Introduction
Cervical cancer is one of the most common malignant
tumors in the world that can affect the physical and mental health
of women (1). Globally, there are
~530,000 new cervical cancer cases and 275,000 cervical
cancer-related deaths each year, and incidence has indicated
steadily/gradually declining patient age at the time of diagnosis
(2). Cervical intraepithelial
neoplasia (CIN) is a precancerous lesion of cervical invasive
carcinoma, which can be divided into three grades, namely CIN1, 2
and 3; the higher the grade of CIN, the greater the probability of
cervical invasive carcinoma development (3). Histology is considered the gold
standard for diagnosing the pathological progress of cervical
cancer development, while CIN2 or worse (CIN2+) is the cutoff for
intervention in clinical practice (4). Additionally, international expert
consensus recommendations require demonstration of high intra- and
inter-laboratory reproducibility, and non-inferior sensitivity and
specificity for the outcome of CIN2+ compared with pathological
testing (5).
Persistent or repeated infection with high-risk
human papillomavirus (HPV) is the main cause of cervical cancer,
which can be prevented and treated (6,7). The
progress from precancerous cervical lesions to cancer diagnosis
requires 5–12 years. Therefore, early screening and treatment of
precancerous cervical lesions are of great significance (8). The E6 and E7 proteins are oncoproteins
produced by high-risk HPV types such as HPV-16 and HPV-18. HPV
types are classified as low-risk or high-risk based on their
association with the development of certain health conditions,
particularly cervical cancer. The classification is primarily
determined by the potential of the virus to cause malignant
transformation in cells. Low-Risk HPV types are less likely to lead
to the development of cancer. High-risk HPV types are more likely
to cause persistent infections that can lead to the development of
cancer (9). These proteins play a
pivotal role in the initiation and progression of cervical cancer.
E6 and E7 are known for their ability to interact with cellular
proteins, disrupting normal regulatory pathways in infected cells.
Understanding the significance of E6 and E7 proteins is crucial in
the context of cervical cancer diagnosis (9). HPV E6/E7 mRNA plays a role in the
transcription and expression of oncogenes and can be used as an
early marker for the development of cervical cancer lesions
(10,11). The detection of HPV E6/E7 mRNA can
serve as a biomarker for identifying infections with high-risk HPV
types and assessing the risk of cervical cancer development.
Although a previous systematic review investigated HPV E6/E7 mRNA
for the detection of CIN2+, it is noteworthy that systematic
reviews are only qualitative analyses of the literature. Therefore,
the diagnostic value of HPV E6/E7 mRNA in screening for CIN2+ still
lacks a more objective meta-quantitative analysis (12). A meta-analysis was conducted as part
of the present study to explore the diagnostic value including
sensitivity, specificity, positive likelihood ratio (LR+), negative
likelihood ratio (LR-), diagnostic odds ratio (DOR) and area under
curve (AUC) of HPV E6/E7 mRNA in screening for CIN2+, aiming to
provide a new marker for the clinical diagnosis of cervical
cancer.
Materials and methods
Literature inclusion and exclusion
criteria
Inclusion criteria: i) Retrospective or prospective
studies evaluating the diagnostic value of E6/E7 mRNA in
differentiating between CIN2+ and CIN2-; ii) histopathology as the
gold standard; and iii) true positive (TP), true negative (TN),
false positive (FP) and false negative (FN) values can be directly
or indirectly extracted from the retrieved literature. Exclusion
criteria: i) Animal studies, case reports and conference papers;
ii) no available data; and iii) duplicate reports or studies based
on the same data.
Search strategy
The PubMed, Embase and Cochrane Library databases
were searched from inception to May 2023. The search terms
included: ‘((((diagnosis[Title/Abstract]) OR
(diagnostic[Title/Abstract])) OR (sensitivity[Title/Abstract])) OR
(specificity[Title/Abstract])) AND (((((((((((((((Human
Papillomavirus Virus[Title/Abstract]) OR (Papillomavirus Virus,
Human[Title/Abstract])) OR (Virus, Human
Papillomavirus[Title/Abstract])) OR (Human
Papillomaviruses[Title/Abstract])) OR (HPV, Human Papillomavirus
Viruses[Title/Abstract])) OR (Human Papilloma
Virus[Title/Abstract])) OR (Human Papilloma
Viruses[Title/Abstract])) OR (Papilloma Virus,
Human[Title/Abstract])) OR (Virus, Human
Papilloma[Title/Abstract])) OR (HPV Human
Papillomavirus[Title/Abstract])) OR (HPV Human
Papillomaviruses[Title/Abstract])) OR (Human Papillomavirus,
HPV[Title/Abstract])) OR (Human Papillomaviruses,
HPV[Title/Abstract])) AND ((Messenger RNA[Title/Abstract]) OR
(mRNA[Title/Abstract]))) AND ((E6[Title/Abstract]) OR
(E7[Title/Abstract])))’.
Literature screening and data
extraction
Literature search, screening and extraction of
relevant material was carried out by two researchers. When there
were questions or disagreements, a third researcher was consulted
before making a decision. The data extraction content included:
Author, year of publication, sample size, sex, age and the values
of TP, FP, TN and FN. If no TP, FP, TN and FN values were reported,
data such as sensitivity, specificity, positive predictive value
and negative predictive value were used to reverse the
extrapolation.
Literature quality assessment
The QUADAS-2 tool (www.quadas.org) was separately used by two academics
for evaluating the quality of published literature (13), and RevMan (version 5.3) (https://training.cochrane.org/online-learning/core-software/revman)
was used to draw a quality evaluation map.
Data synthesis and statistical
analysis
Bivariate model or hierarchical summary receiver
operating characteristic (SROC) model was used to combine
sensitivity and specificity. The I2 value was used to
evaluate the heterogeneity caused by non-threshold effects. If
I2>50%, the random effects model was used, otherwise,
the fixed effects model was used. When I2 is 25–50%,
heterogeneity is low. When I2 is 50–75%, heterogeneity
is at a moderate level, and when I2>75%, there is a
high degree of heterogeneity. Subgroup analysis was performed to
explore the causes of heterogeneity among the included studies. All
analyses were performed with STATA (version 15.1; StataCorp LP).
All statistical tests were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Results of literature search
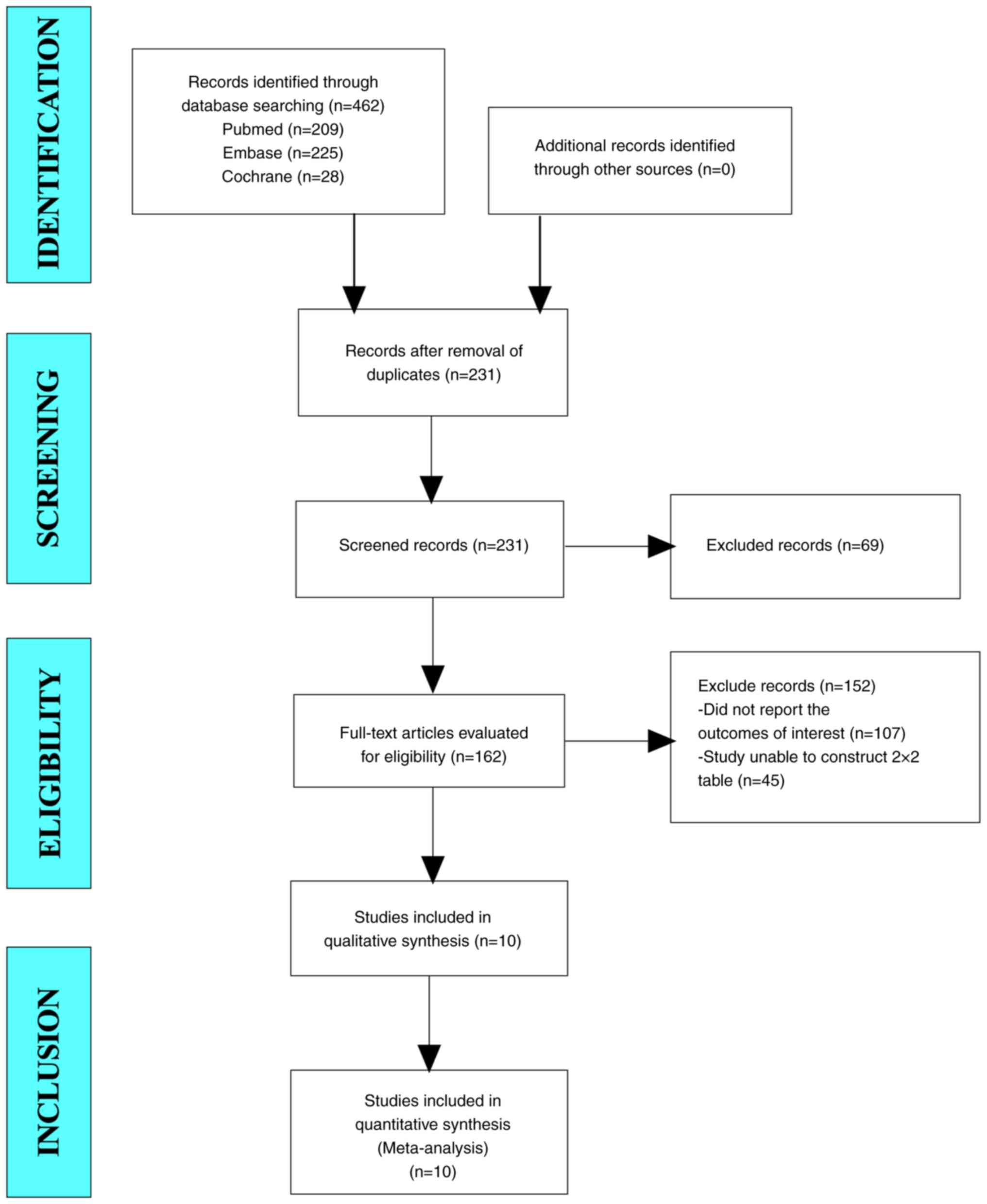
In the current study, a total of 462 studies were
retrieved from the aforementioned databases. After eliminating
duplicate studies, 231 studies were obtained. After browsing titles
and abstracts, 162 studies were obtained. Finally, 10 articles were
included in the present meta-analysis through full-text reading
(Fig. 1).
Baseline characteristics and quality
assessment of the included studies
Baseline characteristics of the included
studies
The present meta-analysis comprised 10 publications.
A total of 2,224 patients were included, of whom there were 1,274
patients with CIN2+ and 950 patients with CIN2-. The age range the
CIN2+ group was 30.0–48.8 years, while the age range of the CIN2-
group was 30.0–45.46 years, which was comparable (Table I) (14–23).
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
|
| Sample size, n | Age, years |
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author(s),
year | CIN 2+ | CIN 2- | CIN 2+ | CIN 2- | TP | FP | FN | TN | Sensitivity, % | Specificity, % | (Refs.) |
|---|
| Andersson et
al, 2011 | 87 | 68 | 32 (21–79) | 79 | 22 | 8 | 46 | 91.0 | 68.0 | (14) |
| Waldstrom and | 126 | 67 | 30 (16–65) | 117 | 41 | 9 | 26 | 92.5 | 38.2 | (15) |
| Ornskov, 2011 |
|
|
|
|
|
|
|
|
|
|
|
| Liu et al,
2013 | 57 | 35 | N/A | N/A | 41 | 9 | 16 | 26 | 71.9 | 74.3 | (16) |
| Shi et al,
2017 | 348 | 102 | 39.7±8.9 | 42.2±9.7 | 248 | 33 | 100 | 69 | 71.3 | 67.6 | (17) |
| Camus et al,
2018 | 10 | 10 | N/A | N/A | 9 | 5 | 1 | 5 | 90.0 | 50.0 | (18) |
| Fan and
Shen, 2018 | 95 | 97 | N/A | N/A | 87 | 18 | 8 | 79 | 91.5 | 81.4 | (19) |
| Han et al,
2018 | 101 | 96 | 48.8±12.5 | 42.8±10.3 | 86 | 32 | 15 | 64 | 85.1 | 66.7 | (20) |
| Pan et al,
2019 | 92 | 209 | 45.46 (20–89) | 85 | 140 | 7 | 69 | 92.4 | 33.0 | (21) |
| Zhang et al,
2020 | 328 | 209 | 43.9±11.1 | 308 | 44 | 20 | 165 | 93.8 | 79.0 | (22) |
| Sun et al,
2021 | 30 | 57 | 35.4±6.5 | 26 | 29 | 4 | 28 | 86.7 | 49.1 | (23) |
Quality assessment of the included
studies
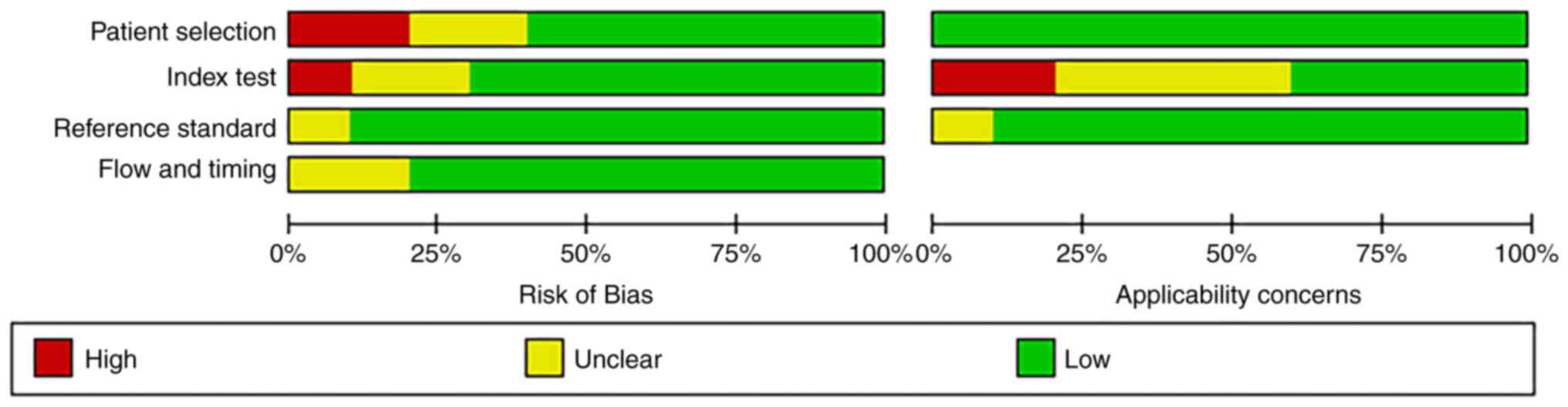
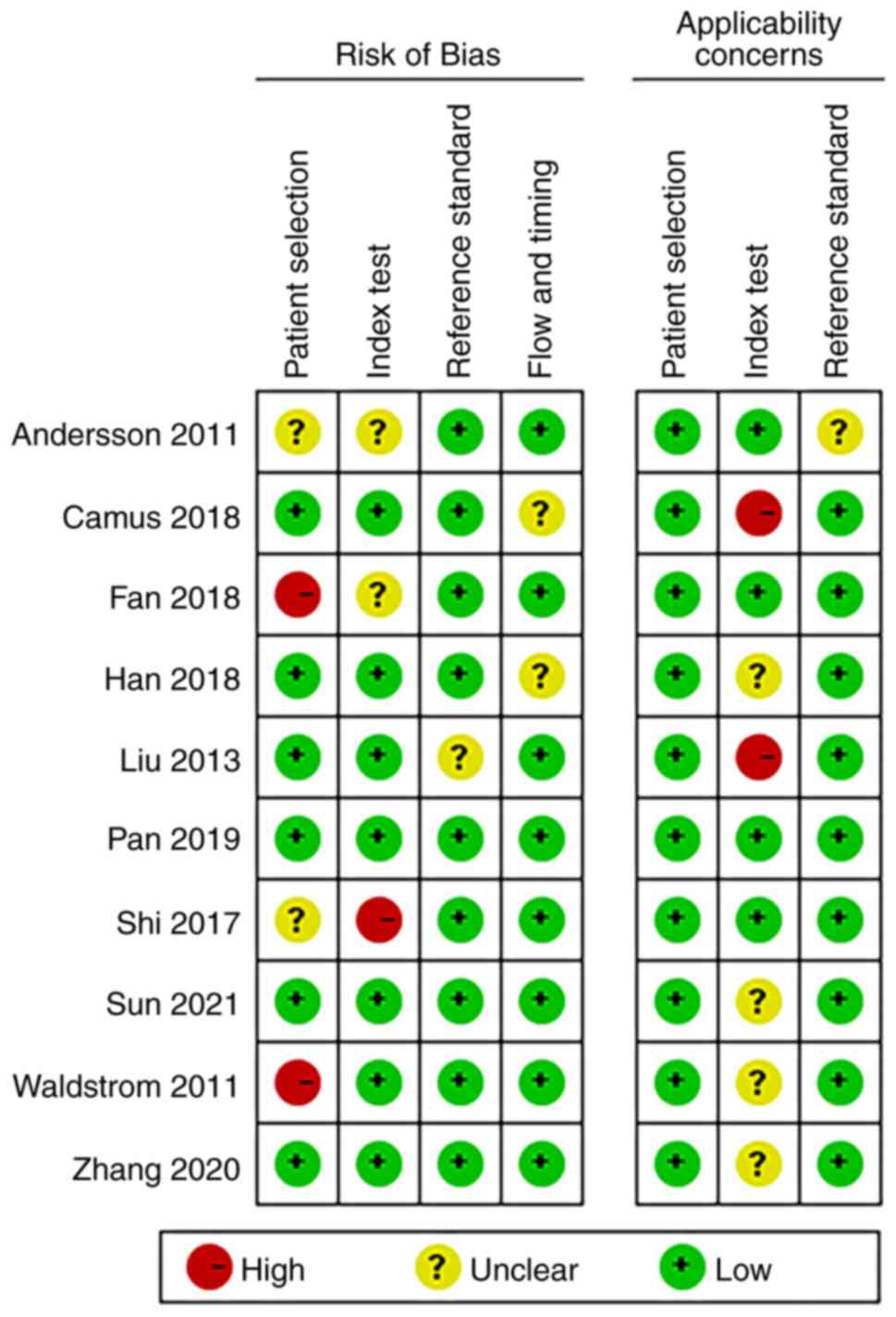
‘Risk of bias’ mainly includes four aspects:
‘Patient selection’, ‘index test’, ‘reference standard’, and ‘flow
and timing’ (13). Of the ‘patient
selection’ assessment, only two studies were high risk (patients
employing selection methods that did not meet the aforementioned
criteria, potentially introducing selection bias), and the rest
were low risk (patients that adhered to the criteria for random or
sequential selection). There was only one study in ‘index test’
showing high risk. Nine studies with regard to the aspect
‘reference standard’ were low-risk and 8 studies with regard to the
aspect ‘flow and timing’ were low-risk. Additionally,
‘applicability concerns’ mainly includes three aspects (13): Patient selection, index test and
reference standard. For ‘index test’, there was also one study that
showed high-risk, and the rest were low risk. Overall, the quality
of the literature included in the present review was acceptable
(Figs. 2 and 3).
Results of meta-analysis
Since the I2 for sensitivity (91.71%),
specificity (93.95%), LR+ (94.7%), LR- (89.3%) and DOR (84.2%) were
>50%, representing a high level of inconsistency among studies,
a sensitivity analysis was conducted to find sources of
heterogeneity (Fig. S1, Fig. S2, Fig.
S3, Fig. S4). The results
showed that the two studies by Shi et al (17) and Zhang et al (22) had a greater impact on the results.
Both studies were excluded and tested for heterogeneity again. The
results of the repeated heterogeneity test showed that the
heterogeneity was significantly reduced.
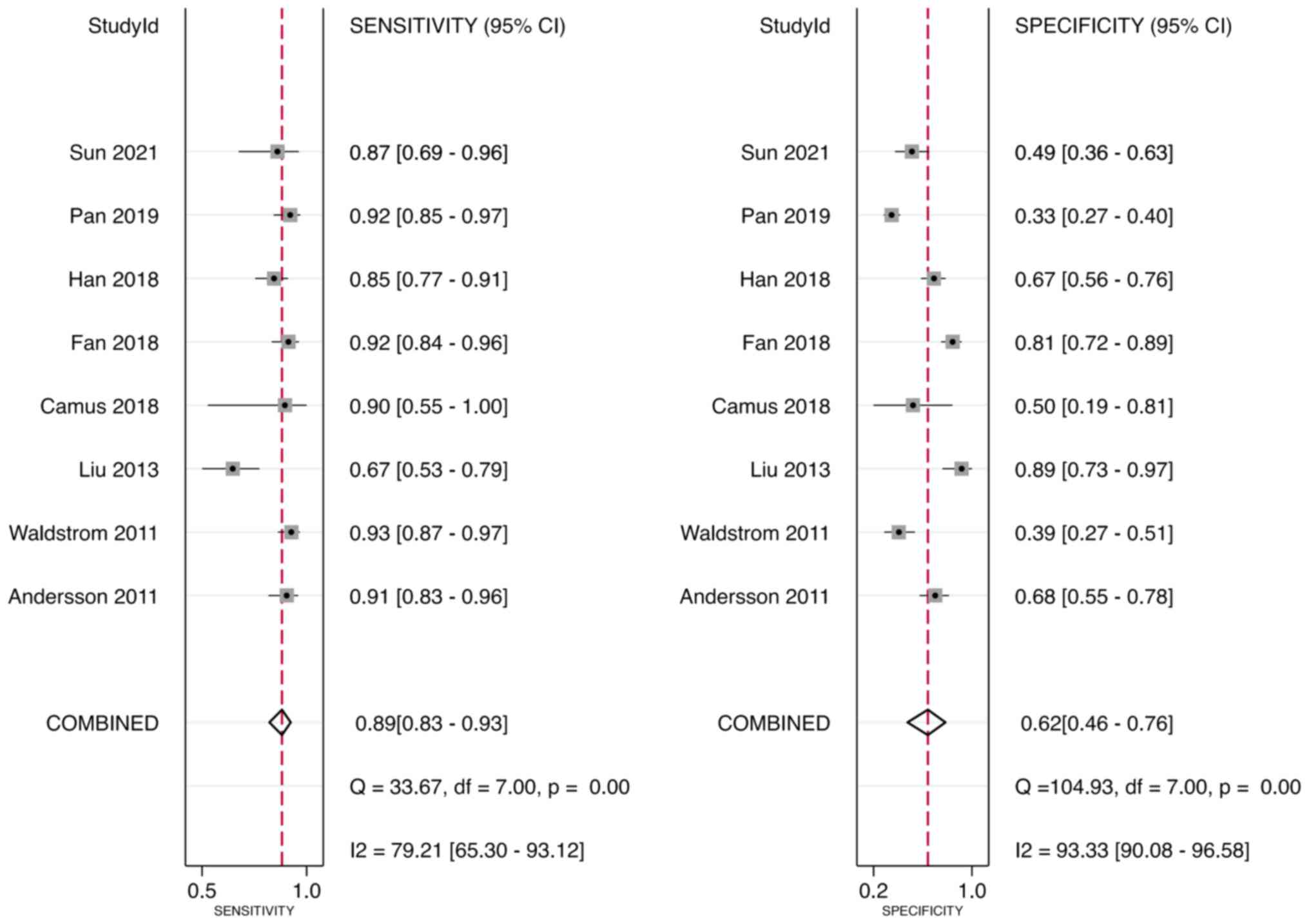
Sensitivity and specificity
Meta-analysis was performed through a random-effect
model due to heterogeneity in sensitivity (I2=79.21%)
and specificity (I2=93.33%). The pooled sensitivity and
specificity of the studies overall were 0.89 (95% CI, 0.83–0.93)
and 0.62 (95% CI, 0.46–0.76), respectively (Fig. 4).
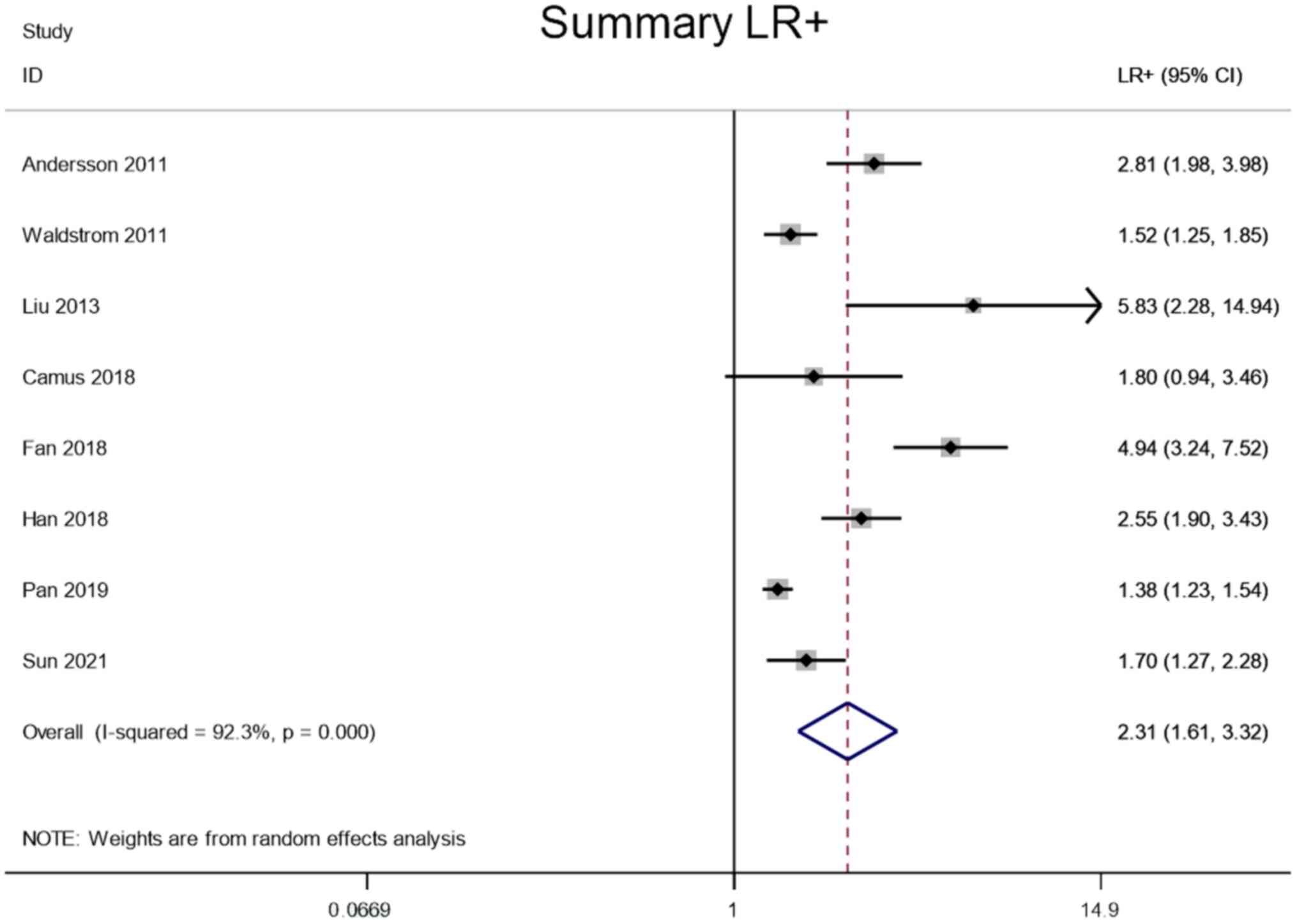
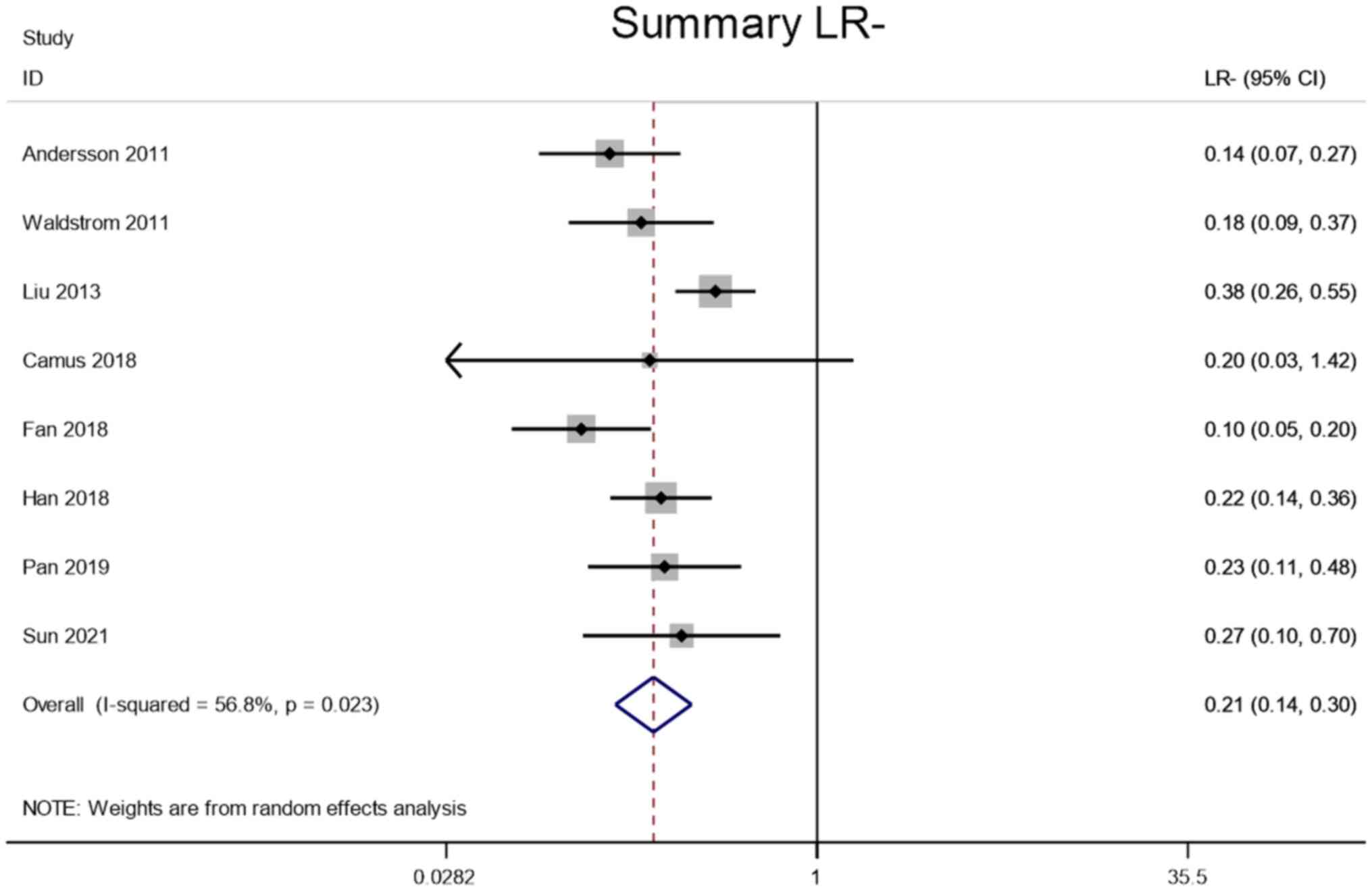
LR+ and LR-
Meta-analysis was performed through a random-effect
model due to lower heterogeneity in LR+ (I2=92.3%) and
LR- (I2=56.8%). The pooled LR+ and LR- of the studies
overall were 2.31 (95% CI, 1.61–3.32) and 0.21 (95% CI, 0.14–0.30),
respectively (Figs. 5 and 6).
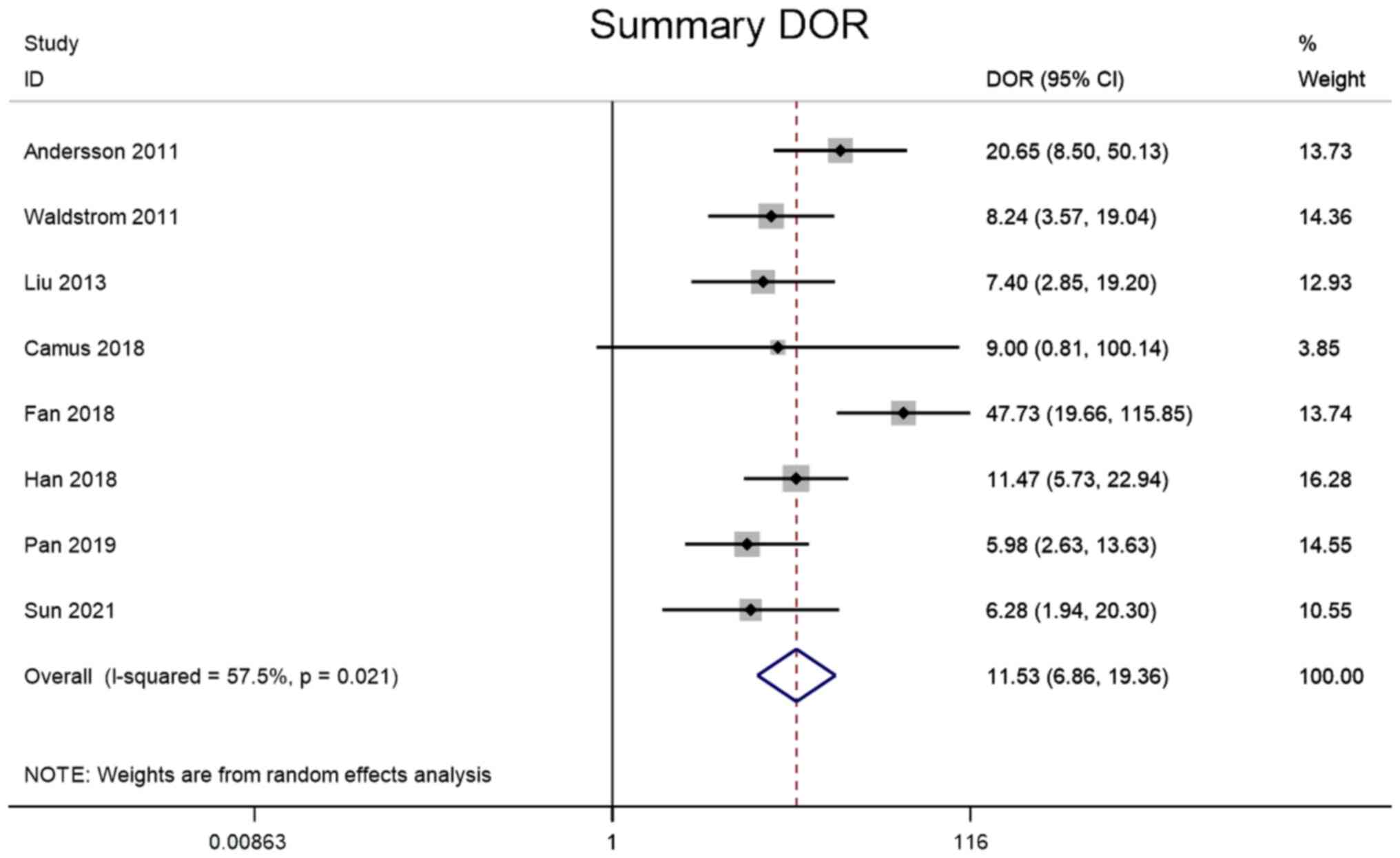
DOR
Meta-analysis was performed through a random-effect
model due to lower heterogeneity in DOR (I2=57.5%). The
pooled DOR of the studies overall was 11.53 (95% CI, 6.86–19.36;
Fig. 7).
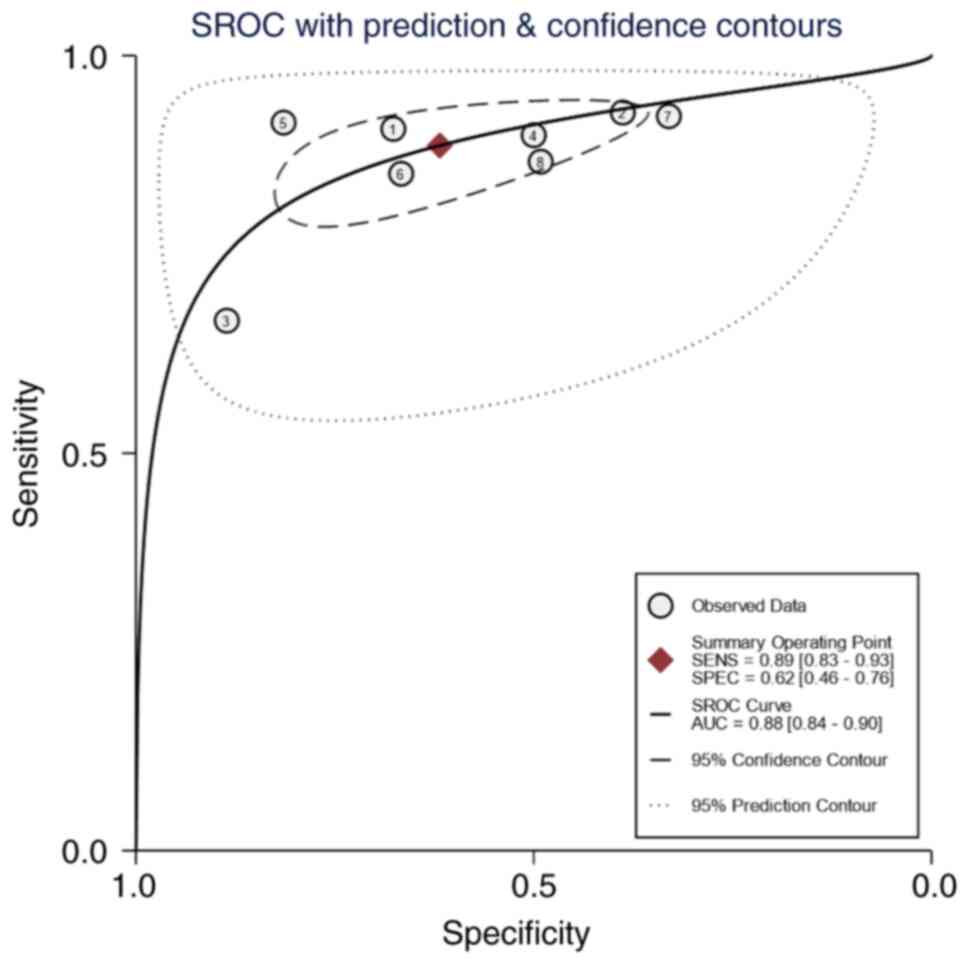
ROC analysis
When the AUC value is 0.5–0.6, it is considered that
the diagnostic tool is ineffective, 0.6–0.7 is poor, 0.7–0.8 is
average, 0.8–0.9 is good and 0.9–1.0 is excellent (24). The SROC curve of the present study
shows that AUC was 0.88 (95% CI, 0.84–0.90), indicating that E6/E7
mRNA has good diagnostic value for cervical cancer screening
(Fig. 8).
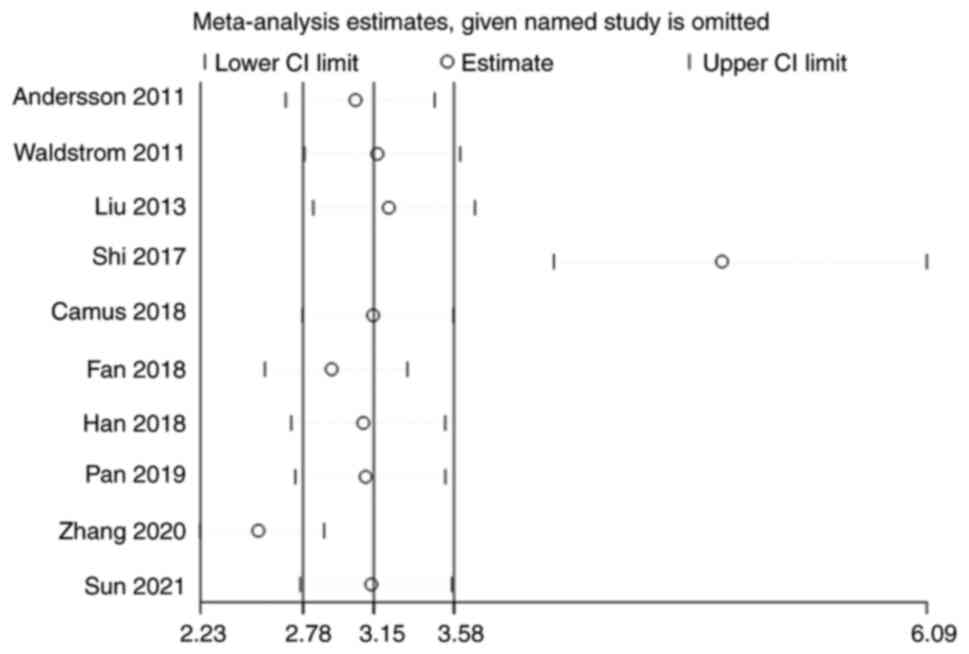
Sensitivity analysis
Sensitivity analysis was carried out by iteratively
excluding each included study individually, followed by
re-conducting the meta-analysis with the remaining studies. The
results of this sensitivity analysis were then compared to the
original analysis to evaluate the influence of each study on the
meta-analysis outcomes. Notably, after the exclusion of the studies
conducted by Shi et al (17)
and Zhang et al (22), the
subsequent meta-analysis exhibited considerable changes compared
with the original analysis. Therefore, it can be inferred that
these two studies had a pronounced impact on the overall results
(Fig. 9).
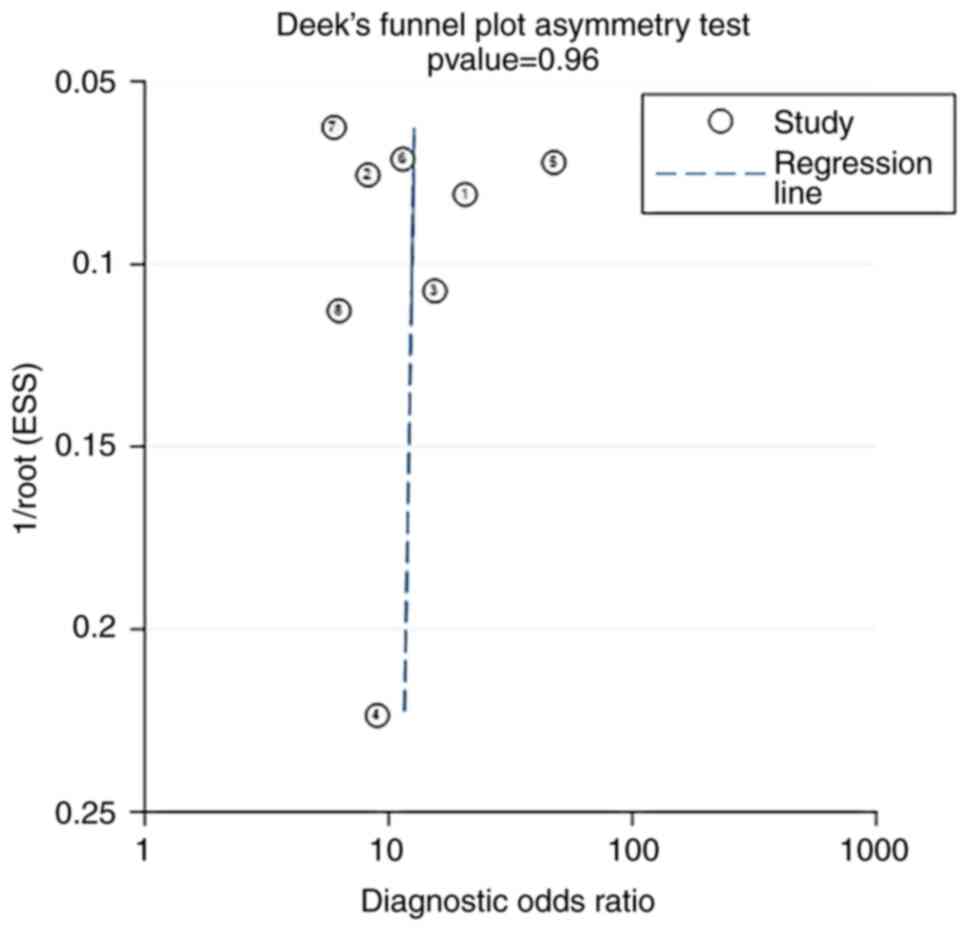
Publication bias
The P-value of the Deek's funnel plot of HPV E6/E7
mRNA for distinguishing between CIN2+ and CIN2 was 0.96, indicating
that there was no obvious publication bias in the current study
(Fig. 10).
Discussion
It is now clear that the occurrence and development
of cervical cancer and CIN are mainly caused by the continuous
infection with high-risk HPV. HPV DNA testing is primarily a way to
check if a patient is infected with HPV. Although it has a high
sensitivity, its specificity is relatively low, and it cannot
evaluate the infection stage of cervical HPV and the activity of
viral oncogenes (25). HPV circular
DNA is free in the nucleus of the host, and viral nucleic acid is
generally integrated in the genome of the host normal cell, which
can cause the inactivation or loss of E2 gene fragment, and then
lead to the mRNA transcription of viral E6 and E7 oncogenes
(19). Basu et al (26) reported that HPV E6/E7 proteins could
bind to p53 and pRb, the key tumor suppressor proteins in cervical
epithelial cells, respectively, and lead to their inactivity,
resulting in abnormal cell cycle regulation and increasing the risk
of malignant degeneration of CIN. An increasing number of studies
have revealed that the expression level of HPV E6/E7 mRNA is
positively associated with the severity of cervical lesions, and
the higher the expression level, the greater the risk of high-grade
CIN progressing to cervical cancer (27,28).
Therefore, the present meta-analysis explored the diagnostic value
of HPV E6/E7 mRNA in screening for CIN2+, aiming to provide a new
marker for clinical diagnosis of cervical cancer.
Firstly, the pooled sensitivity and specificity of
the studies overall were 0.89 (95% CI, 0.84–0.92) and 0.59 (95% CI,
0.46–0.71), respectively. This indicates that HPV E6/E7 mRNA is
highly sensitive in the diagnosis of CIN2+, which helps to reduce
the rate of missed diagnosis. However, lower specificity may lead
to higher misdiagnosis in healthy patients. Additionally, the
pooled DOR of the studies overall was 11.53 (95% CI, 6.85–19.36),
suggesting that HPV E6/E7 mRNA had high diagnostic efficacy.
Notably, the SROC curve of the current study showed that the AUC of
0.88 (95% CI, 0.84–0.90) indicates that E6/E7 mRNA has good
diagnostic value for cervical cancer screening. In a study by Camus
et al (18), the sensitivity
of HPV DNA for CIN2+ diagnosis was 80%, while the AUC was 0.76. In
addition, Zhang et al (29)
reported an HPV DNA sensitivity of 86.5% and an AUC of 0.865. This
suggests that the sensitivity and diagnostic accuracy of HPV E6/E7
mRNA may be higher than that of HPV DNA. When HPV E6/E7 mRNA
detection is positive, cervical cancer histopathological
examination should be performed for early diagnosis and early
intervention.
However, the present study also has certain
limitations. First, most of the included studies were
retrospective, thus potentially introducing selection bias and
limiting the generalizability of the findings to broader
populations or screening settings. Further large-scale randomized
controlled trials are needed to validate the findings. Second, most
of the included studies were single-center, retrospective studies.
Third, while the current study reported no obvious publication bias
based on Deek's funnel plot, publication bias can be challenging to
detect, especially when the number of included studies is limited.
Fourth, the study primarily focused on diagnostic accuracy
measures. However, it does not directly assess clinical outcomes,
such as the impact of HPV E6/E7 mRNA testing on patient management
or the reduction in cervical cancer incidence or mortality. Fifth,
the study does not directly compare HPV E6/E7 mRNA testing with
other screening methods, making it challenging to evaluate whether
this biomarker offers advantages over existing diagnostic
approaches.
HPV E6/E7 mRNA testing has high diagnostic efficacy
for CIN2+. HPV E6/E7 mRNA is highly sensitive in the diagnosis of
CIN2+, which helps to reduce the rate of missed diagnoses. However,
lower specificity may lead to more misdiagnoses in healthy
patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: Not funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FSX and TFR made substantial contributions in
conceving and drafting the manuscript. QGW and RRP made substantial
contributions to acquisition of data. SZC and JL made substantial
contributions to analysis and interpretation of data. FSX and JL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nadile M, Kornel A, Sze NSK and Tsiani E:
A comprehensive review of Genistein's effects in preclinical models
of cervical cancer. Cancers (Basel). 16:352023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cervical Cancer Treatment, . Patient
version. PDQ Cancer Information Summaries. National Cancer
Institute; Bethesda, MD: 2002
|
|
3
|
Ikeda S, Ueda Y, Hara M, Yagi A, Kitamura
T, Kitamura Y, Konishi H, Kakizoe T, Sekine M, Enomoto T and Sobue
T: Human papillomavirus vaccine to prevent cervical intraepithelial
neoplasia in Japan: A nationwide case-control study. Cancer Sci.
112:839–846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A, Colombo N and Committee EG: Cervical cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 28 (Suppl 4):iv72–iv83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meijer CJLM, Berkhof J, Castle PE,
Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J,
Dillner J, et al: Guidelines for human papillomavirus DNA test
requirements for primary cervical cancer screening in women 30
years and older. Int J Cancer. 124:516–520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolf JL, Billingsley CC, Kendler A and
Jackson AL: Cervical stratified mucin-producing intraepithelial
lesion: A systematic review of diagnosis and management. J Low
Genit Tract Dis. 24:259–264. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischer M, Uxa S, Stanko C, Magin TM and
Engeland K: Human papilloma virus E7 oncoprotein abrogates the
p53-p21-DREAM pathway. Sci Rep. 7:26032017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rebolj M, Rimmer J, Denton K, Tidy J,
Mathews C, Ellis K, Smith J, Evans C, Giles T, Frew V, et al:
Primary cervical screening with high risk human papillomavirus
testing: observational study. BMJ. 364:l2402019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Argyri E, Tsimplaki E, Daskalopoulou D,
Stravopodis DJ, Kouikoglou O, Terzakis E and Panotopoulou E: E6/E7
mRNA expression of high-risk HPV types in 849 Greek women.
Anticancer Res. 33:4007–4011. 2013.PubMed/NCBI
|
|
10
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D,
Gao C, Ma D and Liao S: Human papillomavirus vaccine against
cervical cancer: Opportunity and challenge. Cancer Lett.
471:88–102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Derbie A, Mekonnen D, Woldeamanuel Y, Van
Ostade X and Abebe T: HPV E6/E7 mRNA test for the detection of high
grade cervical intraepithelial neoplasia (CIN2+): A systematic
review. Infect Agent Cancer. 15:92020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan D and Giele H: The scratch collapse
test: A QUADAS-2 assessment of a systematic review. J Plast
Reconstr Aesthet Surg. 72:1418–1833. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andersson E, Kärrberg C, Rådberg T,
Blomqvist L, Zetterqvist BM, Ryd W, Lindh M and Horal P:
Type-specific human papillomavirus E6/E7 mRNA detection by
real-time PCR improves identification of cervical neoplasia. J Clin
Microbiol. 49:3794–3799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldstrom M and Ornskov D: Clinical
performance of a human papillomavirus messenger RNA test (Aptima
HPV Assay) on residual material from archived 3-year-old PreservCyt
samples with low-grade squamous intraepithelial lesion. Arch Pathol
Lab Med. 135:1052–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu TY, Xie R, Luo L, Reilly KH, He C, Lin
YZ, Chen G, Zheng XW, Zhang LL and Wang HB: Diagnostic validity of
human papillomavirus E6/E7 mRNA test in cervical cytological
samples. J Virol Methods. 196:120–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi WJ, Liu H, Wu D, Tang ZH, Shen YC and
Guo L: E6/E7 proteins are potential markers for the screening and
diagnosis of cervical pre-cancerous lesions and cervical cancer in
a Chinese population. Oncol Lett. 14:6251–6258. 2017.PubMed/NCBI
|
|
18
|
Camus C, Vitale S, Loubatier C, Pénaranda
G, Khiri H, Plauzolles A, Carcopino X, Halfon P and Giordanengo V:
Quantification of HPV16 E6/E7 mRNA spliced isoforms viral load as a
novel diagnostic tool for improving cervical cancer screening. J
Clin Med. 7:5302018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y and Shen Z: The clinical value of
HPV E6/E7 and STAT3 mRNA detection in cervical cancer screening.
Pathol Res Pract. 214:767–775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han L, Husaiyin S, Zhao F, Rezhake R and
Niyazi M: Clinical value of human papillomavirus E6/E7 mRNA
detection in screening for cervical cancer in women positive for
human papillomavirus DNA or. Clin Lab. 64:1363–1371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan D, Zhang CQ, Liang QL and Hong XC: An
efficient method that combines the ThinPrep cytologic test with
E6/E7 mRNA testing for cervical cancer screening. Cancer Manag Res.
11:4773–4780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang SK, Guo Z, Wang P, Kang LN, Jia MM,
Wu ZN, Chen Q, Cao XQ, Zhao DM, Guo PP, et al: The potential
benefits of HPV E6/E7 mRNA test in cervical cancer screening in
China. Front Oncol. 10:5332532020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Yue Y, Li R, Sun Q, Hu C, Ge X and
Guan Q: Detection of HPV E6/E7 mRNA in the diagnosis of cervical
cancer and precancerous lesions after kidney transplantation. Am J
Transl Res. 13:7312–7317. 2021.PubMed/NCBI
|
|
24
|
Qian S, Zhang S, Lu M, Chen S, Liu L, Liu
S, Jiang F and Zhang J: The accuracy of screening tools for
sarcopenia in older Chinese adults: A systematic review and
meta-analysis. Front Public Health. 12:13103832024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Molden T, Kraus I, Skomedal H, Nordstrom T
and Karlsen F: PreTect HPV-proofer: Real-time detection and typing
of E6/E7 mRNA from carcinogenic human papillomaviruses. J Virol
Methods. 142:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu P, Banerjee D, Mittal S, Dutta S,
Ghosh I, Chowdhury N, Abraham P, Chandna P and Ratnam S:
Sensitivity of APTIMA HPV E6/E7 mRNA test in comparison with hybrid
capture 2 HPV DNA test for detection of high risk oncogenic human
papillomavirus in 396 biopsy confirmed cervical cancers. J Med
Virol. 88:1271–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song Y, Zhang M, Zhang C, Du S and Zhai F:
HPV E6/E7 mRNA combined with thin-prep cytology test for the
diagnosis of residual/recurrence after loop electrosurgical
excision procedure in patients with cervical intraepithelial
neoplasia. Diagn Microbiol Infect Dis. 108:1161192024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duvlis S, Popovska-Jankovic K, Arsova ZS,
Memeti S, Popeska Z and Plaseska-Karanfilska D: HPV E6/E7 mRNA
versus HPV DNA biomarker in cervical cancer screening of a group of
Macedonian women. J Med Virol. 87:1578–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Wang J, Zhang R, Lei F and Lai S:
Application value of detection of high-risk HPV infection in early
cervical cancer patients in disease diagnosis and prognosis
evaluation. Clin Lab. 66:2020. View Article : Google Scholar
|