Introduction
Long non-coding RNAs (lncRNAs) represent one of the
various types of non-protein-coding transcripts. By definition,
lncRNAs are transcripts of >200 nucleotides that are not
translated into proteins (1) and
are composed of intergenic transcripts, enhancer RNAs and sense or
antisense transcripts that overlap other genes (2). LncRNAs play pivotal roles across a
diverse array of biological processes, regulating gene expression
through mechanisms (3) that include
functioning as signaling entities, molecular decoys, guides for
chromatin-modifying enzymes, and scaffolding for multi-protein
complexes. Beyond their critical involvement in the oncogenic
pathways, the importance of lncRNAs in cellular differentiation,
organogenesis, embryonic development, and the adaptive response to
environmental stimuli has been highlighted (4). These findings illuminate the role of
lncRNAs as essential molecules in maintaining homeostasis of
physiological processes (5),
participating in cell cycle control, apoptosis, and the cellular
response to a broad spectrum of physiological and pathological
stimuli. Consequently, lncRNAs emerge not only as potential
therapeutic targets and biomarkersin cancer-related processes
(6,7) such as proliferation, invasion,
migration and angiogenesis, but also as promising drug targets and
diagnostic tools (8) in a wider
biological context.
LncRNA homeobox A cluster antisense RNA 2
(HOXA-AS2), located between the human HOXA3 and HOXA4 genes and
with a length of 1,048 bp, has been extensively detected and
researched across various malignancies since its initial
demonstration as an apoptosis inhibitor in NB4 promyelocytic
leukemia cells in 2013 (9). As an
oncogene, lncRNA HOXA-AS2 exhibits abnormally high expression in a
wide array of solid and hematological malignancies, such as acute
myeloid leukemia (AML) (10),
gallbladder cancer (11) and glioma
(12), promoting the progression of
these cancer types. The mechanisms by which lncRNA HOXA-AS2
inhibits apoptosis and stimulates proliferation have been the most
extensively studied (13–15), indicating that lncRNA HOXA-AS2 not
only affects the proliferation, invasion and migration of cancer
cells but is also closely related to patient prognosis. For
instance, the upregulation of lncRNA HOXA-AS2 in AML (10) demonstrates its oncogenic role by
interacting with the epigenetic inhibitor Enhancer of zeste homolog
2 (EZH2), subsequently repressing the expression of Large Tumor
Suppressor 2 (LATS2). This mechanism elucidates how HOXA-AS2
contributes to the proliferation and inhibits the differentiation
of AML cells, negatively impacting patient survival. The binding of
HOXA-AS2 with EZH2 and the inhibition of LATS2 underscore its
potential as an effective therapeutic target in AML, highlighting
the significance of disrupting this interaction to modulate cell
behavior and tumor progression. Additionally, high expression of
lncRNA HOXA-AS2 is not only closely related to tumor size, staging,
lymph node metastasis and distant metastasis (DM) but may also
promote tumor progression and occurrence by acting as a competitive
endogenous RNA (ceRNA) and affecting the distribution of microRNAs
(miRs), especially in digestive system tumors such as gastric
cancer, hepatocellular carcinoma and pancreatic cancer (16).
In summary, the expression level of lncRNA HOXA-AS2
and its mechanisms in cancer development, particularly its
potential value in prognosis assessment, offers a compelling
research direction. Therefore, the present meta-analysis aimed to
explore the predictive value of lncRNA HOXA-AS2 in various patients
with cancer, to strengthen the concept of lncRNA HOXA-AS2 as a
prognostic biomarker and therapeutic target. Through the findings
of the included studies, the present study aimed to provide a more
comprehensive perspective on understanding the role of lncRNA
HOXA-AS2 and its potential impact on cancer prognosis.
Materials and methods
Search strategy
The literature retrieval was performed by two
independent researchers using the following online databases:
PubMed (https://pubmed.ncbi.nlm.nih.gov/), PubMed Central
(PMC, http://www.ncbi.nlm.nih.gov/pmc/), EMBASE (https://www.embase.com/), Web of Science (https://www.webofscience.com/wos/), China
National Knowledge Infrastructure (CNKI, http://www.cnki.net/) and Wanfang Database (https://www.wanfangdata.com.cn/). The latest
search was performed on January 4th, 2024. The following keywords
were used in the search: ‘lncRNA HOXA cluster antisense RNA 2’ OR
‘lncRNA HOXA-AS2’ OR ‘HOXA-AS2’ OR ‘HOXA cluster antisense RNA 2’
The reference lists of relevant articles were also screened for
additional eligible studies.
Inclusion and exclusion criteria
Eligible articles were identified based on the
following inclusion criteria: i) The expression of lncRNA HOXA-AS2
was detected in any human solid malignant tumor; ii) association
between lncRNA HOXA-AS2 and patient prognosis and/or other clinical
pathological factors was reported; iii) The hazard ratio (HR) with
95% confidence interval (CI) was reported or sufficient data was
provided to calculate the HR; and iv) patients were classified into
high or low expression groups according to the lncRNA HOXA-AS2
expression level. While the literature search did not impose
language restrictions, all studies included in the analysis were
published in English due to their adherence to the inclusion
criteria. Articles were excluded when they did not cover all of the
aforementioned inclusion criteria. Reviews and meta-analyses were
also excluded to prevent data duplication, as they may have
reported on primary studies already included in the synthesis. In
addition, retracted articles were excluded to ensure the integrity
and reliability of the analysis. Articles lacking prognostic
information or with insufficient data were also excluded, as they
did not meet the requirements for the assessment of the impact of
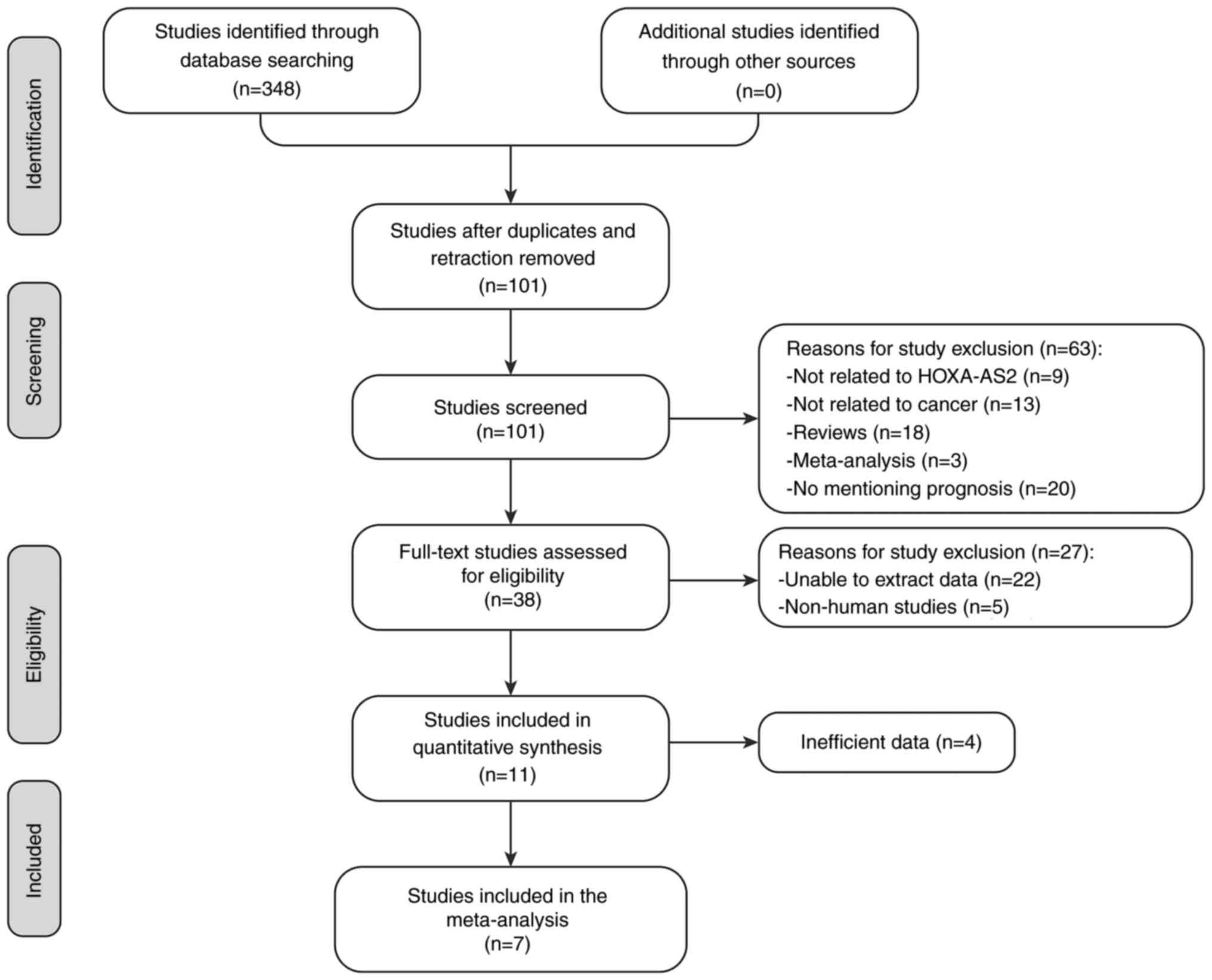
lncRNA HOXA-AS2 expression on cancer prognosis. Fig. 1 shows a detailed depiction of the
literature screening process and the specific reasons for article
exclusion.
Data extraction and quality
assessment
Two investigators independently extracted data from
eligible studies and followed a standardized protocol for
consistency. The data extracted and analyzed included the following
items: i) Name of first author, publication year, publication
country and region, study design, cancer type, sample size,
expression pattern, tumor stage, criterion of high expression
(according to the mean or median value of expression), detection
method, follow-up time, outcome measures and analysis type; ii) HR
with 95% CI for overall prognosis of patients; and iii) patient
characteristics, including number of patients with high and low
HOXA-AS2 expression, age, sex, lymph node metastasis (LNM), tumor
size, tumor stage and distance metastasis.
If a study reported the data from multivariate and
univariate analyses, the HR with the corresponding 95% CI was
directly extracted from the multivariate analysis. The survival
curve of those studies that did not report HRs and 95% CIs directly
were analyzed using Engauge Digitizer version 12.1 (https://markummitchell.github.io/engauge-digitizer/)
and then the HRs and 95% CIs were estimated following the published
method of Tierney et al (17).
The Newcastle-Ottawa Scale (NOS) with a score range
of 0–9 was applied to assess the quality of all included studies. A
high-quality study was identified as having a score of ≥7 (18–20).
Statistical analysis
Stata version 15.0 (StataCorp LP) was used for all
statistical analyses in this meta-analysis, applying a
random-effects model to account for anticipated heterogeneity among
studies. Higgins I2 statistics and Cochran's Q-test were
applied to assess the heterogeneity among studies. Begg's and
Egger's test were utilized to detect the publication bias.
Sensitivity analysis was performed by omitting each study one by
one to assess the effects on the pooled results. P<0.05 was
considered to indicate a statistically significant difference.
Results
Study selection and
characteristics
The literature screening process is illustrated in
Fig. 1. An initial search across
six electronic databases yielded a total of 348 articles, with
PubMed contributing 69 articles, Wanfang 19, Web of Science 78,
EMBASE 89, CNKI 66 and PMC 27. The next step involved the removal
of duplicates and retractions, which reduced the pool by 247
articles, leaving 101 for further screening. Upon reviewing the
titles and abstracts, 9 articles unrelated to lncRNA HOXA-AS2, 13
not related to cancer, 18 reviews, 3 previously published
meta-analyses and 20 articles lacking prognostic information were
excluded. This led to a full-text assessment of 38 articles for
eligibility. Of these, 22 were excluded due to clinicopathologic
characteristics data extraction issues and 5 due to being
cell-based studies. A further 4 articles were excluded due to
insufficient prognosis data. Ultimately, 7 articles were included
in the present meta-analysis (14,21–26),
encompassing a total of 454 patients.
The 7 selected studies were published from 2019 to
2021A total of 454 patients with cancer were enrolled in the pooled
analysis, with a mean subject size of 64.8, ranging from 27 to 116.
All studies measured the expression of lncRNA HOXA-AS2 in tissue
specimens by reverse transcription-quantitative polymerase chain
reaction. Of the included studies, 4 explored the relationship
between lncRNA HOXA-AS2 expression and tumor staging, 4 assessed
the relationship between lncRNA HOXA-AS2 expression and tumor size,
and 4 investigated the presence of DM and LNM was addressed in 3
studies. The NOS scores of the included studies ranged from 7 to 9,
denoting high-quality research. Only 3 studies provided HRs
directly, with the remaining studies presenting Kaplan-Meier
survival curves. The cancer types investigated consisted of
osteosarcoma, NSCLC, PTC, prostate cancer (PCa), hepatocellular
carcinoma, cervical cancer and AML. All included studies were
retrospective and conducted in China. The main characteristics of
the eligible studies are shown in Table
I.
 | Table I.Characteristics of studies included
in the meta-analysis. |
Table I.
Characteristics of studies included
in the meta-analysis.
| First author,
year | Location | Sample size, n | Cancer type | lncRNA HOXA-AS2
expression, n |
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Detection
method | Sample type | Survival
analysis | Cut-off | HR statistic | HR method | HR (95% CI) | Follow-up time,
months | NOS score | Multivariate
analysis | (Refs.) |
|---|
| High | Low |
|---|
| Wang et
al, | Shandong, | 27 | Osteosarcoma | 15 | 12 | RT-qPCR | Tissue | OS | Mean | KM survival | Indirectly | 0.98
(0.25–3.81) | 60 | 7 | NM | (25) |
| 2019 | China |
|
|
|
|
|
|
|
| curve |
|
|
|
|
|
|
| Cui et
al, | Fujian, | 40 | NSCLC | 20 | 20 | RT-qPCR | Tissue | OS | NM | KM survival | Indirectly | 2.53 | 75 | 8 | NM | (24) |
| 2019 | China |
|
|
|
|
|
|
|
| curve |
| (0.67–9.58) |
|
|
|
|
| Jiang et
al, | Beijing, | 68 | PTC | 30 | 38 | RT-qPCR | Tissue | OS | Mean | KM survival | Indirectly | 1.32 | 60 | 7 | NM | (14) |
| 2019 | China |
|
|
|
|
|
|
|
| curve |
| (0.26–6.58) |
|
|
|
|
| Xiao and | Shaanxi, | 68 | Prostate
cancer | 34 | 34 | RT-qPCR | Tissue | OS | Mean | KM survival | Indirectly | 1.23 | 60 | 7 | NM | (26) |
| Song 2020 | China |
|
|
|
|
|
|
|
| curve |
| (0.11–13.85) |
|
|
|
|
| Lu et
al, | Guangxi, | 116 | HCC | 58 | 58 | RT-qPCR | Tissue | OS | Median | KM survival | Directly | 2.478 | 25 | 9 | YES | (21) |
| 2020 | China |
|
|
|
|
|
|
|
| curve |
| (1.09–5.62) |
|
|
|
|
| Qu et
al, | Liaoning, | 108 | AML | 54 | 54 | RT-qPCR | Blood | OS | Median | KM survival | Directly | 2.35 | 60 | 8 | YES | (22) |
| 2020 | China |
|
|
|
|
|
|
|
| curve |
| (1.17–6.05) |
|
|
|
|
| Chen
and, | Guangdong, | 27 | Cervical
cancer | 14 | 13 | RT-qPCR | Tissue | OS | Mean | KM survival | Directly | 2.80 | 120 | 8 | NM | (23) |
| He 2021 | China |
|
|
|
|
|
|
|
| curve |
| (1.00–7.90) |
|
|
|
|
LncRNA HOXA-AS2 expression and patient
overall survival (OS)
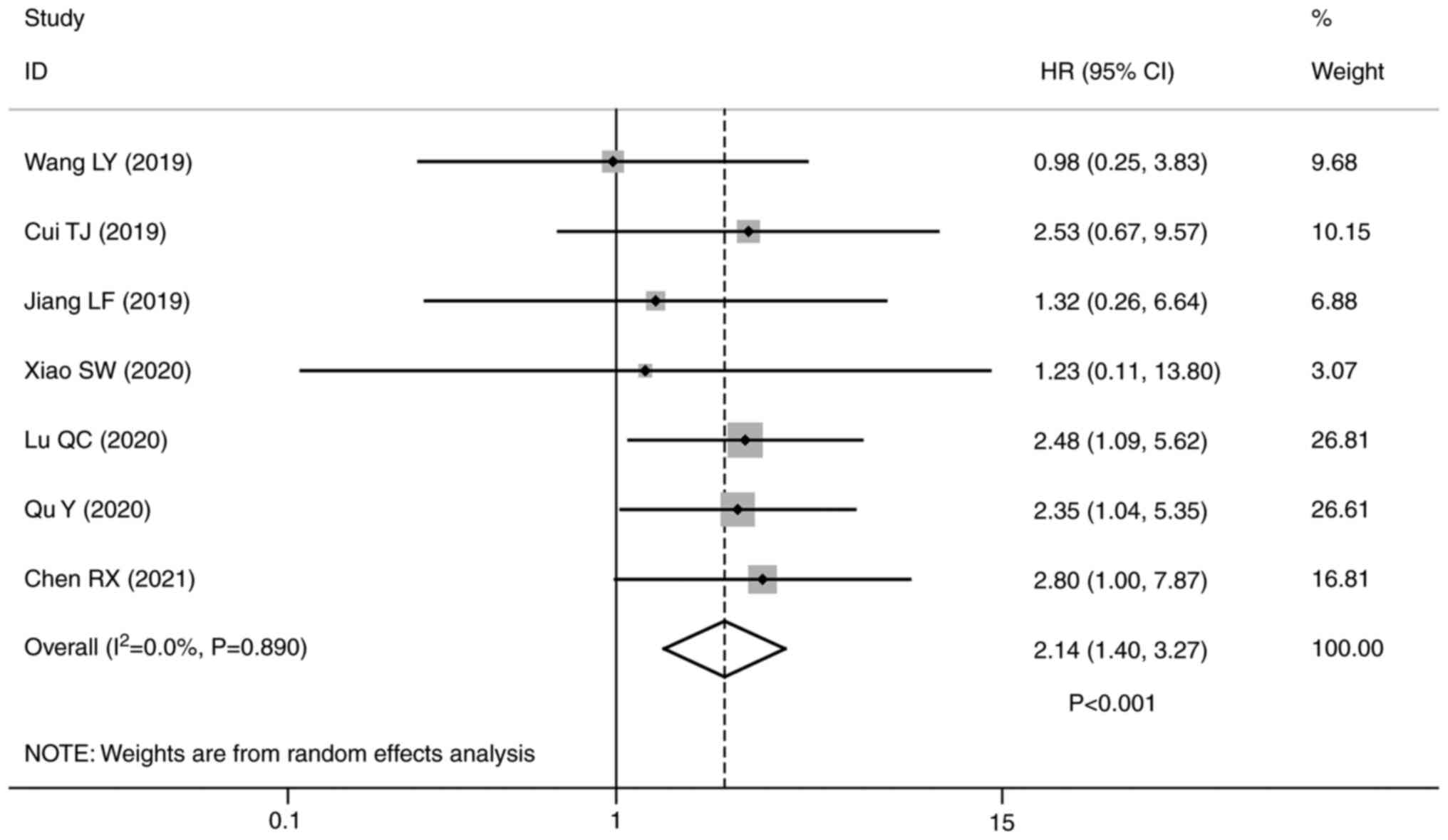
The present meta-analysis investigated the impact of
lncRNA HOXA-AS2 expression levels on the OS of patients with
cancer, incorporating a total of 454 individuals. The pooled
analysis indicated that patients with high lncRNA HOXA-AS2
expression had a significantly worse OS compared with those with
low expression (HR=2.14; 95% CI, 1.40–3.27; P<0.001; Fig. 2).
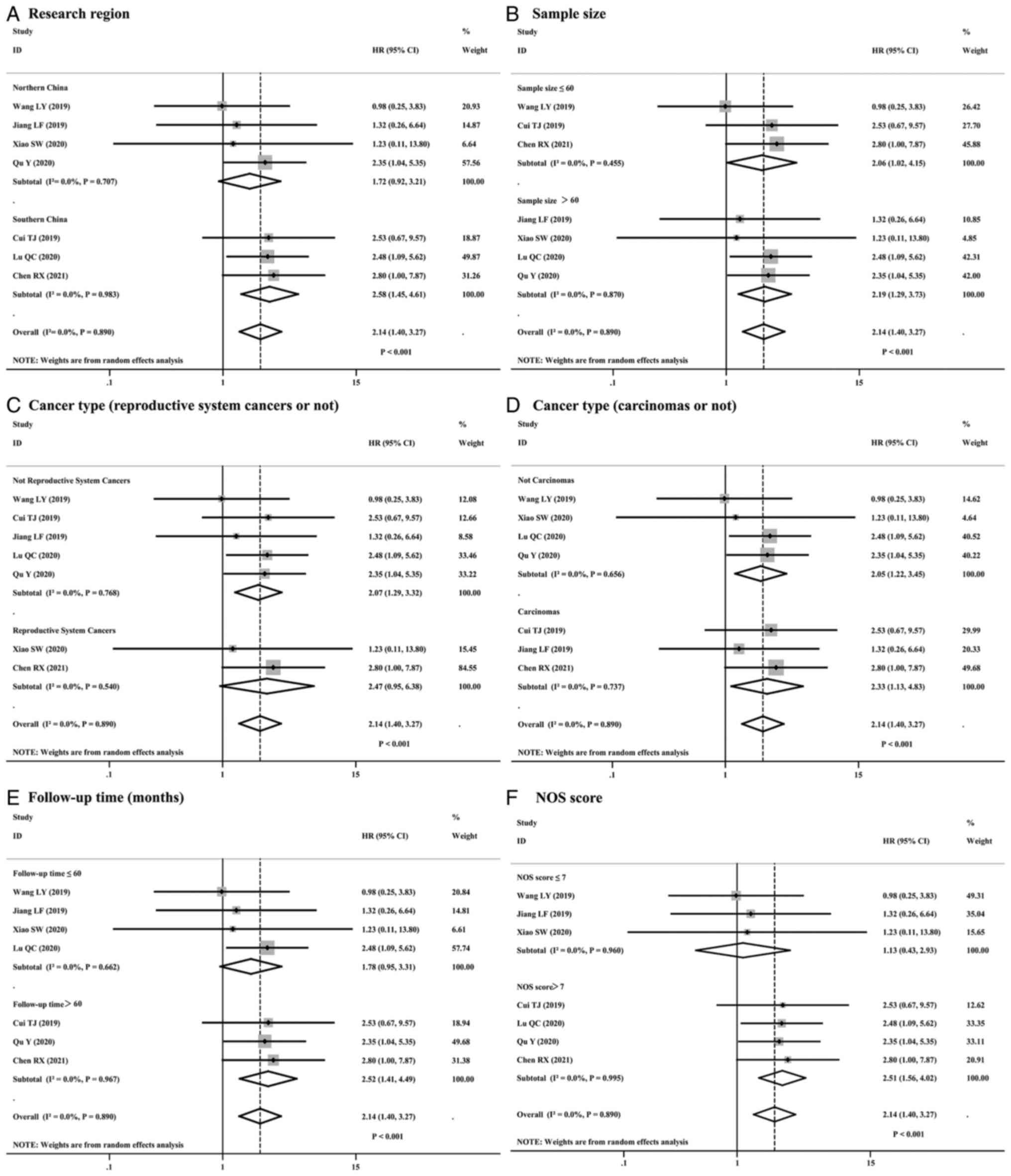
Subgroup analysis further delineated the association
across various demographics and clinical characteristics. A total
of 6 subgroups were analyzed as follows: Research region (Northern
China or Southern China; Fig. 3A),
sample size (n≤60 or n>60; Fig.
3B), cancer type (reproductive system cancer or not, Fig. 3C; carcinoma or not, Fig. 3D), follow-up duration (≤60 or >60
months; Fig. 3E) and NOS score (≤7
or >7; Fig. 3F). It is
noteworthy that, as reported in Table
II, significant associations were found in certain subgroups.
For instance, in Southern China, high expression of lncRNA HOXA-AS2
was significantly correlated with poorer overall survival (OS)
(HR=2.58; 95% CI, 1.45–4.61; P=0.001), as well as in the study
subgroup with sample sizes exceeding 60 (HR=2.19; 95% CI,
1.29–3.73; P=0.004). Furthermore, the analysis revealed significant
associations between high lncRNA HOXA-AS2 expression and OS in the
subgroups of non-reproductive system cancers (HR=2.07; 95% CI,
1.29–3.32; P=0.003) and in the Carcinoma subgroup (HR=2.33; 95% CI,
1.13–4.83; P=0.023). These findings highlight the potential
prognostic significance of lncRNA HOXA-AS2 expression in specific
cancer populations and underscore the value of stratifying patients
in future research. Despite the low heterogeneity observed across
the subgroups (I2=0.0%), these significant results
demonstrate the consistency of the effect across different patient
populations and study designs, affirming the robustness of the
association between high lncRNA HOXA-AS2 expression and poorer OS
across diverse cancer types and study conditions, while also
revealing potential differential factors.
 | Table II.Subgroup meta-analysis of the pooled
HRs for overall survival. |
Table II.
Subgroup meta-analysis of the pooled
HRs for overall survival.
| Stratified
analysis | Number of
studies | Number of
patients | HR (95% CI) | P-value | Heterogeneity
(I2, P) | Model |
|---|
| Region |
|
|
|
|
|
|
|
Northern China | 4 | 271 | 1.72
(0.92–3.21) | 0.087 | 0.0%, 0.707 | Random |
|
Southern China | 3 | 183 | 2.58
(1.45–4.61) | 0.001 | 0.0%, 0.983 | Random |
| Cancer type 1 |
|
|
|
|
|
|
| Not
reproductive system cancer | 5 | 359 | 2.07
(1.29–3.32) | 0.003 | 0.0%, 0.768 | Random |
|
Reproductive system
cancer | 2 | 95 | 2.47
(0.95–6.38) | 0.063 | 0.0%, 0.540 | Random |
| Cancer type 2 |
|
|
|
|
|
|
| Not
carcinoma | 4 | 319 | 2.05
(1.22–3.45) | 0.007 | 0.0%, 0.656 | Random |
|
Carcinoma | 3 | 135 | 2.33
(1.13–4.83) | 0.023 | 0.0%, 0.737 | Random |
| Sample size, n |
|
|
|
|
|
|
|
≤60 | 3 | 94 | 2.06
(1.02–4.15) | 0.043 | 0.0%, 0.455 | Random |
|
>60 | 4 | 360 | 2.19
(1.29–3.73) | 0.004 | 0.0%, 0.870 | Random |
| Follow-up time,
months |
|
|
|
|
|
|
|
≤60 | 4 | 279 | 1.78
(0.95–3.31) | 0.070 | 0.0%, 0.662 | Random |
|
>60 | 3 | 175 | 2.52
(1.41–4.49) | 0.002 | 0.0%, 0.967 | Random |
| NOS score |
|
|
|
|
|
|
| ≤7 | 3 | 163 | 1.13
(0.43–2.93) | 0.806 | 0.0%, 0.960 | Random |
|
>7 | 4 | 291 | 2.51
(1.56–4.02) | <0.001 | 0.0%, 0.995 | Random |
LncRNA HOXA-AS2 expression and
clinicopathological characteristics
In the present study, the relationship between
lncRNA HOXA-AS2 expression levels and various clinicopathological
features were explored in patients with cancer (Table III). In this study, a
random-effects model was uniformly applied to account for observed
and potential heterogeneity across studies in all analyses of
clinicopathological features.
 | Table III.Association of long non-coding RNA
homeobox A cluster antisense RNA 2 expression with
clinicopathological features. |
Table III.
Association of long non-coding RNA
homeobox A cluster antisense RNA 2 expression with
clinicopathological features.
| Clinicopathological
parameters | OR (95% CI) | P-value | Heterogeneity
(I2, P) | Model |
|---|
| Age (elderly vs.
young) | 1.00
(0.63–1.59) | 0.991 | 0.0%, 0.409 | Random |
| Sex (male vs.
female) | 1.55
(0.72–3.34) | 0.258 | 55.9%, 0.078 | Random |
| Lymph node
metastasis (positive vs. negative) | 2.06
(1.07–3.99) | 0.032 | 24.9%, 0.264 | Random |
| Tumor size (large
vs. small) | 2.02
(0.86–4.78) | 0.006 | 69.8%, 0.019 | Random |
| Tumor stage (III +
IV vs. I + II) | 2.71
(1.50–4.89) | 0.001 | 33.5%, 0.211 | Random |
| Distance metastasis
(yes vs. no) | 2.11
(1.15–3.88) | 0.016 | 30.7%, 0.228 | Random |
Association between lncRNA HOXA-AS2
expression and age or sex
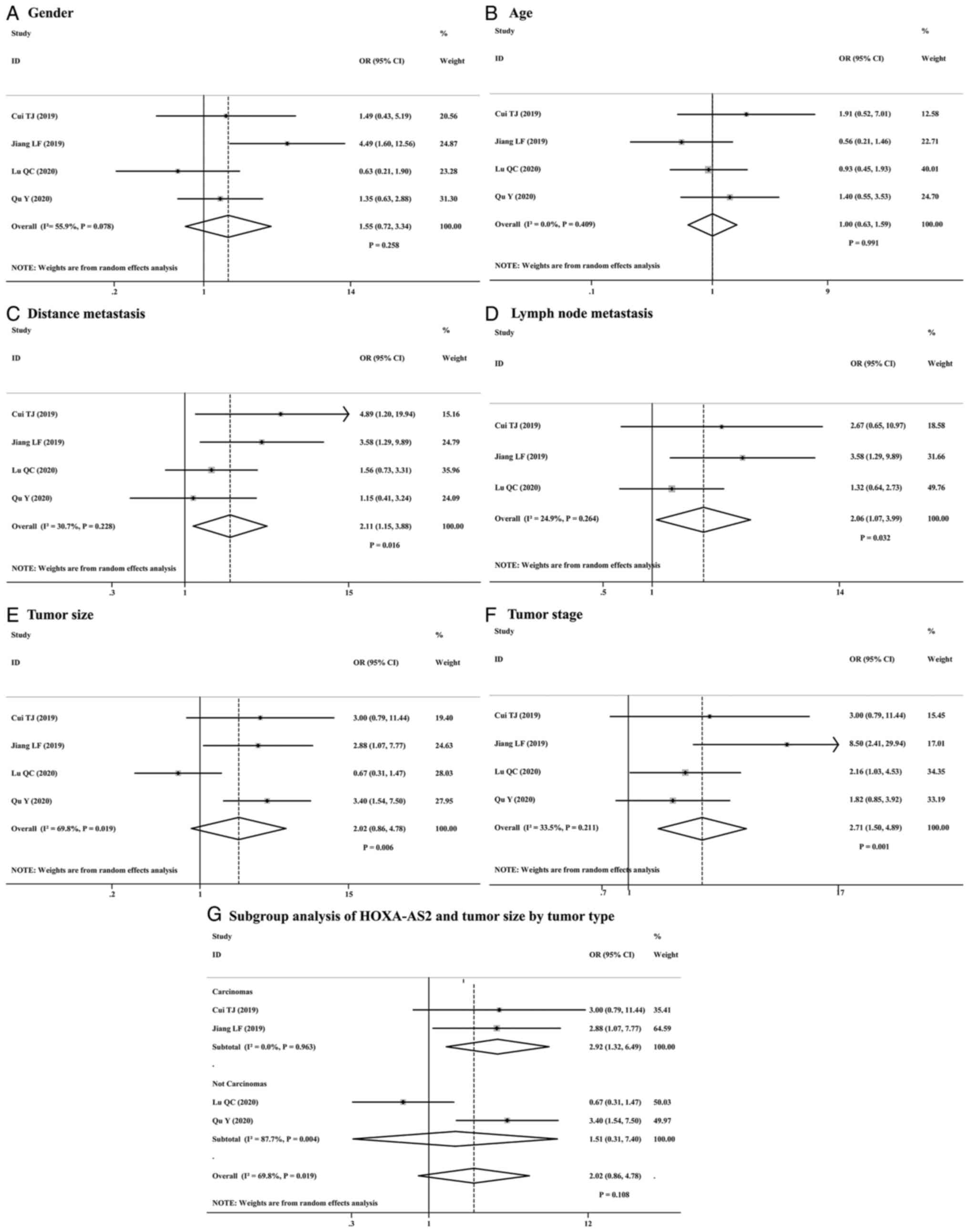
The study found no significant association between
the expression of lncRNA HOXA-AS2 and gender (OR=1.55; 95% CI,
0.72–3.34; P=0.258; Fig. 4A),
despite the presence of moderate heterogeneity
(I2=55.9%; P=0.078), but the P-value was greater than
0.05. Similarly, there was no significant correlation between
patient age and the expression of lncRNA HOXA-AS2 (OR=1.00; 95% CI,
0.63–1.59; P=0.991; Fig. 4B), with
negligible heterogeneity in the studies (I2=0%;
P=0.409).
Association between lncRNA HOXA-AS2
expression and metastatic status
The study revealed that high expression of lncRNA
HOXA-AS2 is significantly associated with an increased risk of
metastasis. Specifically, there was an increased risk for both LNM
and DM (OR for LNM=2.06; 95% CI, 1.07–3.99; P=0.032, and OR for
DM=2.11; 95% CI, 1.15–3.88; P=0.016), respectively (Fig. 4C and D).
Association between lncRNA HOXA-AS2
expression and tumor stage or size
For tumor size, significant heterogeneity was
observed (I2=69.8%, P=0.019), and the results
demonstrated a significant association between high lncRNA HOXA-AS2
expression and larger tumor size (HR=2.02; 95% CI, 0.86–4.78;
P=0.006; Fig. 4E). Regarding tumor
staging, the analysis revealed that high lncRNA HOXA-AS2 expression
is associated with higher tumor stages (HR=2.71; 95% CI, 1.50–4.89;
P=0.001; Fig. 4F), with a
heterogeneity level of 33.5%, although not statistically
significant (P=0.211). Subgroup analysis was conducted to
investigate the sources of heterogeneity, indicating a more
pronounced association between lncRNA HOXA-AS2 expression and tumor
size in carcinomas (OR=2.92; 95% CI, 1.32–6.49; P=0.008; Fig. 4G). In contrast, no such significant
association was found in non-carcinoma tumors (OR=1.51; 95% CI,
0.31–7.40; P=0.612; Fig. 4G),
suggesting that these patients may have contributed to the observed
heterogeneity. In summary, high expression of lncRNA HOXA-AS2 was
significantly associated with adverse clinicopathological
characteristics in patients with cancer, particularly in the risk
of metastasis and tumor progression, and especially in carcinomas.
However, lncRNA HOXA-AS2 expression was unrelated to patient age
and sex.
Sensitivity analysis and publication
bias
To ascertain the robustness of the meta-analysis, a
meticulous sensitivity analysis was conducted using the
leave-one-out method, and the presence of publication bias was
examined using a Begg's funnel plot and Egger's regression
test.
Sensitivity analysis
The leave-one-out sensitivity analysis involved
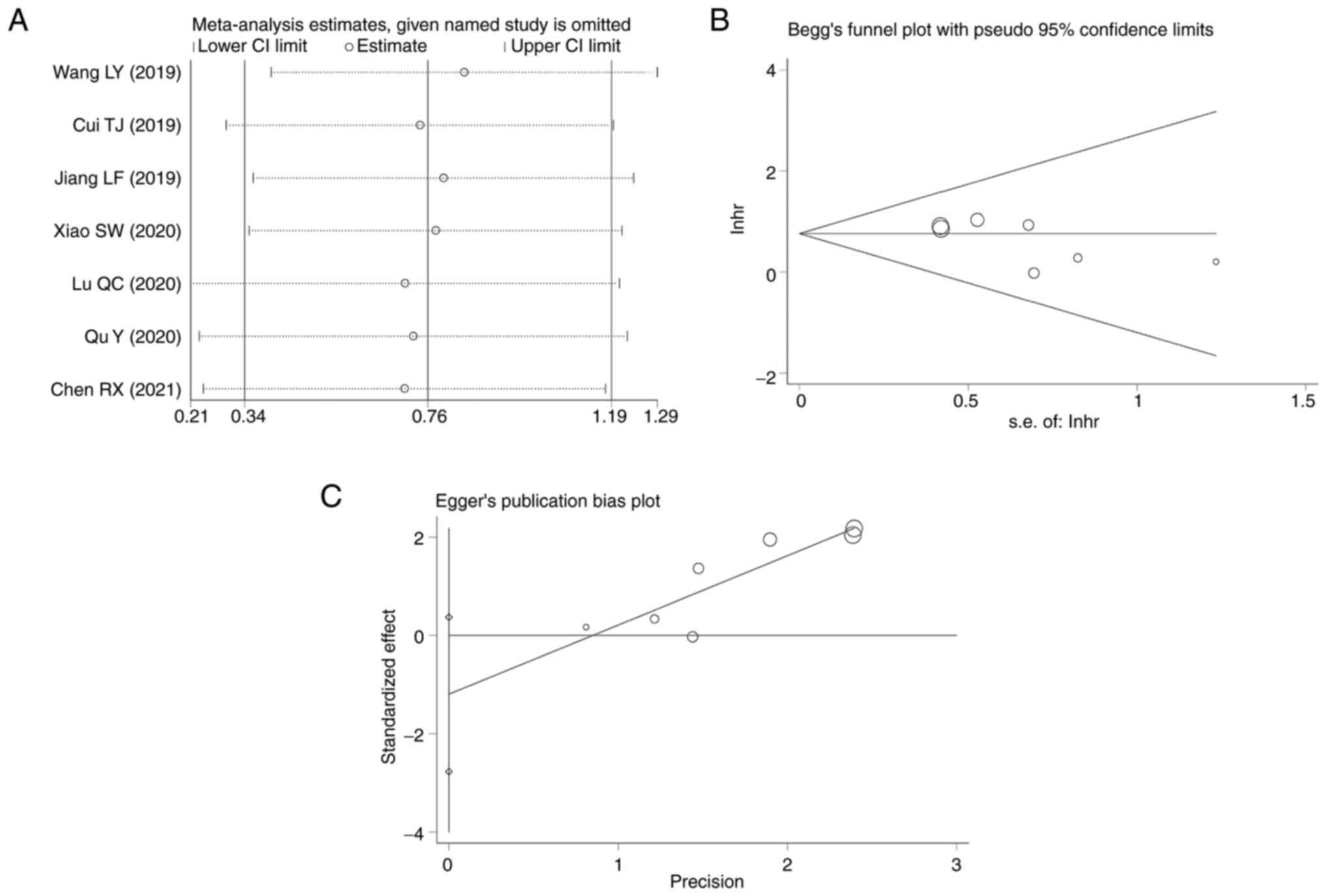
sequentially omitting each study to evaluate its impact on the
overall effect estimate. The analysis revealed that omitting any
single study did not significantly alter the combined HRs (Fig. 5A), which underscored the stability
of the meta-analysis results.
Publication bias
The Begg's funnel plot was scrutinized for asymmetry
to detect publication bias, and the plot presented no overt
asymmetry, suggesting an absence of bias (Fig. 5B). Additionally, the Begg's test
yielded a P-value of 0.133, indicating no significant publication
bias. Egger's regression test (Fig.
5C), which is sensitive to funnel plot asymmetry, corroborated
these findings by showing no significant publication bias
(intercept=−1.199832; P=0.107). While there was a hint of asymmetry
in the Egger's plot, the non-significant P-value implied that the
effect sizes of the included studies were symmetrically distributed
around the overall effect size, thereby providing no substantial
evidence of publication bias.
Collectively, the leave-one-out sensitivity analysis
and the publication bias assessments affirmed the credibility of
the meta-analysis. The consistent results across these analytical
approaches demonstrated the robustness of the conclusions drawn
from the pooled data, free from the undue influence of any single
study or publication bias.
Discussion
Since Zhao et al (9) published their results indicating that
lncRNA HOXA-AS2 repressed apoptosis in trans retinoic acid-treated
NB4 promyelocytic leukemia cells in 2013, it has been demonstrated
(23,27,28)
that lncRNA HOXA-AS2 is upregulated in multiple solid tumors and
promotes various malignant behaviors and clinical manifestations.
In addition to the studies included in the present meta-analysis,
there are additional studies that have explored the oncogenic role
of lncRNA HOXA-AS2 in tumors. For instance, Lian et al
(29) found that lncRNA HOXA-AS2
was upregulated in pancreatic cancer (PC); moreover, the
interaction between HOXA-AS2 and EZH2 and lysine specific
demethylase 1 promoted PC cell proliferation in vitro.
Similar results were also discovered in malignant glioma (30,31),
kidney renal clear cell carcinoma (32) and oral cancer (33). These studies were not included in
the present meta-analysis due to a lack of necessary clinical
data.
In terms of the molecular mechanism, available
research indicates the following regulatory functions of lncRNA
HOXA-AS2: i) ceRNA regulatory mechanism: The role of lncRNA
HOXA-AS2 as a ceRNA is its most well-studied and established
function. Extensive research, as evidenced by numerous
publications, has highlighted lncRNA HOXA-AS2 as a pivotal element
in the ceRNA network across a variety of cancer types, including
AML (34), bladder cancer (35), PCa (27,36)
and lower-grade glioma (26). This
mechanism primarily involves lncRNA HOXA-AS2 sponging various
miRNAs such as miR-520c-3p (34),
miR-125b (35), miR-885-5p
(27), miR-509-3p (26) and miR-184 (36) in different cancer contexts. These
interactions significantly impact the expression of downstream
genes, thereby influencing the progression of cancer. For instance,
studies by Yang et al (27)
and Xiao and Song (26) have shown
that reducing the levels of lncRNA HOXA-AS2 affects cell
proliferation, migration, invasion and epithelial-mesenchymal
transition (EMT), subsequently promoting the development and
progression of PCa. Meanwhile, Chen et al (36) conducted a comprehensive
transcriptomic analysis using multiple datasets from the Chinese
Glioma Genome Atlas and The Cancer Genome Atlas. The results
confirmed the role of lncRNA HOXA-AS2 as a ceRNA, inducing cell
proliferation in lower-grade gliomas. ii) EMT promoting function:
Zhang et al (37)
demonstrated that high expression of lncRNA HOXA-AS2 in gall
bladder cancer could increase the expression levels of Vimentin (a
mesenchymal marker), whereas the expression of E-cadherin (an
epithelial marker) is decreased, resulting in an upregulation of
the migration of cancer cells. iii) Protein binding function: Ding
et al (38) demonstrated
that in gastric cancer, the competitive binding of lncRNA HOXA-AS2
and EZH2 causes the dissociation between EZH2 and the promoter of
the P21, polo-like kinase 3 and DNA damage inducible transcript 3
genes, inhibiting H3K27 trimethylation and leading to the
repression of these tumor suppressing genes. iv) Activator of
adjacent genes: Zhao et al (39) previously found that lncRNA HOXA-AS2
could directly elevate the expression levels of HOXA3 mRNA and
protein but not that of HOXA4 in acute lymphoblastic leukemia
cells.
While the upregulation of HOXA-AS2 in various cancer
types and its role in promoting malignant behaviors and clinical
manifestations have been established, the specific mechanism of its
secretion into the circulatory system remains a focal point of
scientific investigation. The current research on the secretion
mechanism of lncRNA HOXA-AS2 is insufficient. Generally, the
secretion of lncRNAs may involve more complex cellular mechanisms
than cytokines or proteins, including but not limited to pathways
involving extracellular vesicles. A study has shown that
extracellular vesicles, especially exosomes, contain extracellular
lncRNAs and mediate the horizontal transfer of lncRNAs between
tumor cells to disseminate drug resistance (40). Compared with miRNAs, although
lncRNAs are found in plasma-derived exosomal RNA, only a subset of
lncRNAs are selectively loaded into exosomes, which may be
associated with physiological and cellular factors (41). These extracellular vesicles capable
of carrying and transporting RNA molecules, including lncRNAs, may
thus be involved in the secretion process of lncRNA HOXA-AS2.
However, the specific application of this mechanism to lncRNA
HOXA-AS2 remains speculative and requires further investigation.
Despite the lack of direct evidence regarding the secretion
mechanism of lncRNA HOXA-AS2, the present study, along with that of
others, has highlighted the significant potential of lncRNA
HOXA-AS2 as a cancer biomarker, particularly in predicting the
prognosis of patients with cancer. Future studies are required not
only to explore the role of lncRNA HOXA-AS2 in cancer development
but also to unveil how it is secreted into the circulatory system.
This will be crucial for leveraging lncRNA HOXA-AS2 as a
non-invasive biomarker for cancer diagnosis and prognosis
assessment.
In the present comprehensive study investigating the
role of lncRNA HOXA-AS2 expression in cancer, its relationship with
a variety of clinicopathological characteristics was examined. The
present systematic review and meta-analysis, encompassing 454
patients across 7 independent studies, revealed that high lncRNA
HOXA-AS2 expression was significantly negatively associated with OS
in patients with cancer, yielding an OR of 2.14 (95% CI, 1.40–3.27;
P<0.001). Further analysis of the data from 6 critical studies
underscored the complex relationship between lncRNA HOXA-AS2
expression and clinical features. The findings of the present study
indicated that there was no significant association between lncRNA
HOXA-AS2 expression with patient age or sex. Specifically, despite
a moderate level of heterogeneity in the sex of patients
(I2=55.9%, P=0.078), there was no substantial link
between lncRNA HOXA-AS2 expression and sex (OR=1.55; 95% CI,
0.72–3.34; P=0.258). In addition, age displayed very low
heterogeneity (I2=0%) and no significant association
with lncRNA HOXA-AS2 expression (OR=1.00; 95% CI, 0.63–1.59;
P=0.991). Significantly, our analysis confirmed the crucial role of
lncRNA HOXA-AS2 in promoting metastasis and advancing tumor
severity, as evidenced by its strong association with lymph node
and distant metastasis, and with higher tumor stage and size. These
associations align with earlier studies which also reported lncRNA
HOXA-AS2 as a key player in cancer progression. These findings
further emphasized the potential of lncRNA HOXA-AS2 as a prognostic
biomarker for cancer, paving the way for new diagnostic and
therapeutic approaches in oncology.
In updating the literature review to January 4,
2024, the present study incorporated the latest research findings
on lncRNA HOXA-AS2, extending beyond the scope of previous
meta-analyses (20,42,43).
Unlike these prior studies, the present study provided an
exhaustive subgroup analysis, considering six clinically relevant
factors not previously analyzed together. By examining age, sex, DM
and LNM and including eight aspects such as research region, sample
size, involvement of reproductive system cancer and whether the
cancer is a carcinoma or non-carcinoma, the present study offers a
more comprehensive perspective on the multifaceted clinical value
of lncRNA HOXA-AS2. Furthermore, updated statistical methods and
more stringent quality assessment standards were employed, ensuring
a high degree of credibility and applicability of the results.
Notably, we delved into the complex functions of lncRNA HOXA-AS2,
including its ceRNA regulatory mechanism, EMT promoting function,
protein binding capability and its role as an activator of adjacent
genes. These insights provide a nuanced understanding of lncRNA
HOXA-AS2′s involvement in cancer progression and its potential as a
non-invasive biomarker for cancer diagnosis and prognosis
assessment. Unlike previous research, this study also ventures into
the preliminary discussion on the secretion mechanisms of lncRNA
HOXA-AS2 into the circulatory system, a crucial step towards its
application in clinical settings. By exploring these novel aspects,
our research not only significantly updates but also expands the
existing knowledge on lncRNA HOXA-AS2, laying a solid foundation
for future investigations into its clinical applications and
mechanisms of action. This unique contribution marks a significant
step forward in the understanding of lncRNA HOXA-AS2′s potential as
a diagnostic and prognostic tool, highlighting areas that warrant
further exploration and validation in future research.
Despite adhering to strict procedures and rigorous
statistical methods, the present study still faces certain
limitations that should be addressed. Firstly, the total sample
size and the individual sample sizes of the included studies were
relatively small, and the types of cancers covered did not
comprehensively represent all common cancer types. Through precise
selection, the quality of the included studies and specificity to
lncRNA HOXA-AS2 related prognosis studies were ensured. Secondly,
approximately half of the pooled HRs were derived from Kaplan-Meier
survival curves published in the original articles using the
statistical method published by Tierney et al (17), potentially leading to inaccuracies
and heterogeneity in the final results. Thirdly, although the
studies were meticulously selected for inclusion, not all the
studies contained key clinicopathological characteristics such as
tumor size, stage, DM and LNM, limiting the capacity for a more
comprehensive analysis. Fourthly, all the studies included were
from China, and while subgroup analyses based on geographical
regions within China were conducted, these results might not be
sufficiently representative on a global scale. Finally, the cut-off
values for high/low lncRNA HOXA-AS2 expression varied and were not
uniform; thus, more research is needed before applying the findings
of the present study to clinical practice.
In summary, the results of the present meta-analysis
underscored a significant association between high HOXA-AS2
expression and adverse clinicopathological features in patients
with cancer, particularly in relation to risk of metastasis and
tumor progression in carcinomas. The results also reinforced the
potential of lncRNA HOXA-AS2 as a prognostic biomarker in cancer.
Finally, the findings suggested that lncRNA HOXA-AS2 expression is
independent of patient demographic factors, such as age and sex,
highlighting its broad applicability across diverse patient
populations. However, we acknowledge the complexities underlying
the regulatory functions of lncRNA HOXA-AS2 in various cancer
types, which are influenced by the localization and expression of
downstream molecules. Therefore, while the present study provided a
foundational understanding of the association between lncRNA
HOXA-AS2 expression and cancer, it should be seen as a preliminary
step in a much larger investigative landscape. To fully delineate
the clinical value of lncRNA HOXA-AS2 and to solidify its role in
cancer diagnostics and therapeutics, more comprehensive studies of
higher quality are indispensable. Future research is required to
delve deeper into the multifaceted mechanisms of lncRNA HOXA-AS2,
which will be crucial for developing innovative diagnostic and
treatment strategies in cancer care.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Science Fund for
Distinguished Young Scholars (grant no. 82203494), the key project
of the Scientific Research Project of the Hunan Education
Department (grant no. 22A0534), the project of Hunan Province
Health Commission (grant no. 202204015341) and the project of Shao
yang Science and Technology Bureau (grant no. 2021052ZD).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
WG and TX contributed to the study's conception and
design. Data collection from databases was performed by WG, HZ, and
AY. Data analysis was conducted by WG, TX, LT, AY, and HZ. The
initial draft and revisions of the manuscript were co-written by TX
and HZ. WG and AY confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
AML
|
acute myeloid leukemia
|
|
PTC
|
papillary thyroid cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
LNM
|
lymph node metastasis
|
|
HR
|
hazard ratio
|
References
|
1
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:Spec No 1:R17–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choudhari R, Sedano MJ, Harrison AL,
Subramani R, Lin KY, Ramos EI, Lakshmanaswamy R and Gadad SS: Long
noncoding RNAs in cancer: From discovery to therapeutic targets.
Adv Clin Chem. 95:105–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Zhang X, Frazão JB, Condino-Neto A
and Newburger PE: HOX antisense lincRNA HOXA-AS2 is an apoptosis
repressor in all trans retinoic acid treated NB4 promyelocytic
leukemia cells. J Cell Biochem. 114:2375–2383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Hu S, Li L, Peng X and Chen F:
Long noncoding RNA HOXA-AS2 functions as an oncogene by binding to
EZH2 and suppressing LATS2 in acute myeloid leukemia (AML). Cell
Death Dis. 11:10252020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Liu L, Zhang W, Fang J, Li G,
Zhang L, Li J, Deng X, Ma J, Li K and Chen Z: Effects of long
noncoding RNA HOXA-AS2 on the proliferation and migration of
gallbladder cancer cells. J Oncol. 2022:60515122022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong C, Tao B, Li X, Xiang W, Peng L,
Peng T, Chen L, Xia X, You J and Yang X: HOXA-AS2 contributes to
regulatory T cell proliferation and immune tolerance in glioma
through the miR-302a/KDM2A/JAG1 axis. Cell Death Dis. 13:1602022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Q, Lu S, Zhang L and Zhao L: LncRNA
HOXA-AS2 activates the notch pathway to promote cervical cancer
cell proliferation and migration. Reprod Sci. 28:3000–3009. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Wu Z, Meng X, Chu X, Huang H and
Xu C: LncRNA HOXA-AS2 facilitates tumorigenesis and progression of
papillary thyroid cancer by modulating the miR-15a-5p/HOXA3 axis.
Hum Gene Ther. 30:618–631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Z, Xing Y, Yang F, Zhao Z, Shen Y,
Song J and Jing S: LncRNA HOXA-AS2 promotes oral squamous cell
proliferation, migration, and invasion via upregulating EZH2 as an
oncogene. Technol Cancer Res Treat. 20:153303382110391092021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X
and Tai S: LncRNA HOXA-AS2 and its molecular mechanisms in human
cancer. Clin Chim Acta. 485:229–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Cai L, Liu Y, Chen W and Wang Q:
Association between prenatal cadmium exposure and cognitive
development of offspring: A systematic review. Environ Pollut.
254:1130812019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Yu CH, Guo KP, Huang CZ and Mo
LY: Prognostic role of red blood cell distribution width in
patients with sepsis: A systematic review and meta-analysis. BMC
Immunol. 21:402020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang F, Zhang G, Zhang H, Pu X, Chi F,
Zhang D, Xin X, Gao M, Luo W and Li X: HOXA-AS2 may be a potential
prognostic biomarker in human cancers: A meta-analysis and
bioinformatics analysis. Front Genet. 13:9442782022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Q, Gao J, Tang S, Li Z, Wang X, Deng C,
Hu J, Tao Y and Wang Q: Integrated RNA sequencing and single-cell
mass cytometry reveal a novel role of LncRNA HOXA-AS2 in
tumorigenesis and sternness of hepatocellular carcinoma. Onco
Targets Ther. 13:10901–10916. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu Y, Wang Y, Wang P, Lin N, Yan X and Li
Y: Overexpression of long noncoding RNA HOXA-AS2 predicts an
adverse prognosis and promotes tumorigenesis via SOX4/PI3K/AKT
pathway in acute myeloid leukemia. Cell Biol Int. 44:1745–1759.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen R and He P: Long noncoding RNA
HOXA-AS2 accelerates cervical cancer by the miR-509-3p/BTN3A1 axis.
J Pharm Pharmacol. 73:1387–1396. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui TJ, Lin GS, Dai YM, Zheng JP, Chen Z,
Chen Q, Zheng Y and Lin X: LncRNA HOXA-AS2 regulates
microRNA-216a-5p to promote malignant progression of non-small cell
lung cancer. Eur Rev Med Pharmacol Sci. 23 (Suppl 3):S264–S273.
2019.PubMed/NCBI
|
|
25
|
Wang L, Wang L and Zhang X: Knockdown of
lncRNA HOXA-AS2 inhibits viability, migration and invasion of
osteosarcoma cells by miR-124-3p/E2F3. Onco Targets Ther.
12:10851–10861. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao S and Song B: LncRNA HOXA-AS2
promotes the progression of prostate cancer via targeting
miR-509-3p/PBX3 axis. Biosci Rep. 40:BSR201932872020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Zhang F, Cai K and Xu J: Long
noncoding RNA HOXA-AS2 facilitates prostate cancer progression by
inhibiting miR-885-5p to upregulate KDM5B. Kidney Blood Press Res.
48:45–55. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Li M and Zhang Y: Long noncoding RNA
HOXA-AS2 regulates the expression of SCN3A by sponging miR-106a in
breast cancer. J Cell Biochem. 120:14465–14475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lian Y, Li Z, Fan Y, Huang Q, Chen J, Liu
W, Xiao C and Xu H: The lncRNA-HOXA-AS2/EZH2/LSD1 oncogene complex
promotes cell proliferation in pancreatic cancer. Am J Transl Res.
9:5496–5506. 2017.PubMed/NCBI
|
|
30
|
Gao Y, Yu H, Liu Y, Liu X, Zheng J, Ma J,
Gong W, Chen J, Zhao L, Tian Y and Xue Y: Long non-coding RNA
HOXA-AS2 regulates malignant glioma behaviors and vasculogenic
mimicry formation via the MiR-373/EGFR axis. Cell Physiol Biochem.
45:131–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan WW, Yang LT, Liu J, Dai ZY, Wang ZY,
Zhang H, Zhang X, Liang XS, Luo P, Zhang J, et al: A TGF-β
signaling-related lncRNA signature for prediction of glioma
prognosis, immune microenvironment, and immunotherapy response. CNS
Neurosci Ther. Oct 18–2023.(Epub ahead of print). View Article : Google Scholar
|
|
32
|
Zhang Y, Zhuang T, Xin Z, Sun C, Li D, Ma
N and Wang X and Wang X: Construction of a necroptosis-related
lncRNA signature for predicting prognosis and immune response in
kidney renal clear cell carcinoma. Cells. 12:662023. View Article : Google Scholar
|
|
33
|
Doghish AS, Elshaer SS, Fathi D, Rizk NI,
Elrebehy MA, Al-Noshokaty TM, Elballal MS, Abdelmaksoud NM,
Abdel-Reheim MA, Abdel Mageed SS, et al: Unraveling the role of
miRNAs in the diagnosis, progression, and drug resistance of oral
cancer. Pathol Res Pract. 253:1550272024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong X, Fang Z, Yu M, Zhang L, Xiao R, Li
X, Pan G and Liu J: Knockdown of long noncoding RNA HOXA-AS2
suppresses chemoresistance of acute myeloid leukemia via the
miR-520c-3p/S100A4 axis. Cell Physiol Biochem. 51:886–896. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang F, Wu D, Chen J, Chen S, He F, Fu H,
Wu Q, Liu S, Li X and Wang W: Long non-coding RNA HOXA-AS2 promotes
the migration, invasion and stemness of bladder cancer via
regulating miR-125b/Smad2 axis. Exp Cell Res. 375:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen PY, Li XD, Ma WN, Li H, Li MM, Yang
XY and Li SY: Comprehensive transcriptomic analysis and
experimental validation identify lncRNA HOXA-AS2/miR-184/COL6A2 as
the critical ceRNA regulation involved in low-grade glioma
recurrence. Onco Targets Ther. 13:4999–5016. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang P, Cao P, Zhu X, Pan M, Zhong K, He
R, Li Y, Jiao X and Gao Y: Upregulation of long non-coding RNA
HOXA-AS2 promotes proliferation and induces epithelial-mesenchymal
transition in gallbladder carcinoma. Oncotarget. 8:33137–33143.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding J, Xie M, Lian Y, Zhu Y, Peng P, Wang
J, Wang L and Wang K: Long noncoding RNA HOXA-AS2 represses P21 and
KLF2 expression transcription by binding with EZH2, LSD1 in
colorectal cancer. Oncogenesis. 6:e2882017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Q, Zhao S, Li J, Zhang H, Qian C,
Wang H, Liu J and Zhao Y: TCF7L2 activated HOXA-AS2 decreased the
glucocorticoid sensitivity in acute lymphoblastic leukemia through
regulating HOXA3/EGFR/Ras/Raf/MEK/ERK pathway. Biomed Pharmacother.
109:1640–1649. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
EL Andaloussi S, Mäger I, Breakefield XO
and Wood MJ: Extracellular vesicles: Biology and emerging
therapeutic opportunities. Nat Rev Drug Discov. 12:347–357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou G and Chen X: Emerging role of
extracellular microRNAs and lncRNAs. ExRNA. 1:102019. View Article : Google Scholar
|
|
42
|
Wang Q, Zhang W, Deng C, Lin S and Zhou Y:
HOXA-AS2 may predict the prognosis of solid tumors among Chinese
patients: A meta-analysis and bioinformatic analysis. Front Oncol.
12:10308252022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu B, Liu Z, Ji X and Zhang X: Assessment
of the prognostic and clinicopathological significance of HOXA-AS2
in human cancers: A systematic review and meta-analysis. Transl
Cancer Res. 12:605–615. 2023. View Article : Google Scholar : PubMed/NCBI
|