) is the most common
histological type, constituting 85.79% of all cases, followed by
esophageal adenocarcinoma at 11.00% and others types as 3.21%
(3). For locally advanced ESCC,
important treatment options include neoadjuvant chemotherapy or
chemoradiotherapy followed by surgery, as well as definitive
chemoradiotherapy (4). However, the
optimal approach for locally advanced ESCC remains unclear,
necessitating further research. Several clinical trials have
demonstrated the effectiveness of combining immune checkpoint
inhibitors (ICIs) with chemotherapy for locally advanced ESCC
(5–7). Yet, not all cases of ESCC respond to
this combined therapy, with response rates ranging from 16.7 to
58.3%. The resistance mechanism to ICIs or chemotherapy in ESCC has
not been comprehensively investigated. Reports have indicated that
the transformation of non-small cell lung cancer (NSCLC) into small
cell lung cancer (SCLC) can act as a resistance mechanism to ICIs
(8,9). Treatments for advanced esophageal
neuroendocrine carcinoma (ENEC) are similar to those used in SCLC
(10). The similar transformation
of ESCC to ENEC could serve as one possible resistance mechanism to
ICIs. The present study report the case of a patient with a
preoperative diagnosis of ESCC who underwent neoadjuvant treatment
with ICIs in combination with chemotherapy, resulting in a
pathological transformation into ENEC.
Case report
A 58-year-old man presented to Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China in April 2023 with epigastric pain that
had persisted for 1 week. The patient had no significant medical or
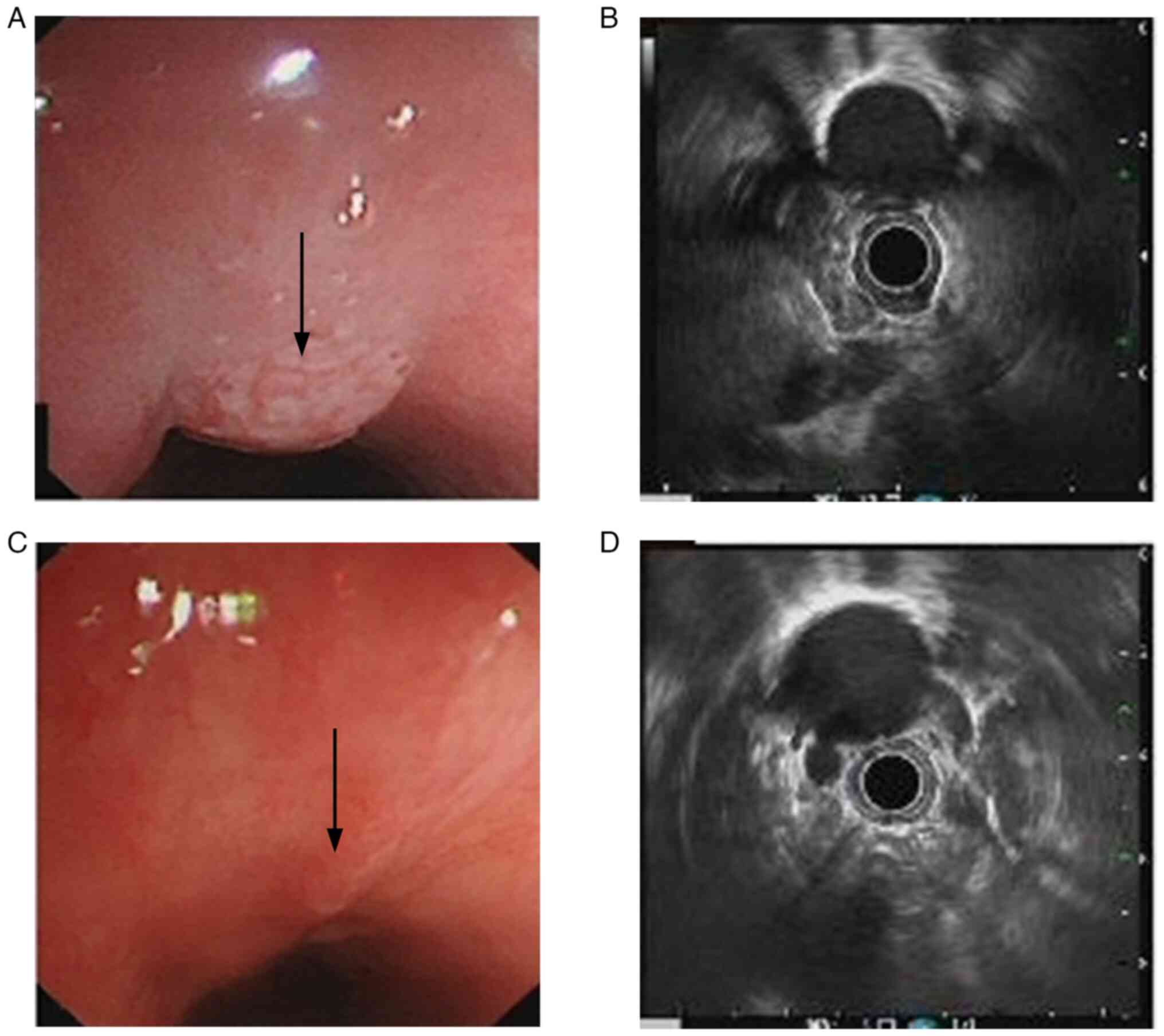
family history. Endoscopic ultrasonography revealed a 6-cm mass 25
cm into the esophagus when measured from the location of the
incisors (Fig. 1A), invading the
muscularis propria, with the thickest section measuring ~5.9 mm and
the outer membrane remaining smooth, with two hypoechoic nodules in
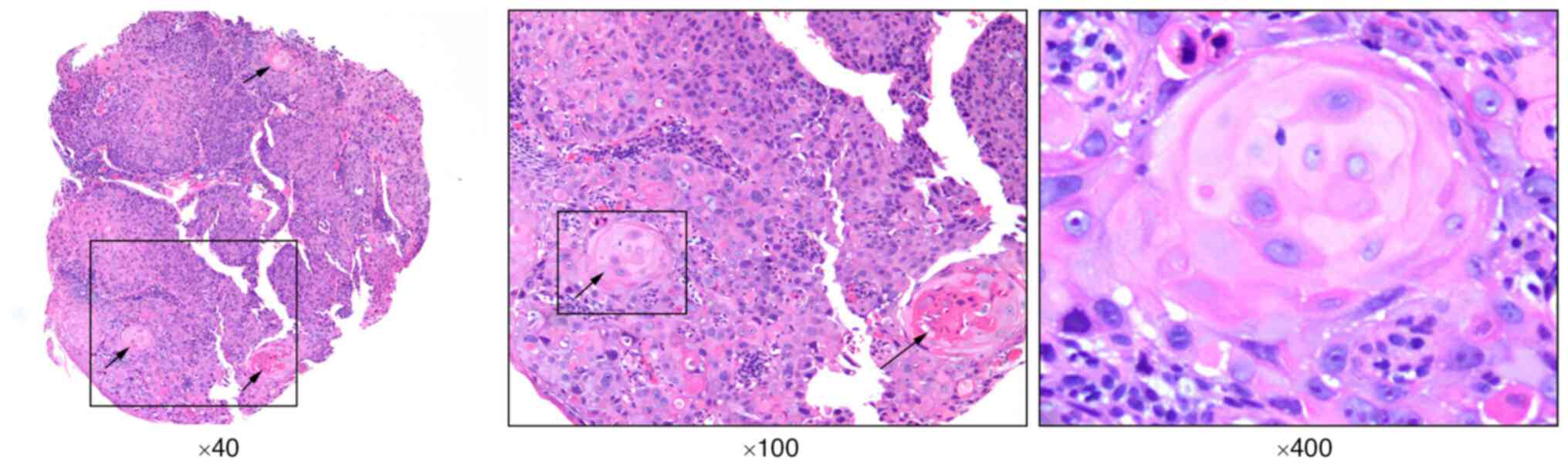
the mediastinum next to the lesioned esophagus (Fig. 1B). Hematoxylin and eosin (H&E)
staining of a biopsy specimen showed heterogeneous hyperplasia of
the squamous epithelium with keratinized pearl formation, leading
to a diagnosis of SCC (Fig. 2).
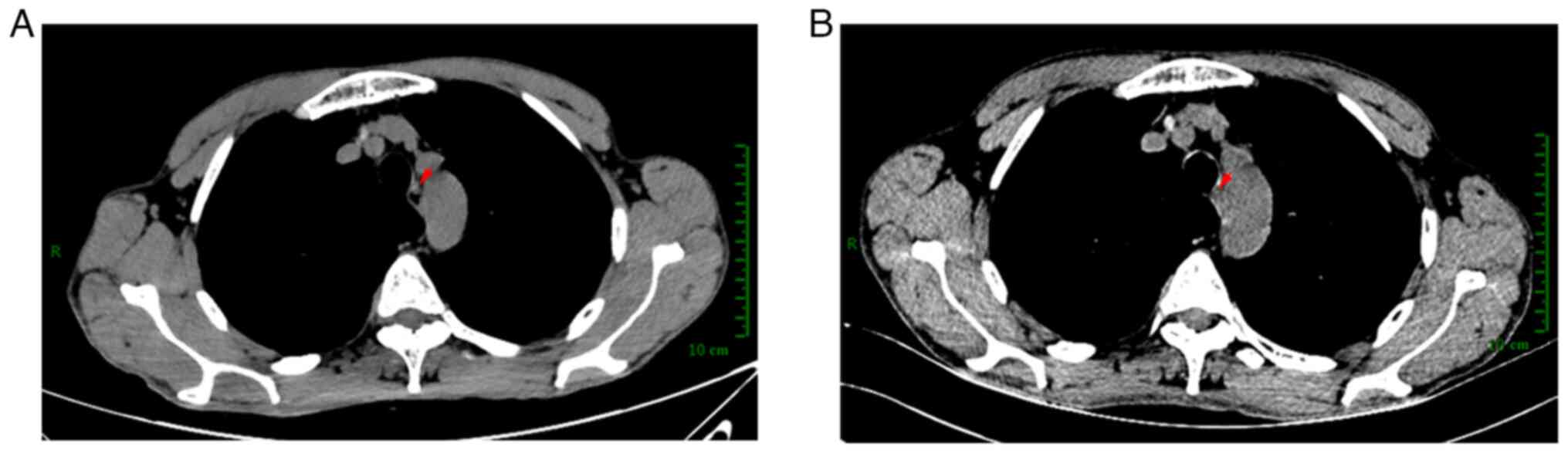
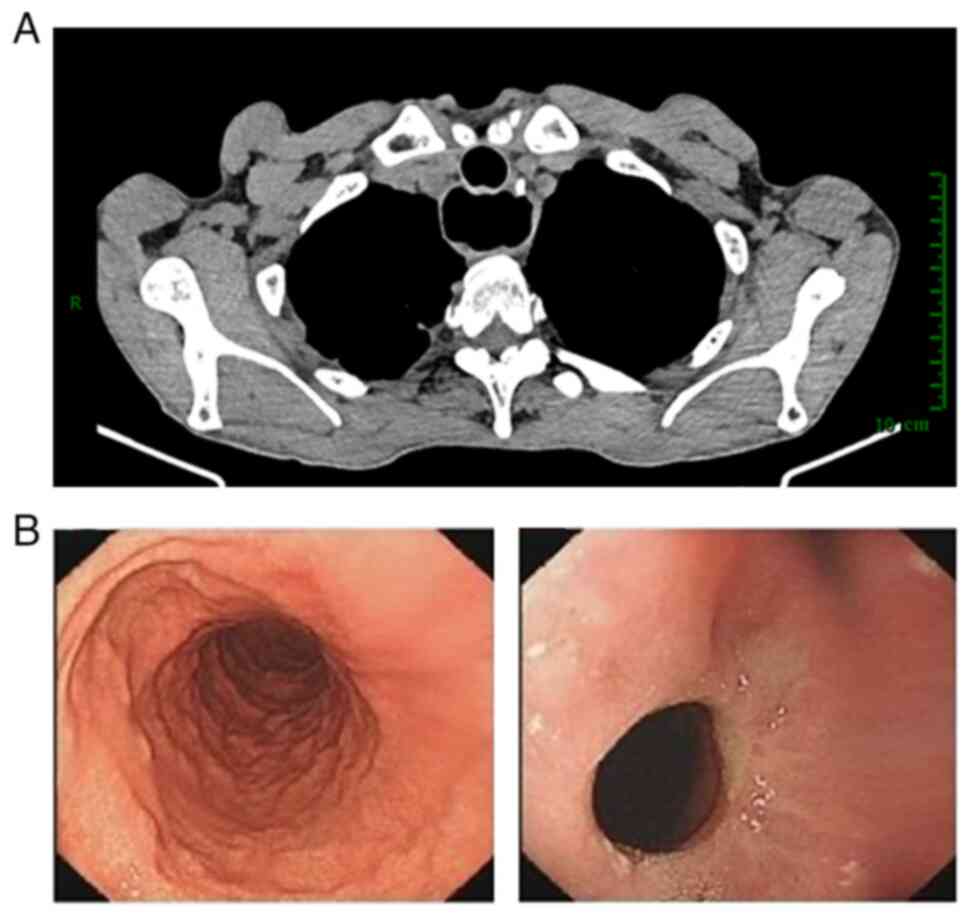
Pretreatment contrast-enhanced computed tomography (CT) revealed
thickening of the middle esophagus wall with mild uneven
enhancement (Fig. 3A), and a
homogeneously enhanced nodular shadow ~3mm in diameter on the left
side of the lesion, with no other metastatic foci observed in the
abdominal CT and cranial magnetic resonance imaging. The levels of
neuron-specific enolase, carbohydrate antigen 19-9, carbohydrate
antigen 125 and carcinoembryonic antigen were normal in the blood
before treatment. According to the 8th edition of American Joint
Committee on Cancer staging, the patient's pretreatment clinical
stage was cT2N1M0, stage II (11).
The patient underwent standard neoadjuvant immunochemotherapy,
receiving 200 mg tislelizumab, an anti-programmed cell death
protein 1 (PD-1) drug, 50 mg/m2 cisplatin and 150
mg/m2 albumin-bound paclitaxel administered
intravenously every 3 weeks from May 2023 until June 2023. The
epigastric pain was elevated during the treatment and no adverse
event was observed. Post-treatment endoscopic ultrasonography
demonstrated a significant reduction of the lesion size, with the
thickest section measuring ~2.9 mm (Fig. 1C and D). Enhanced CT scans showed
that the thickening of the middle esophagus wall had not evidently
changed since before treatment (Fig.
3B). The patient underwent a McKeown esophagectomy 3 weeks
after the last treatment and an R0 resection was achieved. A total
of 20 lymph nodes were biopsied for pathological examination. The
postoperative pathology of the lesion showed a 1.0×0.6 cm
grayish-white, hard-textured area on gross view. The resected
specimen was fixed in 10% formalin and transferred to the
Department of Pathology within 2 h. Histopathologically, the
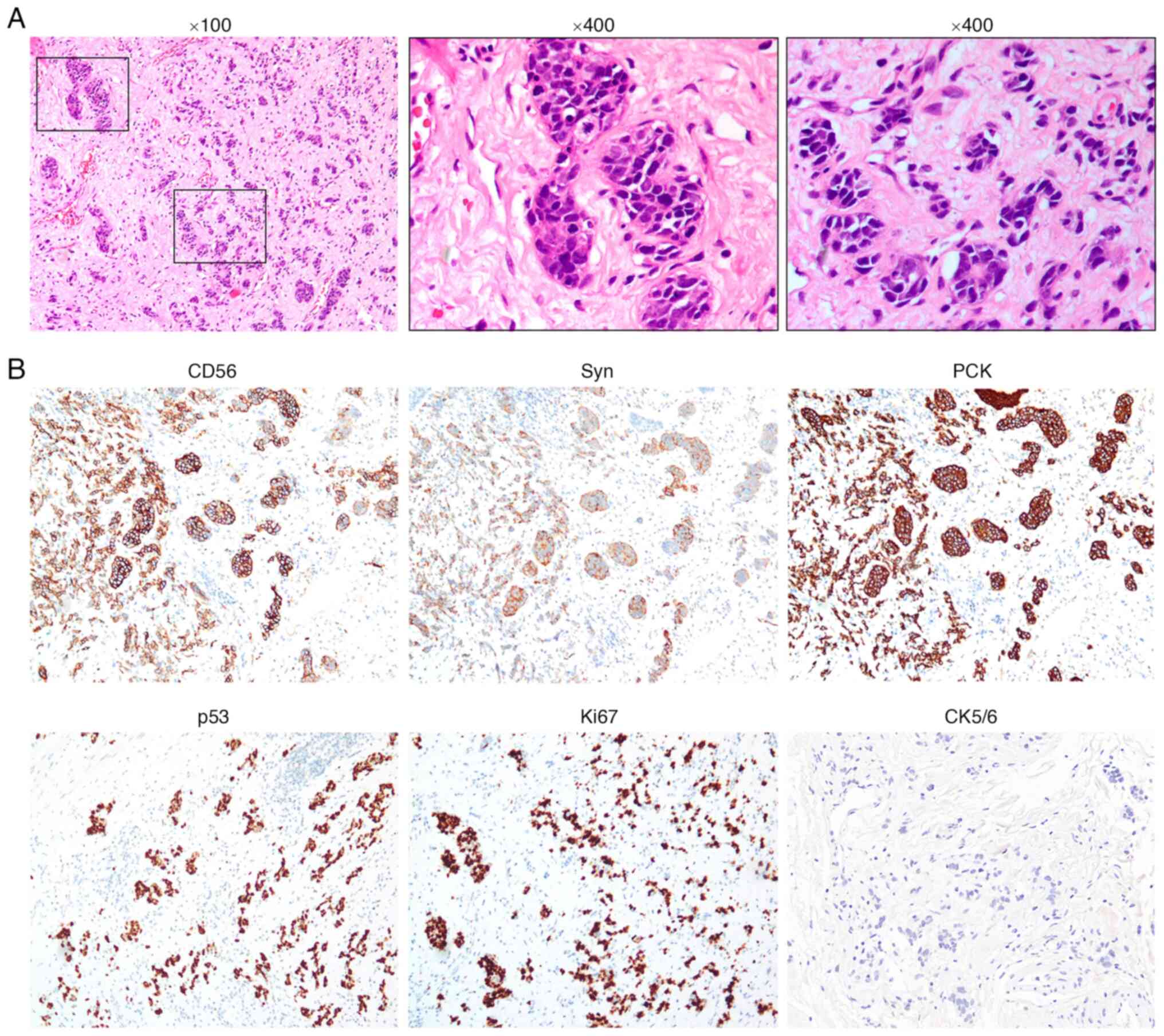
resected specimen displayed neuroendocrine cell tumors
characterized by hyperchromatic nuclei and scant cytoplasm, with
invasion depth restricted to the submucosa (Fig. 4A). To accurately diagnose the
carcinoma type, immunohistochemical staining was performed on the
resected specimen. Immunohistochemistry results showed negativity
for CK5/6 (Fig. 4B) and tumor
protein p40 (Fig. S1), but
positive expression of tumor protein p53 (p53), pan-cytokeratin and
synaptophysin (Syn), patchy CD56-positive cells and Ki67 expression
(Fig. 4B), indicating that the
tumor was an ENEC with no remaining ESCC (12). The results of H&E staining and
immunohistochemistry staining together indicated that p53
expression was localized mainly in the nucleus in nearly all the
tumor cells, while Syn was expressed in the cytoplasm of the tumor
cells. No metastatic lymph nodes were detected. The patient
experienced no recurrence or adverse events during the 5-month
follow-up period. The latest CT scan was performed in October 2023
and an endoscopy was performed in December 2023. The results
revealed no recurrence or metastasis (Fig. 5). The patient did not receive
further treatment after the surgery and follow-up will be performed
every 3 months until recurrence or metastasis is detected.
Discussion
Esophageal cancer is among the most common
malignancies worldwide and ranks within the top 10 cancers in terms
of both morbidity and mortality (1). In China, ESCC is the predominant
pathological type (13). Most
patients are diagnosed with locally advanced ESCC at their first
visit, and the 5-year survival rate for these patients is only 20%
(3). Various strategies have been
implemented to improve ESCC prognosis. The use of ICIs as
neoadjuvant therapy for locally advanced ESCC has shown promising
outcomes. According to the TD-NICE study, the pathological complete
response (PCR) rate is as high as 50% (5). Additionally, a retrospective study of
ICIs in neoadjuvant ESCC treatment reported a maximum PCR rate of
58.3% (6). However, not all ESCC
cases respond to anti-PD-1 therapy, making it crucial to
investigate drug resistance mechanisms. The present case provided a
possible theory. To the best of our knowledge, this is the first
report of ENEC transformation from ESCC through immunochemotherapy.
This case may offer valuable insights into resistance to
immunochemotherapy.
The resistance to ICIs in NSCLC has been explored
(8,9), and a similar transformation may aid in
understanding the resistance mechanism in ESCC. Imakita et
al (14) first reported the
NSCLC-to-SCLC transformation due to immunotherapy, with two
transformation mechanisms proposed. The first hypothesis suggests
that NSCLC cells histologically transform into SCLC cells. The
second hypothesis is that the initial tumor contains both NSCLC and
SCLC components, leading to small cell predominance with
immunotherapy (15). Based on the
present case, two transformation mechanisms for ESCC to ENEC are
proposed. One hypothesis is that the initial tumors comprised both
ESCC and ENEC, with the ESCC component diminishing due to treatment
with paclitaxel, cisplatin and tislelizumab, while the ENEC
component was less sensitive to immunochemotherapy. The practice of
using ICIs for ENEC is rare. There is limited evidence to guide the
treatment of patients with advanced ENEC, as the incidence of ENEC
is only 0.044 per 100,000 individuals (16). The National Comprehensive Cancer
Network guidelines recommend a combination of chemotherapy and
radiotherapy for locally advanced ENEC (10). Currently, only one phase II trial
(NCT03901378) of pembrolizumab combined with etoposide plus
carboplatin or etoposide plus cisplatin therapy in primary
high-grade gastrointestinal NEC is underway, with efficacy and
safety data yet to be reported. In the present study, insufficient
pre-treatment biopsy samples were available for
immunohistochemistry staining of CD56 and Syn to support this
hypothesis. However, no abnormal neuron-specific enolase levels
were detected in the blood before treatment, rendering the evidence
for ENEC weak.
The alternate hypothesis suggests that ESCC cells
underwent histological transformation to ENEC cells due to
immunotherapy. Ho et al (17) proposed the hypothesis that ENEC with
multiple components might develop from totipotent primitive cells.
Reports of esophageal carcinomas with different histological
components support the notion that esophageal carcinomas possess
multidirectional differentiation abilities (18,19). A
report detailing the transformation of ENEC from ESCC through
sequential endoscopy (20) also
supports this hypothesis. Although the specific mechanism of this
tumor transformation due to anti-PD-1 therapy remains unclear, its
possibility cannot be dismissed. Moreover, the impact of
chemotherapeutic agents on tumor transformation should be
considered. The transformation was considered to be less influenced
by paclitaxel and cisplatin than tislelizumab, as paclitaxel
combined with cisplatin has been a standard therapy for ESCC for
~20 years, with no reports of these drugs causing ENEC
transformation. The evolutionary genomic alterations underlying
this resistance mechanism require further detailed exploration. In
studies of NSCLC-to-SCLC transformation, researchers performed
tissue transcriptome sequencing before and after immunotherapy,
identifying similar TP53 mutations (9). Given that the TP53 mutation is one of
the most common mutations in ESCC (21), it may play a crucial role in the
transformation of ESCC into NEC and in resistance to immunotherapy.
The lack of sufficient biopsy tissues for genomic testing presents
a challenge in precision medicine, necessitating further research
into the resistance mechanism of ESCC.
There are some limitations in the present case
report. First, the biopsy specimen before treatment was not
sufficiently large enough for immunohistochemistry staining to
exclude the existence of NEC cells. The molecular alterations that
may drive histological transformation were also unclear. Second,
the images and data were obtained directly from the patient's
medical records. The specific steps of H&E and
immunohistochemical staining on resected specimen were not
available, as the original medical record did not contain related
information The information on the antibodies used in the
immunohistochemistry staining was not included in the original
medical records; thus, it is difficult to indicate the specific
information on antibodies. Apart from that, the image of the
resected specimen on gross view was not provided and this may cause
difficulty in making a clear diagnosis regarding the depth of
invasion or neural or vascular invasion. Third, tissue
transcriptome sequencing before and after immunotherapy are clearly
warranted for further investment on resistance mechanisms.
In summary, the present study reported a case of
ESCC-to-ENEC transformation after neoadjuvant immunochemotherapy,
suggesting that this pathological change may contribute to
resistance to immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GX and NS analyzed data, and wrote the paper as the
co-first authors. KJ conceived the study and edited the manuscript.
GX and NS confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
individual for the publication of any potentially identifiable
images or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen R, Zheng R, Zhang S, Wang S, Sun K,
Zeng H, Li L, Wei W and He J: Patterns and trends in esophageal
cancer incidence and mortality in China: An analysis based on
cancer registry data. J National Cancer Center. 3:21–27. 2023.
View Article : Google Scholar
|
|
4
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS, et al:
Esophageal and esophagogastric junction cancers, version 1.2015. J
Natl Compr Canc Netw. 13:194–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H,
Gong L, Liu H, Tian F, Lu Q, et al: Tislelizumab combined with
chemotherapy as neoadjuvant therapy for surgically resectable
esophageal cancer: A prospective, single-arm, phase II study
(TD-NICE). Int J Surg. 103:1066802022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Jiang Z, Wang Q, Wu T, Guo F, Xu
Z, Yang W, Yang S, Feng S, Wang X, et al: Pathological response and
prognostic factors of neoadjuvant PD-1 blockade combined with
chemotherapy in resectable oesophageal squamous cell carcinoma. Eur
J Cancer. 186:196–210. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao L, Lu J, Zhang P, Hong ZN and Kang M:
Toripalimab combined with docetaxel and cisplatin neoadjuvant
therapy for locally advanced esophageal squamous cell carcinoma: A
single-center, single-arm clinical trial (ESONICT-2). J
Gastrointest Oncol. 13:478–487. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdallah N, Nagasaka M, Abdulfatah E, Shi
D, Wozniak AJ and Sukari A: Non-small cell to small cell lung
cancer on PD-1 inhibitors: Two cases on potential histologic
transformation. Lung Cancer (Auckl). 9:85–90. 2018.PubMed/NCBI
|
|
9
|
Sehgal K, Varkaris A, Viray H, VanderLaan
PA, Rangachari D and Costa DB: Small cell transformation of
non-small cell lung cancer on immune checkpoint inhibitors:
Uncommon or under-recognized? J Immunother Cancer. 8:e0006972020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas WR, David K, Eugene HB, Hemant I,
Deepa TP, Adam JB, Jeremy JE, Hans G and L.H W: AJCC Cancer Staging
Manual. 8th edition. Springer; New York, NY: 2017
|
|
12
|
Odze RD, Lam AK and A O: WHO
Classification of Tumors: Digestive System Tumours. 5th edition.
IARC Press; Lyon: 2019
|
|
13
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J National Canc Center. 2:1–9. 2022. View Article : Google Scholar
|
|
14
|
Imakita T, Fujita K, Kanai O, Terashima T
and Mio T: Small cell lung cancer transformation during
immunotherapy with nivolumab: A case report. Respir Med Case Rep.
21:52–55. 2017.PubMed/NCBI
|
|
15
|
Imakita T, Fujita K, Kanai O, Okamura M,
Hashimoto M, Nakatani K, Sawai S and Mio T: Small cell
transformation of non-small cell lung cancer under immunotherapy:
Case series and literature review. Thorac Cancer. 12:3062–3067.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Hu J, Chen P and Zeng Z: Incidence,
treatment, and survival analysis in esophageal neuroendocrine
carcinoma population. Transl Cancer Res. 9:4317–4329. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho KJ, Herrera GA, Jones JM and Alexander
CB: Small cell carcinoma of the esophagus: Evidence for a unified
histogenesis. Hum Pathol. 15:460–468. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamasaki T, Ishii N, Okuno T, Suekane T,
Inoue T and Nebiki H: A case of esophageal squamous cell carcinoma
with neuroendocrine, basaloid, and ciliated glandular
differentiation. Clin J Gastroenterol. 14:32–38. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robertson NJ, Rahamim J and Smith ME:
Carcinosarcoma of the oesophagus showing neuroendocrine, squamous
and glandular differentiation. Histopathology. 31:263–266. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwagami H, Uedo N and Kitamura M: Case of
esophageal superficial neuroendocrine carcinoma suggestive of
transformation from squamous cell carcinoma. Dig Endosc.
32:8272020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Z, Zhao Y, Kong P, Liu Y, Huang J, Xu
E, Wei W, Li G, Cheng X, Xue L, et al: Integrated multi-omics
profiling yields a clinically relevant molecular classification for
esophageal squamous cell carcinoma. Cancer Cell. 41:181–195.e9.
2023. View Article : Google Scholar : PubMed/NCBI
|