Introduction
Bevacizumab is a humanized immunoglobulin G1
monoclonal antibody (mAb) that was the first angiogenesis inhibitor
to specifically target vascular endothelial growth factor A. It was
approved by the US Food and Drug Administration (FDA) in February
2004. Bevacizumab is typically indicated for the first-line
treatment of adult patients with metastatic colorectal cancer
(mCRC), unresectable advanced, metastatic or recurrent non-small
cell lung cancer (NSCLC) and metastatic breast cancer (1).
Previous pharmacokinetic studies of bevacizumab have
reported this drug to have low clearance rates, a limited volume of
the central compartment, a long elimination half-life (2–4) and
increased area under the curve with inter-patient variations of
4.9-fold (5–7). Although the dosage of bevacizumab is
adjusted by body weight, the trough concentration levels frequently
vary widely from patient to patient and cannot be easily
anticipated. Papachristos et al (8) previously conducted a prospective,
real-world study to investigate the relationship between
bevacizumab exposure and overall survival (OS) of patients with
mCRC. A total of 157 trough concentration samples were analyzed. In
total, three distinct groups of patients were identified, with
patients who experienced longer OS having markedly higher trough
concentration of bevacizumab (8).
This observation suggests that it may be of importance to conduct
therapeutic drug monitoring (TDM) of bevacizumab during routine
clinical treatment.
The selection of a rapid but accurate method for the
measurement of bevacizumab in human plasma samples is crucial for
the evaluation of exposure-response relationships in efficacy and
safety assessments during routine TDM. Classical ligand binding
assays have been used to quantify the levels of therapeutic mAbs in
biological specimens (9). However,
traditional ELISA is not reliable in differentiating between
endogenous IgGs and exogenous mAbs. Cross reactions can occur in
ELISA because of their similarity in amino acid sequences and
protein structures. In such cases, using a mass spectrometry
(MS)-based method for quantifying mAbs may confer advantages over
immunoassays in terms of accuracy. Although a liquid chromatography
(LC)-MS/MS analytical method has been previously developed and
validated for detecting bevacizumab in human plasma (10), routine TDM requires a convenient
pre-treatment process to simplify flow and shorten the time taken.
Iwamoto et al (11)
previously proposed a method for bevacizumab analysis by applying
nano-surface and molecular-orientation limited (nSMOL)
proteolysis.
In the present study, a rapid LC-MS/MS method was
designed and fully validated to quantify bevacizumab in human
plasma using the nSMOL sample pre-treatment technology. Parameters,
including specificity, carry-over, linearity, lower limit of
quantitation (LLOQ), accuracy, precision, stability, matrix effect
and recovery were calculated. The method was applied to design the
trough concentration assay of bevacizumab in eight Chinese patients
with NSCLC.
Materials and methods
Chemicals and reagents
Bevacizumab for injection (100 mg; 4 ml/vial;
Avastin) was purchased from Roche Diagnostics GmbH. ProteoMass™
Pro14-Arg (P14R) MALDI-MS Standard [internal standard
(IS)] was obtained from Sigma-Aldrich; Merck KGaA. The peptides
FTFSLDTSK (purity 99.0%), VLIYFTSSLHSGVPSR (purity 99.0%) and
STAYLQMNSLR (purity 98.0%) were purchased from GenScript as
surrogates after the proteolysis of bevacizumab. A total of 10
individual blank human plasmas (China-Japan Friendship Hospital)
were used for validation of specificity/selectivity and the mixture
plasmas were used for preparation of calibration standards and
quality control samples.
High performance LC (HPLC)-MS grade acetonitrile and
formic acid (purity 98.0%) were purchased from Thermo Fisher
Scientific, Inc. and Sigma-Aldrich; Merck KGaA, respectively.
HPLC-MS grade water was obtained by Milli-Q® Synthesis
(MilliporeSigma). nSMOL™ Antibody BA kit, FG beads™ Trypsin DART™
was purchased from Shimadzu Corporation.
Selection of surrogate and monitoring
peptides
The key to successfully develop a bevacizumab
analytical method is the selection of unique signature peptides.
Fragments of complementarity-determining region sequences of
bevacizumab are typically used as signature surrogate peptides in
bottom-up proteomics approaches (12). It is necessary to confirm beforehand
that the surrogate peptide candidates are unique sequences from
bevacizumab and cannot be generated from human blood samples.
Determination of amino acid sequences of bevacizumab was performed
in DrugBank (https://go.drugbank.com/drugs/DB00112). The surrogate
peptide candidates were determined using Skyline (version
21.2.0.369; skyline.ms/project/home/begin.view) from the variable
regions of the light and heavy chains of bevacizumab. A total of
three candidate peptides were chosen; FTFSLDTSK was used as the
quantification peptide, while VLIYFTSSLHSGVPSR and STAYLQMNSLR were
used as qualitative peptides.
Ultra-HPLC (UPLC)-MS/MS
conditions
LC was performed on an UPLC unit (Shimadzu
Corporation) with a Shimadzu InertSustainBio C18 HP (2.1×100 mm;
3.0-µm particle size). The mobile phase consisted of (A) water
containing 0.1% formic acid and (B) acetonitrile containing 0.1%
formic acid with a gradient elution. At the beginning, the mobile
phase consisted of 5% B and 95% A until 30 sec. Between 0.5 and 3.5
min, the percentage of B changed to 70%. At 3.51 min, the
percentage of B increased to 95% and maintained until 4.5 min. At
4.51 min, the percentage of B returned to 5% till the end of 6.0
min. The overall run time was 6.0 min at 0.2 ml/min flow rate. A
Shimadzu 8050CL triple quadrupole MS equipped with an electro-spray
ionization source (Shimadzu Corporation) was used for mass
spectrometric detection. The detection was operated in the positive
mode with multiple reaction monitoring (MRM). The dwell time was
set to 3.0 msec for each MRM transition. After optimization, the
source parameters were set as follows: Atomizing gas, 3 l/min; dry
gas, 5 l/min; heat gas, 15 l/min; ion spray voltage, 4,000 V;
interface temperature, 300°C; temperature, 150°C and heat block
temperature, 400°C. The MRM transitions and specific parameters for
all surrogate peptides and IS are listed in Table I. Data acquisition and processing
were performed using Labsolutions (version 5.81; Shimadzu
Corporation).
 | Table I.MRM transitions and specific
parameters for the targeted peptides, FTFSLDTSK, VLIYFTSSLHSGVPSR
and STAYLQMNSLR, and IS P14R. |
Table I.
MRM transitions and specific
parameters for the targeted peptides, FTFSLDTSK, VLIYFTSSLHSGVPSR
and STAYLQMNSLR, and IS P14R.
| Selected peptide | Region | MRM transition,
m/z | Q1 Pre Bias, V | CE, V | Q3 Pre Bias, V |
|---|
| FTFSLDTSK | H-chain of |
523.30([M+2H]2+)→797.40(y7+)a | −38 | −18 | −34 |
|
| CDR2 |
523.30([M+2H]2+)→898.50(y8+) | −38 | −20 | −30 |
|
|
|
523.30([M+2H]2+)→650.30(y6+) | −38 | −19 | −34 |
| VLIYFTSSLHSGVPSR | L-chain of |
588.50([M+3H]3+)→776.10(y14++) | −30 | −19 | −32 |
|
| CDR2 |
588.50([M+3H]3+)→939.60(y9+) | −22 | −28 | −26 |
|
|
|
588.50([M+3H]3+)→602.40(y6+) | −22 | −28 | −24 |
| STAYLQMNSLR | H-chain of |
642.60([M+2H]2+)→861.30(y7+) | −32 | −23 | −28 |
|
| CH1 |
642.60([M+2H]2+)→748.30(y7+) | −24 | −22 | −24 |
|
|
|
642.60([M+2H]2+)→620.30(y7+) | −34 | −24 | −24 |
| P14R (IS) | - |
512.10→292.30a | −38 | −20 | −20 |
|
|
| 512.10→389.30 | −38 | −16 | −28 |
|
|
| 512.10→757.50 | −38 | −19 | −38 |
Stock and working solutions,
calibration standards and quality control (QC) sample
Bevacizumab injection (25 mg/ml) was used as a stock
solution to make the calibration standards and QC samples. The
stock solution of the IS P14R (10 nmol) was diluted to 1
ml using Enhanced Reaction Solution from the nSMOL Antibody BA Kit
(cat. no. P/N 225-32250-91; Shimadzu Corporation) to obtain a 10
nmol/ml P14R solution. The surrogate peptides were
prepared by dissolving 1.7 mg FTFSLDTSK (net weight, 1.0 mg), 1.5
mg VLIYFTSSLHSGVPSR (net weight, 1.0 mg) and 1.5 mg STAYLQMNSLR
(net weight, 1.0 mg) in 1 ml methanol/water (1:1, v/v),
respectively. The stock solution of bevacizumab was also further
diluted with blank human plasma to obtain the calibration standards
and QC samples at the designated concentration levels. The final
concentrations of the calibration standards were 5, 10, 20, 50,
100, 200, 300 and 400 µg/ml. The concentrations of the QC samples
in plasma were 10, 50, 200 µg/ml and 5 µg/ml for LLOQ and 400 µg/ml
for upper LOQ (ULOQ). The IS working solutions were prepared by
adding 5 µl P14R (10 nmol/ml) to 995 µl Enhanced
Reaction Solution and 1 ml Reaction Solution, which were freshly
prepared every time. The bevacizumab and P14R stock
solutions were stored at 4 and −80°C, respectively. The calibration
standards and QC samples were immediately stored at −80°C.
Sample pretreatment
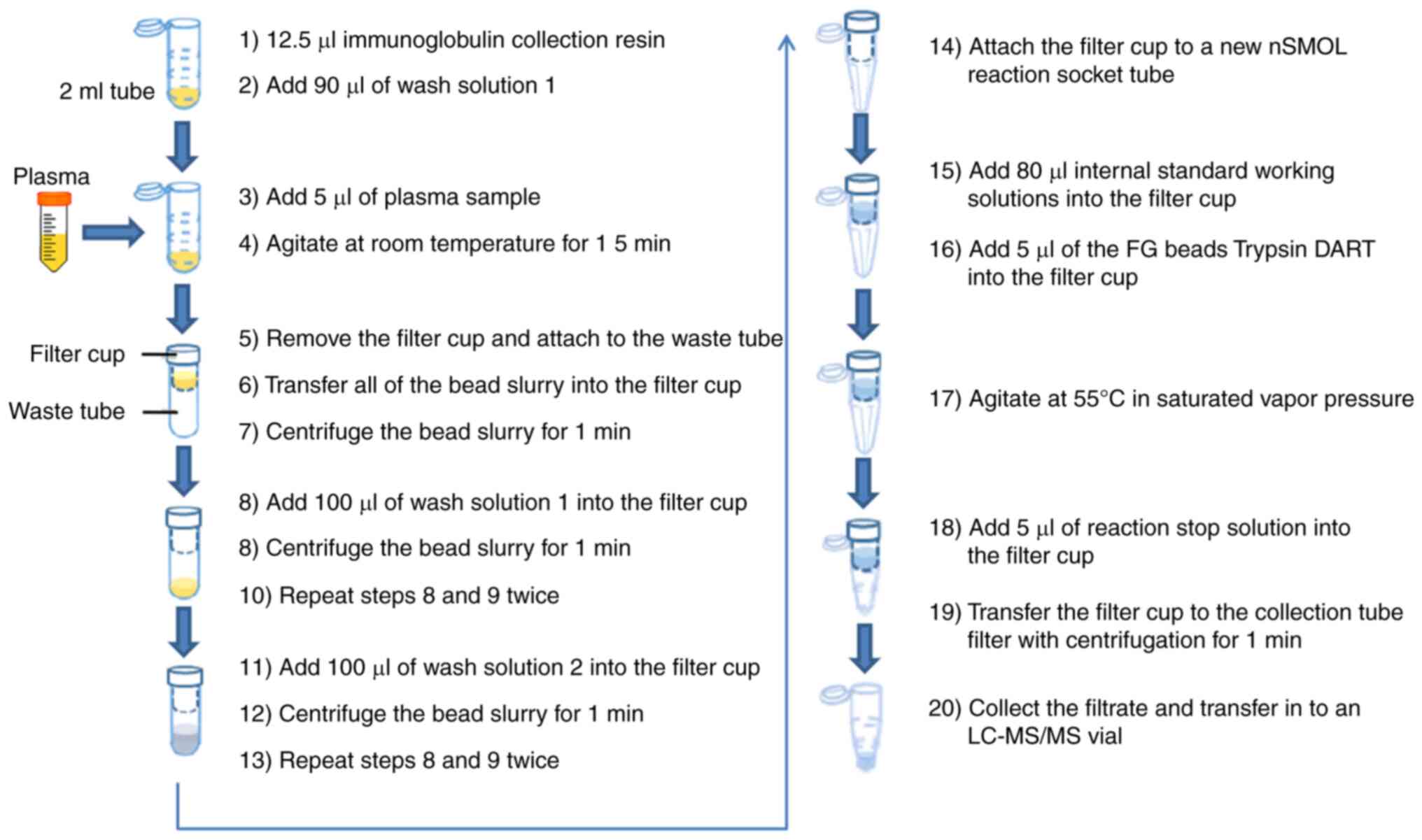
Plasma samples were prepared using an nSMOL antibody
BA kit to obtain the surrogate peptides (11). The study design is summarized in
Fig. 1. The specific experimental
procedures were listed as follows: After sufficiently evenly
diffusing the immunoglobulin collection resin with vortex, 12.5 µl
bead slurry was aliquoted into 2-ml sample tubes before adding 90
µl wash solution 1 (binding solution). A total of 5 µl plasma
sample was added into the 2-ml sample tubes, which included
calibration curve, QC or clinical samples; the mixture was gently
agitated at room temperature for 15 min by a micro tube mixer; the
filter cup was removed from the filter tube (Ultrafree-MC; GV
0.22-µm; MilliporeSigma) and which was attached to the waste tube
(Micro tube 2 ml; Sarstedt, Inc.); all of the bead slurry were
transferred into the filter cup and centrifuged at room temperature
under 10,000 × g for 1 min; 100 µl wash solution 1 (binding
solution) was added into the filter cup and the bead slurry was
centrifuged at room temperature under 10,000 × g for 1 min, which
procedure was repeated twice; 100 µl wash solution 2 was added into
the filter cup and the bead slurry was centrifuged at room
temperature under 10,000 × g for 1 min, which was repeated twice as
well; the filter cup was attached to a new nSMOL reaction socket
tube (Shimadzu Corporation); after sufficiently evenly diffusing 5
µl of FG beads Trypsin DART with vortex, the Trypsin DART and 80 µl
IS working solutions were then added into the filter cup; the
solution was gently (700 rpm) agitated at 55°C in saturated vapor
pressure for 5 h for enzymatic digestion; 5 µl of reaction stop
solution was added into the filter cup; the filtrate was collected
and transferred to an LC-MS/MS vial, after the filter cup was
transferred to the collection tube with centrifugation at 12,000 ×
g for 1 min.
Method validation
The method was fully validated for
specificity/selectivity, linearity, precision, accuracy, recovery,
matrix effect and stability according to The International Council
for Harmonisation of Technical Requirements for Pharmaceuticals for
Human Use guideline-Bioanalytical method validation M10 (13), the European Medicines
Agency-Guideline on bioanalytical method validation (14) and the US FDA (15).
The specificity/selectivity of this method was
investigated by analyzing 10 individual human blank plasma samples.
Each blank sample was tested for interferences by using the
proposed pre-treatment procedures and LC-MS/MS conditions.
The carry-over of surrogate peptides and IS were
evaluated by injecting blank samples after the ULOQ samples to
compare the peak area of blank samples and LLOQ samples at the
retention time.
The linearity and LLOQ for the three surrogate
peptides were assessed by plotting their peak area ratios with the
IS vs. their respective concentrations. The criterion was that the
deviation of each back-calculated concentration had to be within
±20% of the nominal value except for the LLOQ, which had to be
within ±25%.
Accuracy and precision were determined by analyzing
five concentration levels (5, 10, 50, 200 and 400 µg/ml) for
bevacizumab at LLOQ, low QC (LQC), middle QC (MQC), high (HQC) and
ULOQ in the plasma. A total of five replicates of every QC sample
were analyzed on six separate validation days. The intra- and
inter-day accuracy should be within ±20% of the theoretical
concentrations (LLOQ within ±25%). The intra- and inter-day
precision should be <20% for QC samples (LLOQ should be
<25%).
The stability of bevacizumab in the human plasma
samples was evaluated under different temperature and timing
conditions. The short-term stability was evaluated by determining
QC samples at three concentration levels, which were kept at room
temperature for a period that exceeded the routine pre-treatment
time of the samples (~6 h). The auto-sampler stability was measured
by re-analyzing the QC samples kept in the auto-sampler (4°C) for
72 h. The freeze and thaw stability of the QC samples was tested
after three freeze (−80°C) and thaw (room temperature) cycles.
Their long-term stability was assessed by analyzing storage at a
low temperature (−80°C) for 26 days.
The validation processes of the matrix effect and
recovery were performed according to the method of Jiang et
al (16). The matrix effects
for surrogate peptides with their IS P14R were assessed
by using 10 different individual human plasma samples and comparing
the ratio of the peak area in the presence of matrix (measured by
analyzing blank matrix after extraction and then spiked with
FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR) to the peak area in
the absence of matrix (pure analyte and IS solution). The matrix
effect was evaluated by comparing the average peak areas of spiked
samples after extraction to those of corresponding working
solutions at the same concentration. The inter-individual
variability of the IS-normalized matrix factor expressed by
relative standard deviation (RSD) should be <15%.
Recoveries of the pre-treatment method were analyzed
at three QC concentrations by comparing the mean peak areas of
pre-treated QC samples (n=10) with those of post-extracted blank
plasma samples (n=10) spiked with working solutions. The recoveries
of the IS were determined using the same method.
Data processing
Data acquisition and processing were conducted by
using Labsolutions software (version 5.81; Shimadzu Corporation).
Descriptive statistics [mean, SD, RSD and relative error (RE)] were
calculated by using Microsoft Office (version 2016; Microsoft
Corporation).
Patients
The analytical method was used to monitor human
plasma levels of bevacizumab in patients with NSCLC. All the
procedures were conducted in accordance with the Declaration of
Helsinki, and the present study was approved by the Medical Ethics
Committee of China-Japan Friendship Hospital (Beijing, China). A
total of 8 patients with NSCLC were enrolled in this research from
June 2020 to December 2020 in China-Japan Friendship Hospital.
Plasma samples were collected by venipuncture into evacuated
EDTA-anticoagulated blood collection tubes after injecting
bevacizumab over 3 periods of treatment and centrifuged at 1,811 ×
g for 5 min. All samples were stored at −80°C until analysis.
Statistical analysis
Descriptive statistics were performed for the data
of patients. Microsoft Excel (Microsoft® Office® 2016, Microsoft
Corp., USA) was utilized for data analysis (mean values, SDs and
RSDs). A weighted least square fit was used for the standard
calibrations.
Results
Method validation
Specificity/selectivity
The analysis of 10 healthy independent blank human
plasma samples showed that there were no endogenous interference
peaks observed during the retention time of the surrogate peptides.
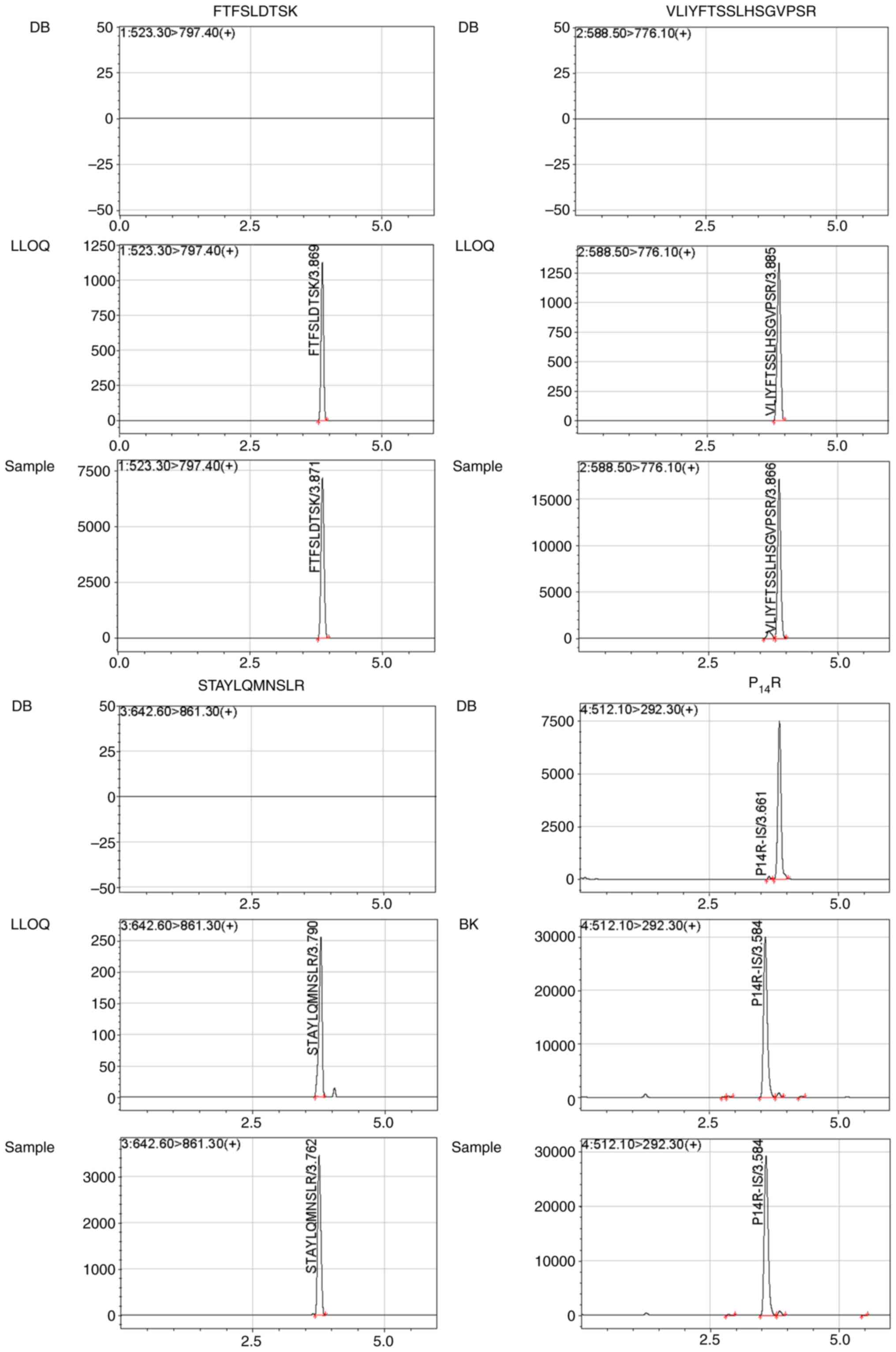
Representative chromatograms of double blank with no surrogate
peptide of interest or IS (Fig. 2)
showed the absence of any interference during the retention time
for surrogate peptides and IS. Blank human plasma from 10
individuals were individually extracted to determine if any
interferences detected at the retention times were <20% for
FTFSLDTSK, VLIYFTSSLHSGVPSR and STAYLQMNSLR, and if they were 5%
for the IS in LLOQ samples.
Carry-over
There was no interference observed at the retention
time of the surrogate peptides and IS after ULOQ sample
injection.
Linearity and LLOQ
Calibration curves were created by plotting the peak
area ratios of the various surrogate peptides to IS vs. nominal
concentration of the analyte standards with a weighting factor of
1/x2 applied to all calibration curves. The
correlation coefficient (r2) of all the calibration
curves was >0.99. The typical regression equations of these
curves were calculated as follows: i) FTFSLDTSK in human plasma
(y=0.00116185×-0.00128682;r2=0.9907); ii)
VLIYFTSSLHSGVPSR in human plasma(y=0.00684308×+0.00390712;
r2=0.9953); and iii) STAYLQMNSLR in human plasma
(y=0.00132500×-0.000746389; r2=0.9971).
The LLOQ of 5 µg/ml bevacizumab in human plasma was
eligible with 11.80–18.84% of RSD and −2.95–2.47% of RE. Typical
chromatograms of LLOQs for FTFSLDTSK, VLIYFTSSLHSGVPSR and
STAYLQMNSLR in plasma are shown in Fig.
2.
Accuracy and precision
The accuracy and precision for FTFSLDTSK,
VLIYFTSSLHSGVPSR and STAYLQMNSLR after bevacizumab proteolysis are
summarized in Table II. The intra-
and inter-day accuracy range was −7.48–9.04%, and the intra- and
inter-day precision range was 1.63–18.84% in five QC samples (LLOQ,
LQC, MQC, HQC and ULOQ). The validated method was reliable and
reproducible for the quantification of bevacizumab.
 | Table II.Intra- and inter-day accuracy and
precision of QCs, LLOQ and ULOQ samples in plasma. |
Table II.
Intra- and inter-day accuracy and
precision of QCs, LLOQ and ULOQ samples in plasma.
| Nominal
concentration, µg/ml | Intra-day
a (n=5) | RSD, % | RE, % |
Inter-daya (n=5, 6 daysb) | RSD, % | RE, % |
|---|
| FTFSLDTSK |
|
|
|
|
|
|
| 5 | 4.85±0.59 | 12.20 | −2.95 | 5.02±0.81 | 16.18 | 0.41 |
| 10 | 9.36±1.02 | 10.84 | −6.43 | 9.69±1.28 | 13.18 | −3.09 |
| 50 | 47.74±3.08 | 6.45 | −4.52 | 51.43±5.92 | 11.51 | 2.86 |
|
200 | 196.09±7.08 | 3.61 | −1.95 | 192.79±16.41 | 8.51 | −3.60 |
|
400 | 402.44±18.24 | 4.53 | 0.61 | 370.10±32.57 | 8.80 | −7.48 |
|
VLIYFTSSLHSGVPSR |
|
|
|
|
|
|
| 5 | 5.10±0.70 | 13.70 | 1.92 | 5.12±0.74 | 14.39 | 2.47 |
| 10 | 9.70±0.34 | 3.48 | −3.02 | 9.42±1.212 | 12.93 | −5.76 |
| 50 | 51.10±4.48 | 8.76 | 2.20 | 48.86±4.47 | 9.15 | −2.28 |
|
200 | 218.08±8.62 | 3.95 | 9.04 | 192.04±21.09 | 10.98 | −3.98 |
|
400 | 394.60±6.44 | 1.63 | −1.35 | 375.16±27.13 | 7.23 | −6.21 |
| STAYLQMNSLR |
|
|
|
|
|
|
| 5 | 4.90±0.58 | 11.80 | −2.04 | 5.09±0.96 | 18.84 | 1.71 |
| 10 | 9.66±1.05 | 10.85 | −3.37 | 10.04±1.46 | 14.53 | 0.44 |
| 50 | 52.76±4.77 | 9.04 | 5.52 | 49.81±5.35 | 10.73 | −0.38 |
|
200 | 204.29±13.40 | 6.56 | 2.15 | 192.66±16.84 | 8.74 | −3.67 |
|
400 | 415.61±24.71 | 5.95 | 3.90 | 399.65±31.18 | 7.80 | −0.09 |
Stability
Next, the stability of bevacizumab in human plasma
was investigated under different storage and processing conditions
(Table III). Bevacizumab was
found to be stable in human plasma following three freeze-thaw
cycles (from −80°C to 25°C) and storage at −80°C for 26 days. In
addition, bevacizumab remained stable during short-term storage at
25°C for 6 h and auto-sampler stability was maintained at 4°C for
72 h.
 | Table III.Stability results of selected
peptides (FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR) in plasma in
different conditions. |
Table III.
Stability results of selected
peptides (FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR) in plasma in
different conditions.
| Nominal
concentration, µg/ml |
FTFSLDTSKa | RSD, % | RE, % |
VLIYFTSSLHSGVPSRa | RSD, % | RE, % |
STAYLQMNSLRa | RSD, % | RE, % |
|---|
| Short-term (25°C
for 6 h) |
|
|
|
|
|
|
|
|
|
| 10 | 9.74±1.11 | 11.35 | −2.57 | 9.84±1.25 | 12.69 | −1.62 | 9.70±1.18 | 12.21 | −3.00 |
| 50 | 49.75±2.82 | 5.67 | −0.50 | 47.86±1.27 | 2.65 | −4.29 | 51.08±5.24 | 10.27 | 2.15 |
|
200 | 186.33±3.38 | 1.81 | −6.84 | 189.08±7.56 | 4.00 | −5.46 | 219.35±14.93 | 6.81 | 9.67 |
| Auto-sampler (4°C
for 72 h) |
|
|
|
|
|
|
|
|
|
| 10 | 9.20±1.14 | 12.36 | −8.04 | 11.21±0.81 | 7.18 | 12.10 | 9.81±1.40 | 14.30 | −1.87 |
| 50 | 49.80±4.26 | 8.56 | −0.40 | 48.44±3.79 | 7.83 | −3.12 | 55.81±4.79 | 8.58 | 11.63 |
|
200 | 192.51±7.40 | 3.84 | −3.75 | 185.69±5.06 | 2.72 | −7.16 | 213.34±4.50 | 2.11 | 6.67 |
| Freeze thaw
(−80-25°C for 3 cycles) |
|
|
|
|
|
|
|
|
|
| 10 | 9.17±1.18 | 12.86 | −8.26 | 9.18±0.59 | 6.43 | −8.17 | 9.35±0.98 | 10.49 | −6.51 |
| 50 | 47.21±5.71 | 12.09 | −5.59 | 46.44±1.67 | 3.61 | −7.12 | 45.63±3.46 | 7.57 | −8.74 |
|
200 | 190.14±11.93 | 6.27 | −4.93 | 191.43±4.24 | 2.21 | −4.28 | 189.56±9.47 | 4.99 | −5.22 |
| Long term (−80°C
for 26 days) |
|
|
|
|
|
|
|
|
|
| 10 | 10.03±0.98 | 9.78 | 0.27 | 8.79±0.76 | 8.62 | −12.08 | 9.31±1.11 | 11.94 | −6.90 |
| 50 | 48.81±3.68 | 7.55 | −2.37 | 44.88±4.72 | 10.52 | −10.24 | 47.86±4.78 | 9.99 | −4.29 |
|
200 | 191.94±7.10 | 3.70 | −4.03 | 191.56±6.63 | 3.46 | −4.22 | 189.65±14.77 | 7.79 | −5.18 |
Matrix effect and recovery
The range of IS-normalized matrix effects in human
plasma was 41.87–44.24% for FTFSLDTSK, 53.48–59.52% for
VLIYFTSSLHSGVPSR and 47.67–49.65% for STAYLQMNSLR. The matrix
effect of every surrogate peptide was in the same level for the
three different QC concentrations. The recovery range in plasma was
22.11–23.59% for FTFSLDTSK, 21.78–24.65% for VLIYFTSSLHSGVPSR and
22.74–23.29% for STAYLQMNSLR. The RSD of IS-normalized matrix
effects and recovery was <20% (Table IV).
 | Table IV.Matrix effect and recovery of
FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR and P14R. |
Table IV.
Matrix effect and recovery of
FTFSLDTSK, VLIYFTSSLHSGVPSR, STAYLQMNSLR and P14R.
| Nominal
concentration of bevacizumab, µg/ml | Selected peptides
FTFSLDTSK |
VLIYFTSSLHSGVPSR | STAYLQMNSLR | IS P14R |
|---|
|
|
|
|
|---|
| Mean ± SD | RSD,% | Mean ± SD | RSD,% | Mean ± SD | RSD, % | Mean ± SD | RSD,% |
|---|
| Matrix effect, %
(n=10) |
|
|
|
|
|
|
|
|
| 10 | 32.98±3.27 | 4.82 | 46.06±6.53 | 6.15 | 37.21±3.86 | 5.04 | 82.30±3.28 | 10.14 |
| 50 | 31.83±1.67 | 6.28 | 43.84±3.44 | 5.27 | 38.46±2.57 | 4.95 |
|
|
|
200 | 34.80±1.31 | 4.1 | 42.12±0.99 | 3.24 | 36.93±1.26 | 3.47 |
|
|
| IS-normalized
matrix factor, % (n=10) |
|
|
|
|
|
|
|
|
| 10 | 41.87±3.47 | 5.49 | 59.52±7.16 | 6.55 | 48.03±4.96 | 4.93 |
|
|
| 50 | 41.89±2.54 | 8.65 | 57.60±3.21 | 7.66 | 49.65±4.28 | 7.25 |
|
|
|
200 | 44.24±4.18 | 6.41 | 53.48±3.90 | 6.24 | 47.67±3.95 | 5.6 |
|
|
| Recovery, %
(n=10) |
|
|
|
|
|
|
|
|
| 10 | 22.86±1.10 | 15.61 | 23.15±1.42 | 14.17 | 22.74±1.28 | 12.79 | 77.47±4.49 | 6.05 |
| 50 | 22.11±1.39 | 5.24 | 21.78±1.15 | 7.84 | 23.29±1.47 | 8.79 |
|
|
|
200 | 23.59±0.97 | 3.78 | 24.65±0.80 | 2.34 | 22.91±0.94 | 2.97 |
|
|
Application of the method in the TDM
of patients with NSCLC
The validated LC-MS/MS method was applied
successfully in a trough concentration assay after injecting eight
patients with NSCLC bevacizumab over three periods of treatment.
Representative chromatograms of a plasma sample from a patient with
NSCLC at 2 h after injecting bevacizumab for three periods of
treatment are shown in Fig. 2.
Patient characteristics are shown in Table V. The bevacizumab trough
concentration in the eight patients with NSCLC and dose are shown
in Table VI.
 | Table V.Patient characteristics. |
Table V.
Patient characteristics.
| Patient | Age, years | Sex | Height, cm | Weight, kg | BMI,
kg/m2 | BSA,
m2 | Disease stage | Combined
pharmacotherapy |
|---|
| 1 | 36 | F | 172 | 52 | 17.6 | 1.562 | IV-B | PEM, CBDCA,
ICO |
| 2 | 61 | F | 162 | 70 | 26.5 | 1.734 | IV-B | ICO |
| 3 | 50 | F | 170 | 82 | 28.4 | 1.934 | IV-B | OSI |
| 4 | 66 | M | 175 | 72.5 | 23.7 | 1.843 | IV-A | PTX |
| 5 | 64 | F | 168 | 53.6 | 19.0 | 1.558 | IV-B | AFA |
| 6 | 68 | F | 160 | 56.5 | 22.1 | 1.546 | IV-B | OSI |
| 7 | 82 | F | 155 | 49 | 20.4 | 1.48 | IV | STM |
| 8 | 56 | M | 168 | 63 | 22.3 | 1.678 | IV-A | PEM, CBDCA |
 | Table VI.Quantification of bevacizumab in the
plasma of patients, adjusted doses, tumor response and adverse
events. |
Table VI.
Quantification of bevacizumab in the
plasma of patients, adjusted doses, tumor response and adverse
events.
| Patient | Dose of
bevacizumab, mg | Trough
concentration, µg/ml | Tumor response
after three courses of treatment | Bevacizumab-induced
adverse events |
|---|
| 1 | 400 | 35.36 | PD | N/A |
| 2 | 500 | 32.05 | PD | Gum bleeding |
| 3 | 500 | 62.27 | SD | Gum bleeding |
| 4 | 500 | 33.48 | SD | N/A |
| 5 | 400 | 23.22 | SD | Gum bleeding,
hemorrhinia |
| 6 | 500 | 40.65 | PD | N/A |
| 7 | 300 | 29.23 | PD | N/A |
| 8 | 500 | 37.60 | SD | Hemorrhinia |
Patient demographic
characteristics
Table V shows the
characteristics of the eight patients. Patients had been diagnosed
with either postoperative recurrence or metastatic NSCLC, all of
which were classified as adenocarcinoma. In total, 20 genes
associated with lung cancer were identified by next-generation
sequencing (NovaSeq 6000; Illumina, Inc.) for personality usage of
molecular targeted drugs, including ALK, ATM, BRAF, BRCA1, BRCA2,
DDR2, EGFR, ERBB2, HRAS, KIT, KRAS, MET, NARS, NTRK1, NTRK2, NTRK3,
PDGFRA, PIK3CA, RET and ROS1, in the pathology laboratory of the
China-Japan Friendship Hospital. The genetic results of the
patients were retrieved from their electronic medical records.
Patient 1, 2, 3 and 5 had EGFR 19 exon deletion. Patient 4 and 6
had EGFR 20 exon and 21 exon mutation, respectively. Patient 7 had
an ERBB2 20 exon insertion mutation and patient 8 had no gene
mutations.
Bevacizumab trough concentration and
adverse events
In total, eight analytes collected after ≥3 periods
of treatment were treated as the bevacizumab trough concentration.
The median bevacizumab trough concentration was found to be 34.42
µg/ml, with a range of 23.22–62.27 µg/ml. Tumor responses in all of
the patients were evaluated. Patient 1, 2, 6 and 7 were considered
as progressive disease, whereas patient 3, 4, 5 and 8 were shown to
exhibit stable disease. The most common adverse effects observed
were gum bleeding and hemorrhinia with 50% incidence rate.
Discussion
In the present study, a determination method of
bevacizumab in human plasma by UPLC-MS/MS method was fully
validated. The method meets the requirements for the quantitative
measurements of bevacizumab human plasma sample, which was also
successfully applied in a trough concentration analysis of eight
patients with NSCLC.
A short and stable isotope-labeled peptide,
P14R, was chosen as the IS in the present study due to
availability. In addition, it has been previously used for the
quantification of antibody-based drugs for biological analysis
(17). The method reported in the
present study allows for a throughput of ≥100 samples per day on a
single LC-MS/MS platform due to a relatively short injection to
injection time of 6 min during the chromatography step.
P14R was a chemically-synthesized stable isotope labeled
(SIL) peptide which was an alternative selection. SIL peptides with
flanking amino acids on their C- and N-terminals were used as IS to
minimize the variability during sample processing and
detection.
Evaluation of processing recovery contains four
different parts, namely pre-digestion recovery, digestion recovery,
post-digestion recovery and overall digestion. Each procedure can
result in recovery loss. The recovery of the present method was at
20–25%, which to the best of our knowledge, has not been previously
reported. Although it remains unclear at present which of the
procedures resulted in recovery loss, sensitivity of the present
method was sufficient for the detection of bevacizumab in human
plasma samples. Optimizing this aspect of the protocol is required
in future studies.
Subsequently, in the present study, eight patients
were injected with 7.5 mg/kg body weight bevacizumab. In
particular, five patients were injected with 500 mg bevacizumab,
with a trough concentrations range of 32.054–62.266 µg/ml, which
was variable among individuals. The trough concentrations in the
two patients injected with 400 mg bevacizumab were 33.726 and
23.217 µg/ml, respectively, whereas the trough concentration of one
patient injected with 300 mg bevacizumab was 29.225 µg/ml. The
individual differences of bevacizumab trough concentrations
suggested that TDM is promoted for bevacizumab in adjusting the
therapeutic strategy. Additional plasma samples from patients with
NSCLC are required for detecting the bevacizumab trough
concentration and explore the TDM range for individualized drug
administration.
TDM-guided approach has been proved to improve
efficacy and reduce toxicity for several classes of small-molecule
and therapeutic antibody drugs. In the case of bevacizumab in
NSCLC, the TDM approach shows the potential to provide
possibilities for patients with NSCLC to achieve the goal of
personalized treatment. Additional samples are required to
investigate the rational bevacizumab TDM range for different
subtypes of NSCLC and to identify the influence factors of
bevacizumab pharmacokinetics.
Acknowledgements
Not applicable.
Funding
The present study was supported by National High Level Hospital
Clinical Research Funding (grant no. 2022-NHLHCRF-LX-01-0303), CAMS
Innovation Fund for Medical Sciences (grant no. 2021-I2M-1-012),
Research Fund of China-Japan Friendship Hospital (grant no.
2019-2-QN-66), Bethune Charitable Foundation (grant no.
B-19-H-20200622) and National Key Research and Development Program
of China (grant no. 2020YFC2005504).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BL and XLZ contributed to the study conception and
design and prepared the materials. BL optimized the methodology and
wrote the original draft. MY conducted clinical evaluation. XXW,
WQC and LS performed the method validation. XBZ, PML and LHL were
responsible for recording clinical data and interpretation of the
data. HKL, XYL and GW performed the data analysis and statistics.
XLZ reviewed and edited the manuscript and gave final approval of
the version to be published. BL, MY and PML acquired funding. BL,
MY and XLZ confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Research involving human subjects complied with all
relevant national regulations, institutional policies and is in
accordance with the tenets of the Helsinki Declaration, and has
been approved by the Institutional Review Board of China-Japan
Friendship Hospital (approval no. 2021-111-K69; Beijing,
China).
Patient consent for publication
Written informed consent was obtained from all
individuals included in the present study for publication of their
data and associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UPLC-MS/MS
|
ultra-performance liquid
chromatography tandem mass spectrometry
|
|
LLOQ
|
lower limit of quantitation
|
|
OS
|
overall survival
|
|
mCRC
|
metastatic colorectal cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
TDM
|
therapeutic drug monitoring
|
|
mAb
|
monoclonal antibodies
|
|
nSMOL
|
nano-surface and molecular-orientation
limited
|
|
MRM
|
multiple reaction monitoring
|
|
P14R
|
Pro14-Arg
|
|
QC
|
quality control
|
|
LQC
|
low quality control
|
|
MQC
|
middle quality control
|
|
HQC
|
high quality control
|
|
IS
|
internal standard
|
|
RE
|
relative error
|
|
RSD
|
relative standard deviation
|
References
|
1
|
Shih T and Lindley C: Bevacizumab: An
angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. 28:1779–1802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu JF, Bruno R, Eppler S, Novotny W, Lum B
and Gaudreault J: Clinical pharmacokinetics of bevacizumab in
patients with solid tumors. Cancer Chemother Pharmacol. 62:779–786.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Gupta M, Jin D, Xin Y, Visich J and
Allison DE: Characterization of the long-term pharmacokinetics of
bevacizumab following last dose in patients with resected stage II
and III carcinoma of the colon. Cancer Chemother Pharmacol.
71:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tabrizi MA, Tseng CM and Roskos LK:
Elimination mechanisms of therapeutic monoclonal antibodies. Drug
Discov Today. 11:81–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao B, Yeap S, Clements A, Balakrishnar B,
Wong M and Gurney H: Evidence for therapeutic drug monitoring of
targeted anticancer therapies. J Clin Oncol. 30:4017–4025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glade Bender JL, Adamson PC, Reid JM, Xu
L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D,
Chen HX, et al: Phase I trial and pharmacokinetic study of
bevacizumab in pediatric patients with refractory solid tumors: A
children's oncology group study. J Clin Oncol. 26:399–405. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu JY, Wu XN, Ding L, Zhao YB, Ai B, Li Y,
Hu X and Cheng G: Phase I safety and pharmacokinetic study of
bevacizumab in Chinese patients with advanced cancer. Chin Med J
(Engl). 123:901–906. 2010.PubMed/NCBI
|
|
8
|
Papachristos A, Kemos P, Kalofonos H and
Sivolapenko G: Correlation between bevacizumab exposure and
survival in patients with metastatic colorectal cancer. Oncologist.
25:853–858. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ternant D, Cézé N, Lecomte T, Degenne D,
Duveau AC, Watier H, Dorval E and Paintaud G: An enzyme-linked
immunosorbent assay to study bevacizumab pharmacokinetics. Ther
Drug Monit. 32:647–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Legeron R, Xuereb F, Chaignepain S, Gadeau
AP, Claverol S, Dupuy JW, Djabarouti S, Couffinhal T, Schmitter JM
and Breilh D: A new reliable, transposable and cost-effective assay
for absolute quantification of total plasmatic bevacizumab by
LC-MS/MS in human plasma comparing two internal standard
calibration approaches. J Chromatogr B Analyt Technol Biomed Life
Sci. 1070:43–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwamoto N, Umino Y, Aoki C, Yamane N,
Hamada A and Shimada T: Fully validated LCMS bioanalysis of
bevacizumab in human plasma using nano-surface and
molecular-orientation limited (nSMOL) proteolysis. Drug Metab
Pharmacokinet. 31:46–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Todoroki K, Mizuno H, Sugiyama E and
Toyo'oka T: Bioanalytical methods for therapeutic monoclonal
antibodies and antibody-drug conjugates: A review of recent
advances and future perspectives. J Pharm Biomed Anal.
179:1129912020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Zheng X, Liu DY, Zhao Q, Wu YW,
Tan FL, Wang YX, Jiang J and Hu P: Therapeutic effects and adverse
drug reactions are affected by icotinib exposure and CYP2C19 and
EGFR genotypes in Chinese non-small cell lung cancer patients.
Asian Pac J Cancer Prev. 15:7195–7200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu D, Jiang J, Zhang L, Tan F, Wang Y,
Zhang D and Hu P: Clinical pharmacokinetics of icotinib, an
anti-cancer drug: Evaluation of dose proportionality, food effect,
and tolerability in healthy subjects. Cancer Chemother Pharmacol.
73:721–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu
Z, Xue C, Zhang J, Zhang J, Ma Y, et al: Network meta-analysis of
erlotinib, gefitinib, afatinib and icotinib in patients with
advanced non-small-cell lung cancer harboring EGFR mutations. PLoS
One. 9:e852452014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang H, Zeng J, Titsch C, Voronin K,
Akinsanya B, Luo L, Shen H, Desai DD, Allentoff A, Aubry AF, et al:
Fully validated LC-MS/MS assay for the simultaneous quantitation of
coadministered therapeutic antibodies in cynomolgus monkey serum.
Anal Chem. 85:9859–9867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brun V, Masselon C, Garin J and Dupuis A:
Isotope dilution strategies for absolute quantitative proteomics. J
Proteomics. 72:740–749. 2009. View Article : Google Scholar : PubMed/NCBI
|