Introduction
Adolescents and young adults (AYAs) describes
patients with cancer aged 15–39 years, according to the National
Cancer Institute (1) and >1.2
million AYA patients with cancer are newly diagnosed each year
globally (2). An increasing body of
evidence has reported that the molecular biology of tumors in AYAs
is unique compared with that in other ages (3,4). In
the case of papillary thyroid carcinoma (PTC), more advanced tumor
stages as well as different treatment resistance behaviors are
observed when compared with several other cancers such as melanoma,
breast cancer and colorectal cancer (5).
Thyroid cancer has one of the highest incidences
within endocrine malignancies at 10.1 per 100,000 women and 3.1 per
100,000 men globally (6), with ~80%
of thyroid cancers classified as PTC (7). Thus, PTC is one of the primary drivers
behind the increase in overall incidence of cancer in AYA, making
PTC a valuable topic for study (8).
To date, a consensus has not been reached on whether prophylactic
central neck dissection should be performed (9), and investigations into this topic may
fuel optimal PTC treatment decisions that may greatly benefit AYA
patients.
In light of studies that have reported notably
different clinical and molecular features in the AYA group of
patients with PTC (10,11), the present study compared the
clinicopathological characteristics between AYA and older adult
patients with PTC. Furthermore, considering that no existing
literature has measured neck involvement risk quantitatively for
AYA patients with PTC, to the best of our knowledge, the present
research predicted and stratified the risk of central lymph node
metastasis (CLNM) and lateral lymph node metastasis (LLNM) for AYA
patients to guide individual management strategies of neck
regions.
Materials and methods
Patient recruitment
Medical records of 989 patients with confirmed PTC
treated surgically at Ningbo Medical Center Lihuili Hospital
(Ningbo, China) between 2019 and 2022 were retrospectively
analyzed. The inclusion criteria were as follows: i) Aged >15
years; ii) diagnosis of PTC; iii) biopsy performed; iv) surgical
treatment received as the only treatment; and v) no distant
metastasis. Exclusion criteria were as follows: i) PTC not
histologically proven. Several were poorly differentiated thyroid
cancer or anaplastic thyroid cancer (n=24); ii) missing clinical or
pathological data (n=9); and iii) other head or neck cancers
present (n=4). Post-exclusion, 952 patients with diagnosed PTC who
underwent thyroidectomy were analyzed.
Surgical procedure, CLNM and LLNM, and
follow-up
From the Electronic Medical Records System,
demographic data, serum indices and fine-needle aspiration (FNA)
details were collected for analysis. All patients underwent either
a total thyroidectomy or thyroid lobectomy with blood drawn and
tested prior to procedure. Furthermore, all patients underwent
comprehensive whole-body imaging to rule out distant metastasis
pre-operation. Central lymph node dissection (CLND) was standard
for both treatment and prevention, with positive results confirmed
by ≥2 expert pathologists. Lateral lymph node dissection was
reserved for patients with suspected lateral neck involvement
either preoperatively via ultrasonography or FNA or based on
surgical judgment. If post-surgery ultrasonography or FNA within 6
months revealed LLNM in patients who only had CLND, they were
retroactively classified as having had lateral neck involvement
during their initial surgery. Postoperative pathology was
consistently reported using standardized methods within 7 working
days following surgery, and for follow-up, each patient underwent a
neck ultrasound at 6 months post-surgery, along with other
necessary imaging assessments to evaluate postoperative neck
conditions. All observed metastatic lymph nodes were
ipsilateral.
Statistical analysis
Pearson's χ2 and the independent t-test
were used for the analysis of categorical and continuous variables,
respectively. Logistic uni- and multivariate regression analyses
were conducted to screen out independent risk factors of CLNM and
LLNM in AYA, which were further used to create a nomogram. All
aforementioned statistical analyses were performed using SPSS
(version 24.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference. The performance of these
models was assessed using the concordance index (C-index), receiver
operating characteristic (ROC) curve and calibration curve. These
assessments were performed using R software (version 3.5.1; R Core
Team).
Results
Basic demographics and
clinicopathological features of patients with PTC within different
age groups
Of the 952 patients with PTC, 463 (48.6%) aged 15–39
years were in the AYA group, whilst 489 (51.4%) aged >39 years
were in the older adult group. Basic clinical data for both groups
is presented in Table I.
Significant differences between groups were observed in primary
tumor and cervical areas. Compared with the older adult group, the
AYA group had significantly larger tumor sizes (0.98±0.80 cm vs.
0.8±0.63 cm; P<0.001), and significantly higher rates of
ipsilateral Hashimoto thyroiditis (23.1% vs. 18.0%; P=0.028) and
thyroid capsular invasion (TCI; 43.0% vs. 33.1%; P=0.001). However,
ipsilateral nodular goiter (iNG) was significantly less prevalent
in the AYA group compared with the older adult group (24.6% vs.
33.2%, P=0.006). Furthermore, AYA patients had significantly higher
rates of both CLNM (61.8% vs. 35.0%; P<0.001) and LLNM (28.3%
vs. 18.7%; P=0.009), with significantly larger positive central
lymph node (CLN) sizes and counts (P=0.034 and P<0.001,
respectively), in comparison with the older adult group.
 | Table I.Clinicopathological characteristics of
patients with papillary thyroid carcinoma within different
groups. |
Table I.
Clinicopathological characteristics of
patients with papillary thyroid carcinoma within different
groups.
| A, All PTC patients
that underwent thyroidectomy (n=952) |
|---|
|
|---|
|
|
| AYA and old adult
groups | Patients within AYA
group |
|---|
|
|
|
|
|
|---|
| Characteristic | All patients
(n=952) | AYA group
(n=463) | Older adult group
(n=489) | P-value | 15–29 years
(n=156) | 30–39 years
(n=307) | P-value |
|---|
| Sex |
|
|
| 0.045 |
|
| 0.081 |
| Male | 338 (35.5) | 177 (38.2) | 161 (32.9) |
| 51 (32.7) | 126 (41.0) |
|
|
Female | 614 (64.5) | 286 (61.8) | 328 (67.1) |
| 105 (67.3) | 181 (59.0) |
|
| Maximum tumor
diameter, cm | 0.92±0.66 | 0.98±0.80 | 0.80±0.63 | <0.001 | 1.09±0.96 | 0.92±0.69 | 0.025 |
| PTC with Hashimoto
thyroiditis |
|
|
| 0.028 |
|
| 0.357 |
| No | 757 (79.5) | 356 (76.9) | 401 (82.0) |
| 116 (74.4) | 240 (78.2) |
|
| Yes | 195 (20.5) | 107 (23.1) | 88 (18.0) |
| 40 (25.6) | 67 (21.8) |
|
| PTC with nodular
goiter |
|
|
| 0.006 |
|
| 0.582 |
| No | 676 (71.0) | 349 (75.4) | 327 (66.8) |
| 120 (76.9) | 229 (74.6) |
|
| Yes | 276 (29.0) | 114 (24.6) | 162 (33.2) |
| 36 (23.1) | 78 (25.4) |
|
| Thyroid capsular
invasion |
|
|
| 0.001 |
|
| 0.075 |
|
Absent | 591 (62.1) | 264 (57.0) | 327 (66.9) |
| 80 (51.3) | 184 (59.9) |
|
|
Present | 361 (37.9) | 199 (43.0) | 162 (33.1) |
| 76 (48.7) | 123 (40.1) |
|
| Bilateral
disease |
|
|
| 0.474 |
|
| 0.249 |
|
Absent | 773 (81.2) | 372 (80.3) | 401 (82.0) |
| 130 (83.3) | 242 (78.8) |
|
|
Present | 179 (18.8) | 91 (19.7) | 88 (18.0) |
| 26 (16.7) | 65 (21.2) |
|
| Multifocality |
|
|
| 0.849 |
|
| 0.414 |
|
Absent | 657 (69.0) | 318 (68.7) | 339 (69.3) |
| 111 (71.2) | 207 (67.4) |
|
|
Present | 295 (31.0) | 145 (31.3) | 150 (30.7) |
| 45 (28.8) | 100 (32.6) |
|
| Tumor location |
|
|
| 0.543 |
|
| 0.399 |
| Upper
portion | 270 (28.4) | 121 (26.1) | 149 (30.5) |
| 37 (23.7) | 84 (27.4) |
|
|
Middle/Lower portion | 682 (71.6) | 342 (73.9) | 340 (69.5) |
| 119 (76.3) | 223 (72.6) |
|
| CLNM |
|
|
| <0.001 |
|
| 0.179 |
| No | 495 (52.0) | 177 (38.2) | 318 (65.0) |
| 53 (34.0) | 124 (40.4) |
|
|
Yes | 457 (48.0) | 286 (61.8) | 171 (35.0) |
| 103 (66.0) | 183 (59.6) |
|
|
| B, Positive CLNM
only (n=457) |
|
|
|
| AYA and old
adult groups | Patients within
AYA group |
|
|
|
|
|
|
Characteristic | All patients
(n=952) | AYA group
(n=463) | Older adult
group (n=489) | P-value | 15–29 years
(n=156) | 30–39 years
(n=307) | P-value |
|
| Number of
positive |
|
|
| <0.001 |
|
| 0.394 |
| CLN |
|
|
|
|
|
|
|
|
1-2 | 226 (49.5) | 121 (42.3) | 105 (61.4) |
| 39 (37.9) | 82 (44.8) |
|
|
3-4 | 118 (25.8) | 74 (25.9) | 44 (25.7) |
| 31 (30.1) | 43 (23.5) |
|
| ≥5 | 113 (24.7) | 91 (31.8) | 22 (12.9) |
| 33 (32.0) | 58 (31.7) |
|
| Maximum diameter of
positive CLN |
|
|
| 0.034 |
|
| 0.573 |
| <1.0
cm | 349 (76.4) | 211 (73.8) | 138 (80.7) |
| 78 (75.7) | 133 (72.7) |
|
| ≥1.0
cm | 108 (23.6) | 75 (26.2) | 33 (19.3) |
| 25 (24.3) | 50 (27.3) |
|
| LLNM |
|
|
| 0.009 |
|
| 0.386 |
| No | 344 (75.3) | 205 (71.7) | 139 (81.3) |
| 77 (74.8) | 128 (69.9) |
|
|
Yes | 113 (24.7) | 81 (28.3) | 32 (18.7) |
| 26 (25.2) | 55 (30.1) |
|
AYA patients were further split into two subgroups:
15–29 years (younger AYA group) and 30–39 years (older AYA group).
Only tumor size differed significantly between the groups, with a
greater maximum tumor diameter (MTD) observed in the younger
subgroup compared with that in the older subgroup (1.09±0.96 cm vs.
0.92±0.69 cm; P=0.025), as shown in Table I.
Different clinicopathological features
between patients with or without CLNM or LLNM in the AYA group
Out of the 463 AYA patients, 286 (61.8%) had central
neck involvement and 81 (17.5%) had lateral neck involvement. When
comparing AYA patients based on central involvement, 43.4% of those
with CLNM were male, significantly higher than those without CLNM
(P=0.004, Table II). Those with
CLNM also had significantly larger tumors (1.13±0.86 cm vs.
0.73±0.61 cm; P<0.001) and significantly exhibited factors like
TCI (P<0.001), bilateral disease (P<0.001), multifocality
(P<0.001) and iNG (P=0.033) more frequently, compared with AYA
patients without CLNM.
 | Table II.Clinicopathological characteristics
of adolescents and young adult patients. |
Table II.
Clinicopathological characteristics
of adolescents and young adult patients.
|
| AYA patients
(n=463) | AYA patients with
CLNM (n=286) |
|---|
|
|
|
|
|---|
| Characteristic | No-CLNM
(n=177) | CLNM (n=286) | P-value | No-LLNM
(n=205) | LLNM (n=81) | P-value |
|---|
| Sex |
|
| 0.004 |
|
| 0.304 |
|
Male | 53 (29.9) | 124 (43.4) |
| 85 (41.5) | 39 (48.1) |
|
|
Female | 124 (70.1) | 162 (56.6) |
| 120 (58.5) | 42 (51.9) |
|
| Thyroid capsular
invasion |
|
| <0.001 |
|
| 0.010 |
|
Absent | 149 (84.2) | 115 (40.2) |
| 92 (44.9) | 23 (28.4) |
|
|
Present | 28 (15.8) | 171 (59.8) |
| 113 (55.1) | 58 (71.6) |
|
| Bilateral
disease |
|
| <0.001 |
|
| 0.090 |
|
Absent | 158 (89.3) | 214 (74.8) |
| 159 (77.6) | 55 (67.9) |
|
|
Present | 19 (10.7) | 72 (25.2) |
| 46 (22.4) | 26 (32.1) |
|
| Maximum tumor
diameter | 0.73±0.61 | 1.13±0.86 | <0.001 | 0.96±0.61 | 1.56±1.18 | <0.001 |
| Multifocality |
|
| <0.001 |
|
| 0.013 |
|
Absent | 151 (85.3) | 167 (58.4) |
| 129 (62.9) | 38 (46.9) |
|
|
Present | 26 (14.7) | 119 (41.6) |
| 76 (37.1) | 43 (53.1) |
|
| Tumor location |
|
| 0.704 |
|
| 0.012 |
| Upper
portion | 48 (27.1) | 73 (25.5) |
| 161 (78.5) | 52 (64.2) |
|
|
Middle/Lower portion | 129 (72.9) | 213 (74.5) |
| 44 (21.5) | 29 (35.8) |
|
| PTC with nodular
goiter |
|
| 0.033 |
|
| <0.001 |
| No | 143 (80.8) | 206 (72.0) |
| 163 (79.5) | 43 (53.1) |
|
|
Yes | 34 (19.2) | 80 (28.0) |
| 42 (20.5) | 38 (46.9) |
|
| PTC with Hashimoto
thyroiditis |
|
| 0.353 |
|
| 0.257 |
| No | 132 (74.6) | 224 (78.3) |
| 157 (76.6) | 67 (82.7) |
|
|
Yes | 45 (25.4) | 62 (21.7) |
| 48 (23.4) | 14 (17.3) |
|
| Number of positive
CLN |
|
| - |
|
| <0.001 |
|
1-2 | - | 121 (42.3) |
| 99 (48.3) | 22 (27.2) |
|
|
3-4 | - | 74 (25.9) |
| 60 (29.3) | 14 (17.3) |
|
| ≥5 | - | 91 (31.8) |
| 46 (22.4) | 45 (55.6) |
|
| Maximum diameter of
positive |
|
| - |
|
| <0.001 |
| CLN |
|
|
|
|
|
|
| <1.0
cm | - | 211 (73.8) |
| 188 (91.7) | 23 (28.4) |
|
| ≥1.0
cm | - | 75 (26.2) |
| 17 (8.3) | 58 (71.6) |
|
Among the 286 AYA patients with positive CLNM,
differences were analyzed based on LLNM presence. Those with
positive LLNM had a significantly higher occurrence of TCI,
multifocality and iNG (P=0.010, P=0.013 and P<0.001,
respectively), and displayed significantly larger tumors than those
with negative LLNM (1.56±1.18 cm vs. 0.96±0.61 cm; P<0.001).
Furthermore, AYA patients with LLNM had significantly greater
counts and larger positive CLN sizes than those without LLNM (both
P<0.001; Table II).
Construction of risk prediction model
of CLNM for patients within AYA group
Univariate and multivariate analyses were performed
to identify independent risk factors for CLNM. Factors with
P<0.05 underwent further multivariate analysis. A total of five
factors were demonstrated to be independent risk factors for CLNM
in the AYA patients: Male sex, TCI presence, multifocality,
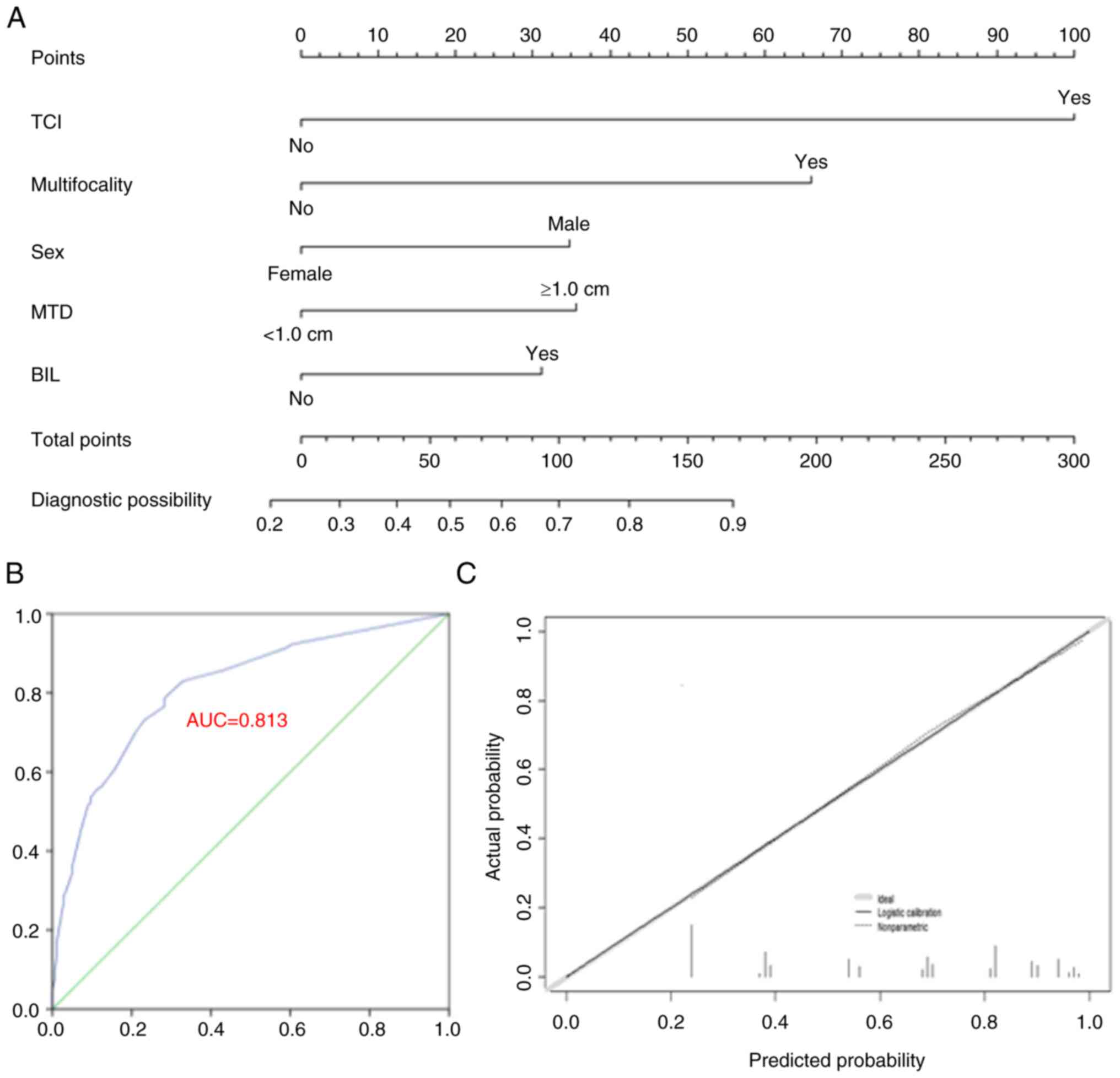
bilateral disease and MTD ≥1.0 cm (Table III). These factors contributed to
the CLNM prediction model (Fig.
1A). The accuracy of the model was verified with 1,000
bootstrap resamples, yielding a C-index of 0.813 (95% CI,
0.774–0.853) and 0.804 after bootstrapping. The ROC curve and
calibration plot are presented in Fig.
1B and C, indicating consistent actual and predicted CLNM
probabilities.
 | Table III.Univariate and multivariate analyses
of cervical lymph node metastasis and lateral lymph node metastasis
for AYA patients. |
Table III.
Univariate and multivariate analyses
of cervical lymph node metastasis and lateral lymph node metastasis
for AYA patients.
| A, Analyzing all
AYA patients to screen independent factors for CLNM |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor
selected | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex (Male vs.
female) | 1.791
(1.203–2.665) | 0.004 | 1.856
(1.144–3.010) | 0.012 |
| Thyroid capsular
invasion (yes vs. no) | 7.913
(4.956–12.633) | <0.001 | 7.262
(4.389–12.016) | <0.001 |
| Bilateral disease
(Yes vs. no) | 2.798
(1.621–4.829) | <0.001 | 1.885
(1.002–3.546) | 0.049 |
| Maximum tumor
diameter (≥1.0 cm vs. <1.0 cm) | 2.728
(1.759–4.232) | <0.001 | 1.956
(1.169–3.274) | 0.011 |
| Tumor location
(Upper vs. middle/lower) | 0.921
(0.602–1.409) | 0.704 |
|
|
| Multifocality (Yes
vs. no) | 4.138
(2.566–6.675) | <0.001 | 3.662
(2.110–6.356) | <0.001 |
| PTC with nodular
goiter (Yes vs. no) | 1.633
(1.037–2.573) | 0.034 | 1.137
(0.658–1.966) | 0.646 |
| PTC with Hashimoto
thyroiditis (Yes vs. no) | 0.812
(0.523–1.261) | 0.353 |
|
|
|
| B, Analyzing AYA
patients with positive CLNM to screen out independent factors for
LLNM |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
| Factor
selected | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Sex (Male vs.
female) | 1.311
(0.782–2.198) | 0.305 |
|
|
| Thyroid capsular
invasion (Yes vs. no) | 2.053
(1.178–3.580) | 0.011 | 2.129
(0.948–4.784) | 0.067 |
| Bilateral disease
(Yes vs. no) | 1.634
(0.924–2.890) | 0.092 |
|
|
| Maximum tumor
diameter (≥ 1.0 vs. <1.0 cm) | 3.225
(1.892–5.496) | <0.001 | 2.740
(1.276–5.885) | 0.010 |
| Tumor location
(Upper vs. middle/lower) | 2.041
(1.162–3.585) | 0.013 | 1.417
(0.632–3.181) | 0.398 |
| Multifocality (Yes
vs. no) | 1.921
(1.142–3.232) | 0.014 | 1.809
(0.852–3.843) | 0.123 |
| PTC with nodular
goiter (Yes vs. no) | 3.430
(1.973–5.960) | <0.001 | 2.851
(1.306–6.225) | 0.009 |
| PTC with Hashimoto
thyroiditis (Yes vs. no) | 0.683
(0.353–1.323) | 0.259 |
|
|
| Maximum diameter of
positive CLN (≥1.0 vs. <1.0 cm) | 27.887
(13.952–55.743) | <0.001 | 27.131
(12.372–59.496) | <0.001 |
| Number of positive
CLN (≥3 vs. <3) | 2.505
(1.429–4.389) | 0.001 | 1.618
(0.746–3.510) | 0.223 |
Using the developed nomogram, each AYA patient
received a CLNM risk score by summing the scores of the five
factors. Patients were divided into two risk subgroups based on
their scores, demonstrating significantly different central neck
involvement rates (P<0.001; Table
IV): i) Low CLNM risk [total score (TS) <50): CLNM rate of
25.9% (42/162) and ii) high CLNM risk (TS ≥50): CLNM rate of 81.1%
(244/301).
 | Table IV.Risk stratification of CLNM for
adolescents and young adult patients. |
Table IV.
Risk stratification of CLNM for
adolescents and young adult patients.
| CLNM | Low risk (TS
<50; n=162) | High risk (TS ≥50;
n=301) | P-value |
|---|
| Negative | 120 (74.1) | 57 (18.9) | <0.001 |
| Positive | 42 (25.9) | 244 (81.1) |
|
Construction of risk prediction model
of LLNM for AYA patients with positive CLNM
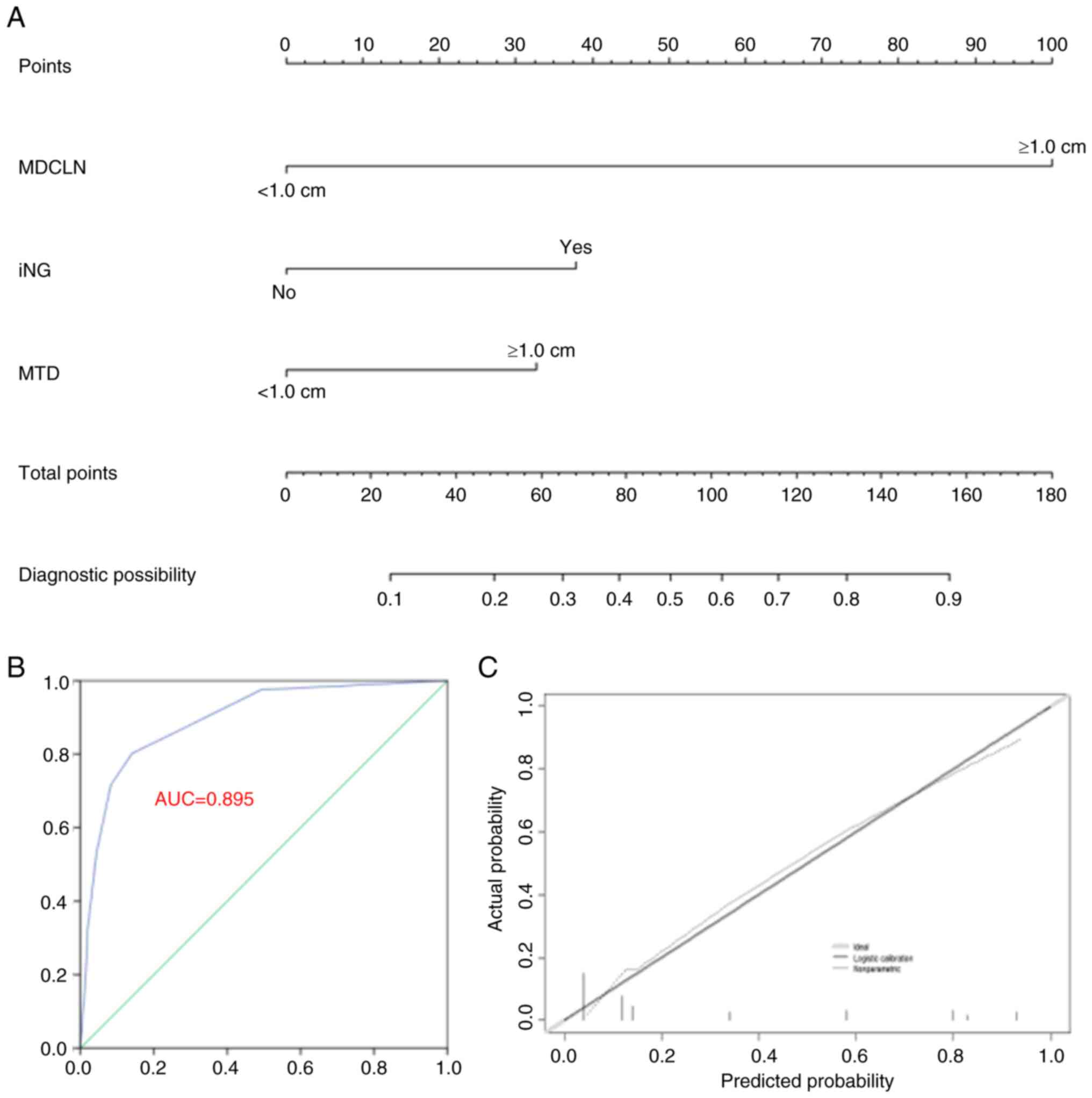
Multivariate analysis identified MTD ≥1.0 cm,
maximum diameter of positive CLN ≥1.0 cm and iNG presence as
independent LLNM risk factors for AYA patients with CLNM (Table III). A prediction model for LLNM
was established using these three factors (Fig. 2A). The C-index values were 0.895
(95% CI, 0.854–0.936) and 0.886 post-bootstrapping. The accuracy of
the model is shown in the ROC curve and calibration plot (Fig. 2B and C).
Based on the LLNM prediction model, AYA patients
with CLNM were categorized into three subgroups with varying LLNM
rates (P<0.001, Table V): i) Low
LLNM risk (TS=0): LLNM rate of 1.9% (2/106); ii) moderate LLNM risk
(0<TS<100): LLNM rate of 20.0% (21/105); and iii) high LLNM
risk (TS≥100): LLNM rate of 77.3% (58/75).
 | Table V.Risk stratification of LLNM for
adolescents and young adult patients with positive central LNM. |
Table V.
Risk stratification of LLNM for
adolescents and young adult patients with positive central LNM.
| LLNM | Low risk (TS=0;
n=106) | Moderate risk
(0< TS <100; n=105) | High risk (TS ≥100;
n=75) | P-value |
|---|
| Negative | 104 (98.1) | 84 (80.0) | 17 (22.7) | <0.001 |
| Positive | 2 (1.9) | 21 (20.0) | 58 (77.3) |
|
Cervical involvement risk assessment
flow chart for AYA patients
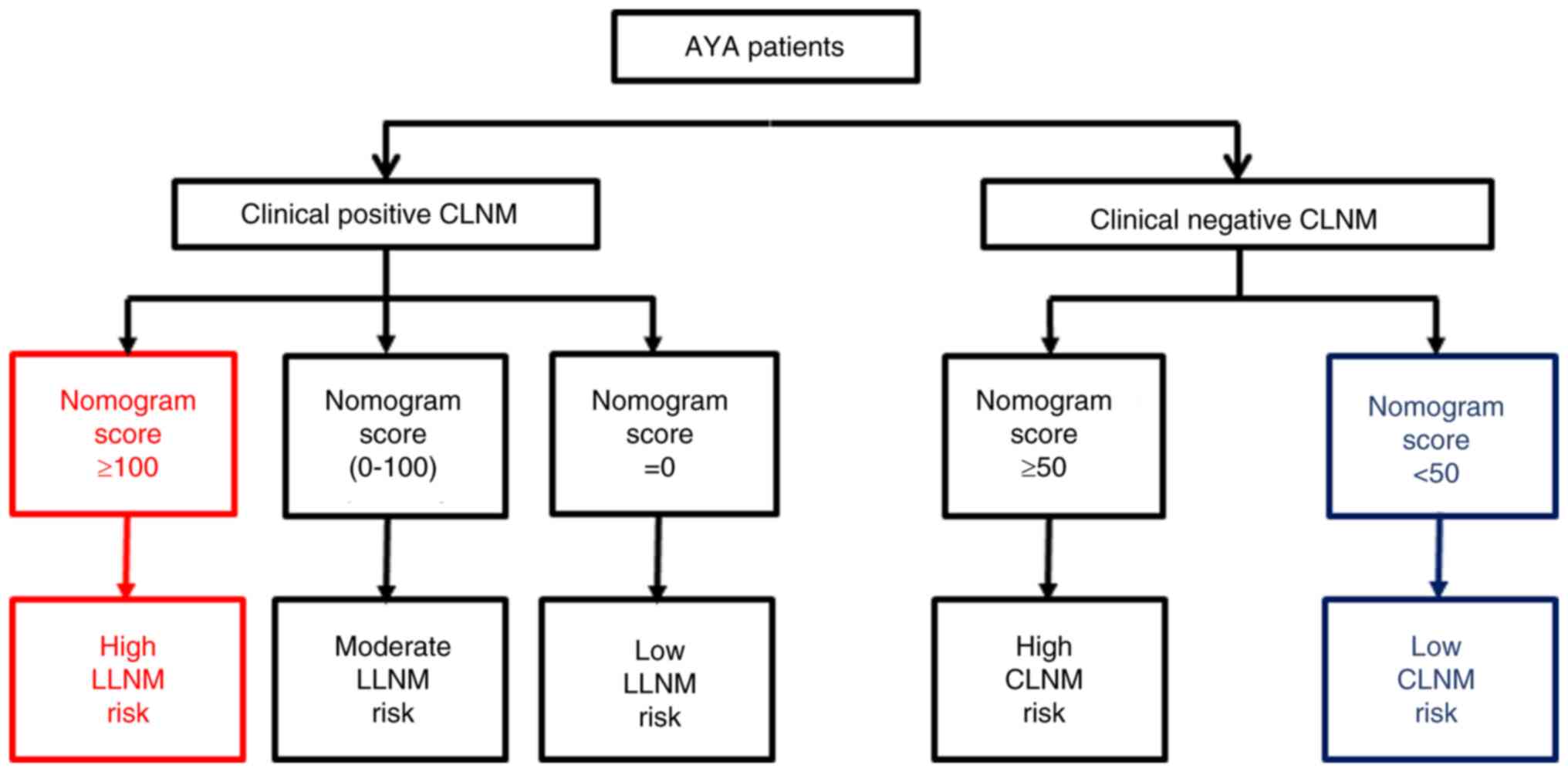
The nomograms assessing CLNM and LLNM risk for AYA
patients were combined into a comprehensive cervical risk
evaluation chart (Fig. 3). In
brief, for high-risk patients, options such as prophylactic CLND or
more intensive postoperative monitoring may be considered. In the
case of intermediate-risk patients, the choice between closer
follow-up and prophylactic CLND should be made after thorough
discussion with the patient, taking their preferences into account.
For low-risk patients, a conservative ‘wait and see’ approach is
advocated, negating the need for interventions like prophylactic
cervical cleansing. In cases where no lymph node metastasis is
detected through preoperative imaging, postoperative pathology
(including lymph node specimens from prophylactic CLND) or in the
6-month postoperative follow-up imaging, the absence of lymph node
metastasis is inferred at the initial diagnosis. These patients
without lymph node metastasis would fall under low LLNM risk (total
score=0) and would thus follow the recommendation for low-risk
patients.
Other blood indexes
There were four thyroid-related hormone levels
between AYA females and males that demonstrated significant
variations. Triiodothyronine (T3) (1.58±0.22 nmol/l vs. 1.71±0.19
nmol/l; P<0.001), Thyroxine (T4) (96.42±16.89 nmol/l vs.
100.74±16.34 nmol/l; P=0.020), free T3 (FT3) (4.27±0.48 pmol/l vs.
4.55±0.44 pmol/l; P<0.001) and Thyroglobulin Antibody (TgAb)
(106.23±227.03 kU/L vs. 49.85±186.09 kU/L; P=0.008) (Table SI).
Discussion
The present study demonstrated that PTC tumors in
patients aged 15–39 years (AYAs) were more aggressive, with AYA
primary tumor sites differing significantly from older patients:
They had larger tumors, more frequent TCI, ipsilateral Hashimoto
thyroiditis and iNG. This suggests a faster and more complex
disease progression in AYAs. Moreover, lymph node involvement in
both central and lateral regions was higher in AYAs. They also had
more extensive CLNM, indicating greater local tumor invasiveness.
Within the AYA group, primary tumor and lymph node conditions were
consistent across ages 15–29 and 30–39 years.
Use of more aggressive treatments such as
prophylactic CLND for PTC is debated (12). An active surveillance approach
instead of traditional surgery has been gaining traction lately for
certain PTC types, but comprehensive factors to be considered for
different approaches remain unclear. Age, a vital factor to
consider in PTC clinical staging as per the 8th edition of the
American Joint Committee on Cancer Tumor-Node-Metastasis staging
(13), may serve as a viable
starting point. Although select few research, such as those by
Vriens et al (11) and
Miccoli et al (14),
reported AYAs to have lower staging and improved prognoses, more
studies reported advanced PTC with neck involvement in this
demographic (15), which align with
the findings of the current study. Managing the cervical lymph node
region is thus crucial for AYAs, and this should be based on
investigations into lymph node metastasis risk to account for the
larger tumor size and more frequent multifocality seen in AYA
patients with PTC (10,14).
For AYA patients, of the five key risk factors for
CLNM, four (MTD ≥1.0 cm, presence of TCI, multifocality and
bilateral disease) are seen as indicators of advanced tumor
progression and are often linked with CLNM in patients with PTC, as
reported in several studies (9,16,17).
Male AYAs are at high risk for central neck metastasis, a factor
not previously associated with patients with PTC (18), hence more studies are needed to
support this finding. At this stage, we hypothesize that such a
discrepancy between the sexes may relate to differing thyroid
hormone levels. The analysis of blood indexes in AYA males and
females in the present study (Table
SI) demonstrated significant variations in T3, T4, FT3 and TgAb
levels, hinting at new research avenues on CLNM risk.
The present study also identified high-risk factors
for LLNM in AYA patients with positive CLNM. A total of three main
factors were recognized, including two related to primary tumors
(MTD ≥1.0 cm and presence of iNG) and one associated with central
neck regions (maximum diameter of positive CLN ≥1.0 cm). Based on
these risks, two prediction models were developed for assessing
CLNM and LLNM risks in AYA patients. Based on the distribution of
the total score described in the newly-created nomogram for
predicting CLNM risk, AYA patients were split into two groups. The
overall CLNM rate was 61.8% (286/463). The low-risk group had a
CLNM rate of 25.9% (42/162) and the rate of the high-risk group was
81.1% (244/301). Furthermore, AYA patients with positive CLNM were
categorized into three groups with LLNM rates of 1.9, 20.0 and
77.3%. This classification is supported by previous studies
(19,20), demonstrating its effectiveness in
screening patients with extremely low LLNM risk (only 2/106
patients in this subgroup showed positive lateral neck
involvement).
Two prediction models were merged to form a CLNM
risk assessment for AYA patients. For AYA patients without clinical
signs of CLNM, preventive central neck dissection should be
considered for those at high CLNM risk; however, for patients with
low CLNM risk, the decision should be based on the surgeon's
assessment and patient preference. If no surgery is performed,
closer monitoring is advised. For AYA patients with detected
positive CLNM, no preventive measures are necessary for those at
low LLNM risk; however, patients with high LLNM risk may require
close observation and possibly preventive lateral neck
dissection.
The present study has certain limitations. Firstly,
the patient sample was obtained from one center with a limited case
count. For stronger evidence, a larger, multicenter sample is
required. The research was also retrospective, so the predictive
model needs validation in a prospective trial. Furthermore, only
lymph node metastasis for subgroup endpoints was assessed. Future
research should have a broader postoperative follow-up to
understand the long-term outcomes for these subgroups. Genetic
testing was also not performed and therefore, the role of several
mutations, such as Braf-V600E and TERT were not assessed in the
current study. Moreover, due to the short median follow-up time, it
was not possible to provide significant disease-free survival and
overall survival rates for patients with PTC.
In conclusion, patients with PTC aged 15–39 years
were more at risk for larger tumor sizes, ipsilateral Hashimoto
thyroiditis, thyroid capsular invasion, CLNM, LLNM and larger CLN
sizes and counts. Therefore, a stratification chart was developed
for AYA patients with PTC to quantify the risk of both CLNM and
LLNM, assisting with the clinical decisions for these patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research was supported by the Science and Technology
Program for Public Wellbeing of Ningbo (grant. no. 2022S051).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LW and GC conceived and designed the study and
analyzed and interpreted data. YY conceived and designed the study.
LW and YY wrote the manuscript. JL, ZJ and YZ collected, analyzed
and interpreted data. LW and GC confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The authors take responsibility for the accuracy and
integrity of the present work, addressing any related concerns. The
study adhered to the Declaration of Helsinki and was approved by
the ethics committee of Ningbo Medical Center Lihuili Hospital
(Ningbo, China; approval no. KY2022SL341-01). Informed consent for
the present retrospective review was waived by the same committee.
The present study is registered at the Chinese Clinical Trials
Registry (trial registration no. ChiCTR2200064921).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adolescent and Young Adult Oncology
Progress Review Group, . Closing the gap: Research and care
imperatives for adolescents and young adults with cancer. National
Institutes of Health; Bethesda, MD: 2006
|
|
2
|
Janssen SHM, van der Graaf WTA, van der
Meer DJ, Manten-Horst E and Husson O: Adolescent and Young Adult
(AYA) Cancer Survivorship Practices: An Overview. Cancers (Basel).
13:48472021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bleyer A, Barr R, Hayes-Lattin B, Thomas
D, Ellis C and Anderson B: The distinctive biology of cancer in
adolescents and young adults. Nat Rev Cancer. 4:288–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tricoli JV, Boardman LA, Patidar R,
Sindiri S, Jang JS, Walsh WD, McGregor PM III, Camalier CE,
Mehaffey MG, Furman WL, et al: A mutational comparison of adult and
adolescent and young adult (AYA) colon cancer. Cancer.
124:1070–1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tricoli JV, Blair DG, Anders CK, Bleyer
WA, Boardman LA, Khan J, Kummar S, Hayes-Lattin B, Hunger SP,
Merchant M, et al: Biologic and clinical characteristics of
adolescent and young adult cancers: Acute lymphoblastic leukemia,
colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer.
122:1017–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pizzato M, Li M, Vignat J, Laversanne M,
Singh D, La Vecchia C and Vaccarella S: The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN estimates for
incidence and mortality rates in 2020. The Lancet Diabetes and
Endocrinology. 4:264–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenbaum MA and McHenry CR: Contemporary
management of papillary carcinoma of the thyroid gland. Expert
review of anticancer therapy. 9:317–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller KD, Fidler-Benaoudia M, Keegan TH,
Hipp HS, Jemal A and Siegel RL: Cancer statistics for adolescents
and young adults, 2020. CA Cancer J Clin. 70:443–459. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z, Heng Y, Lin J, Lu C, Yu D, Tao L
and Cai W: Nomogram for predicting central lymph node metastasis in
papillary thyroid cancer: A retrospective cohort study of two
clinical centers. Cancer Res Treat. 52:1010–1018. 2020.PubMed/NCBI
|
|
10
|
Hod N, Hagag P, Baumer M, Sandbank J and
Horne T: Differentiated thyroid carcinoma in children and young
adults: Evaluation of response to treatment. Clin Nucl Med.
30:387–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vriens MR, Moses W, Weng J, Peng M,
Griffin A, Bleyer A, Pollock BH, Indelicato DJ, Hwang J and Kebebew
E: Clinical and molecular features of papillary thyroid cancer in
adolescents and young adults. Cancer. 117:259–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dedhia PH, Saucke MC, Long KL, Doherty GM
and Pitt SC: Physician perspectives of overdiagnosis and
overtreatment of low-risk papillary thyroid cancer in the US. JAMA
Netw Open. 5:e2287222022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miccoli P, Minuto MN, Ugolini C, Panicucci
E, Massi M, Berti P and Basolo F: Papillary thyroid cancer:
Pathological parameters as prognostic factors in different classes
of age. Otolaryngology-Head and Neck Surgery. 138:200–203. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mulla M and Schulte KM: Central cervical
lymph node metastases in papillary thyroid cancer: A systematic
review of imaging-guided and prophylactic removal of the central
compartment. Clin Endocrinol (Oxf). 76:131–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng JW, Yang XH, Wu BQ, Sun DL, Jiang Y
and Qu Z: Predictive factors for central lymph node and lateral
cervical lymph node metastases in papillary thyroid carcinoma. Clin
Transl Oncol. 21:1482–1491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Nie F, Wang G, Liu T, Dong T and
Sun Y: Value of combining clinical factors, conventional
ultrasound, and contrast-enhanced ultrasound features in
preoperative prediction of central lymph node metastases of
different sized papillary thyroid carcinomas. Cancer Manag Res.
13:3403–3415. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heng Y, Yang Z, Zhou L, Lin J, Cai W and
Tao L: Risk stratification for lateral involvement in papillary
thyroid carcinoma patients with central lymph node metastasis.
Endocrine. 68:320–328. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Chen S, Hou X, Liao Q, Chen G and
Zhao Y: Predictive factors of lateral lymph node metastasis in
papillary thyroid microcarcinoma. Pathol Oncol Res. 25:1245–1251.
2019. View Article : Google Scholar : PubMed/NCBI
|