Introduction
Multiple myeloma (MM) is a mature B-cell neoplasm
characterized by the presence of clonal plasma cells in bone
marrow. Extramedullary MM is associated with aggressive disease and
poor prognosis owing to its increased proliferation and therapeutic
resistance (1). Although the
emergence of monoclonal antibodies (mAbs), such as daratumumab,
elotuzumab, and isatuximab, has dramatically improved the prognosis
of MM, the optimal sequence of treatment regimens remains
controversial (2).
Daratumumab, an anti-CD38 antibody, reduces the
number of natural killer (NK) cells, requiring more than six months
for recovery (3). Another
monoclonal antibody, elotuzumab, is an anti-signaling lymphocytic
activation molecule family member 7 (SLAMF7) antibody that
activates NK cells (4,5). Several studies have shown that
pre-treatment with daratumumab attenuates the effectiveness of
elotuzumab (6,7). To date, there have been no reports
discussing the use of different mAbs for MM associated with
extramedullary disease. Herein, we report a unique case of
daratumumab-resistant MM with extramedullary disease that was
successfully treated with elotuzumab, pomalidomide, and
dexamethasone (EPd).
Case report
A 66-year-old Japanese male with a history of
hypertension, dyslipidemia, and type 2 diabetes was referred to our
hospital for multiple osteolytic tumors of the skull, ribs, and
ilium. Laboratory tests revealed anemia (hemoglobin, 12.1 g/dl) and
increased immunoglobulin G (IgG, 2,866 mg/dl). Serum levels of
β2 microglobulin and lactate dehydrogenase (LDH) were
3.4 mg/l and 182 U/l, respectively. Immunoelectrophoresis
demonstrated the presence of monoclonal component IgG κ-type.
Urinary Bence Jones protein was not detected. Serum free light
chain κ level was 12.2 mg/l; λ level, 3.1 mg/l; and κ/λ ratio,
3.94. Bone marrow aspirate showed 52% infiltration of monoclonal
plasma cells. Cytogenetic analysis revealed a normal male karyotype
(Fig. 1A) and fluorescence in
situ hybridization (FISH) was negative for del(17p), t(4;14)
(Fig. 1B). FISH for t(14;16) was
also negative, but gain/amp(1q21) was not tested. The patient was
diagnosed with stage II MM according to the Revised International
Staging System (8).
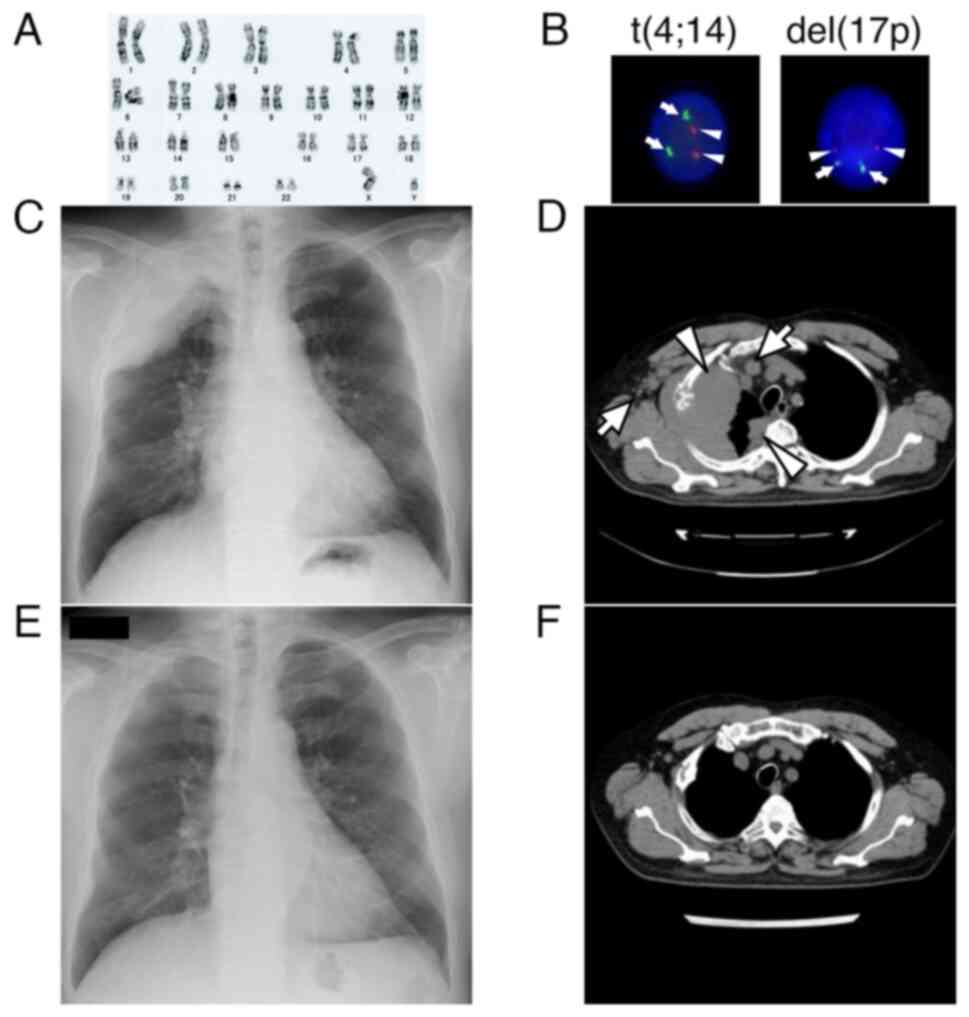 | Figure 1.Results of karyotyping and FISH
analysis, and radiographic images. (A) Cytogenetic analysis showing
normal male karyotype (46, XY). (B) FISH analysis showing no signal
for t(4;14) and del(17p). For t(4;14), the red signal (arrowheads)
indicates FGFR and the green signal (arrows) indicates
IGH. For del(17p), the red signal (arrowheads) indicates
TP53 and the green signal (arrows) indicates D17Z1
(magnification, ×500). (C) Chest radiograph showing a massive tumor
occupying the right apex. (D) Chest CT showing a bulky tumor
extending to the right thorax with pleural dissemination
(arrowheads). Lymph node metastases to the right axilla and
mediastinum were also noted (arrows). (E) Chest radiograph on day
13 of EPd, showing marked regression of the tumor. (F) Chest CT 3
months after the initiation of EPd showing disappearance of the
disease. CT, computed tomography; del, deletion; EPd, elotuzumab,
pomalidomide and dexamethasone; FISH, fluorescence in situ
hybridization. |
Treatment with four cycles of bortezomib,
lenalidomide, and dexamethasone, followed by autologous stem cell
transplantation resulted in a very good partial response according
to the 2016 International Myeloma Working Group uniform response
criteria (9). Thereafter,
maintenance therapy was continued with lenalidomide and
dexamethasone. Thirty-four months later, although the IgG level was
well controlled (548 mg/dl), anterior thoracic pain developed.
Computed tomography (CT) revealed new osteolytic lesions in the
sternum; therefore, the treatment was changed to daratumumab,
bortezomib, and dexamethasone (DBd) at 16 mg/kg on days 1, 8, and
15; 1.3 mg per square of body-surface area twice weekly for two
weeks; and 20 mg on the day of and the day after bortezomib,
respectively (10), resulting in
alleviation of his pain and tumor shrinkage.
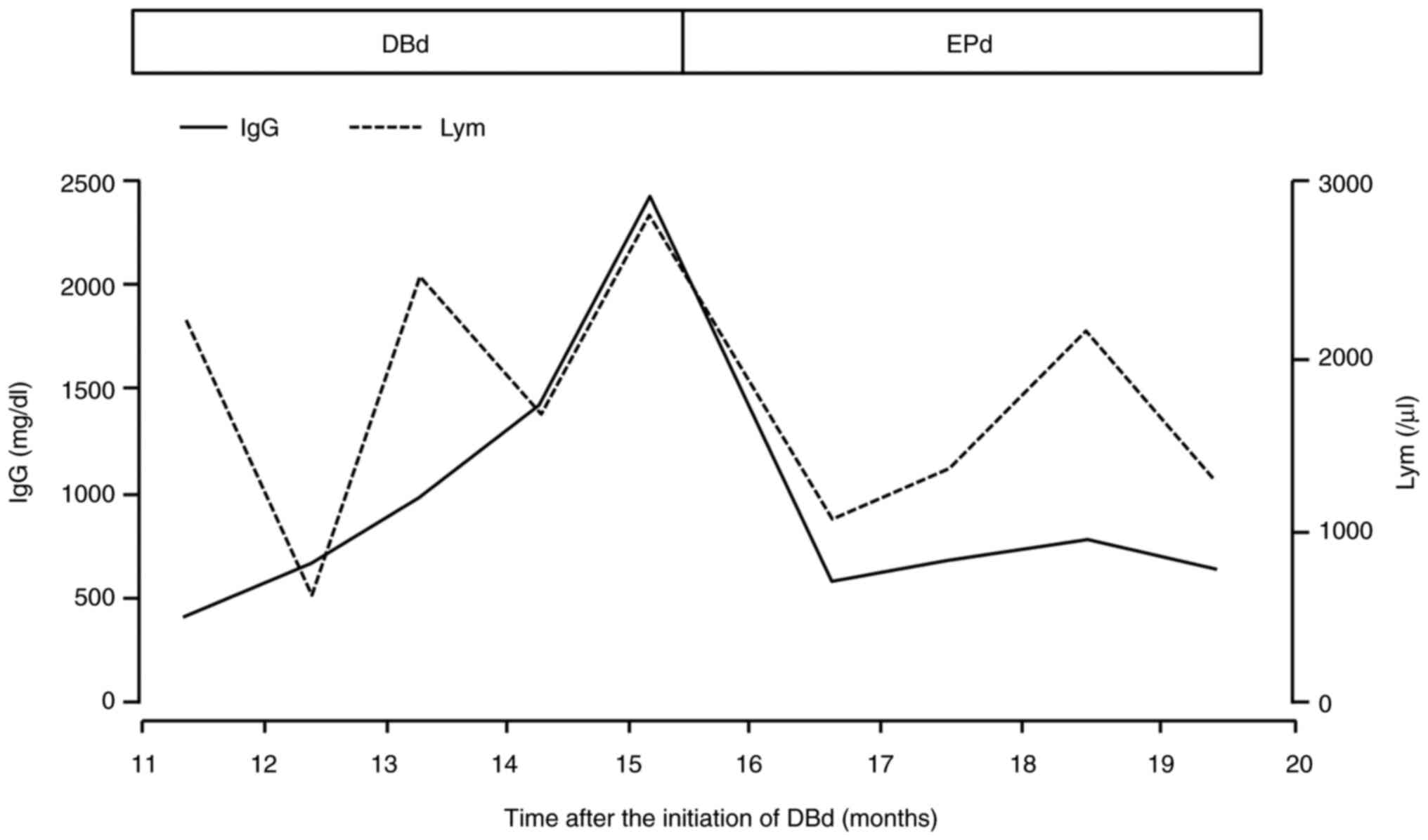
Fifteen months after the initiation of DBd, severe
right-sided chest pain developed, and the IgG level was elevated to
2,266 mg/dl. The lymphocyte count was elevated to 3,072/µl
(Fig. 2). Lymphocytes were
morphologically normal, while neither lymphocytes subset analysis
nor NK cell activity was performed. Serum β2
microglobulin and κ/λ ratio were 4.5 mg/dl and 0.80, respectively.
Chest radiography revealed a massive tumor occupying the apex of
the right lung (Fig. 1C). A chest
CT scan showed a bulky tumor arising from the right rib, extending
to the right thorax, and pleural dissemination with lymph node
metastasis to the right axilla and mediastinum (Fig. 1D). Neither bone marrow examination
including FISH analysis nor biopsy of extramedullary mass was
performed due to the patient's refusal. Upon clinical relapse of MM
with extramedullary disease, the treatment was changed to EPd
(elotuzumab, 10 mg/kg on days 1, 8, 15 and 22 during cycles 1 and
2, and 20 mg/kg on day 1 of each cycle thereafter; pomalidomide, 4
mg per day on days 1 through 21; dexamethasone, 40 mg once weekly)
(11), which resulted in
expeditious resolution of the chest pain. Notably, chest
radiography on day 13 of treatment revealed marked regression of
the tumor in the right apex (Fig.
1E), and serum IgG levels rapidly decreased to 492 mg/dl
(Fig. 2). Three months after the
initiation of EPd, a subsequent CT scan demonstrated disappearance
of the disease (Fig. 1F).
Discussion
Even in the era of monoclonal antibodies and
proteasome inhibitors, the prognosis in MM with extramedullary
disease still remains poor (12,13).
In a retrospective study assessing elotuzumab-based regimens for
the treatment of extramedullary MM, progression-free survival and
overall survival were short (3.8 and 12.9 months, respectively)
(14). Here, we present the first
case of DBd-resistant MM with extramedullary disease that was
successfully treated with EPd.
The mechanism of action of elotuzumab depends on the
action of NK cells. Elotuzumab binds to SLAMF7, which is expressed
at high levels in both MM and NK cells, and exerts cytotoxic
effects by activating NK cells and antibody-dependent cellular
cytotoxicity (ADCC) (4,5). In contrast, daratumumab, an anti-CD38
antibody, reduces the NK cell count in peripheral blood mononuclear
cells by binding to CD38 expressed on NK cells (15). It takes six months for NK cells to
recover in number after cessation of daratumumab infusion (3). These results suggest that continuous
elotuzumab may have weakened effects when used following treatment
with daratumumab.
Hoylman et al (6) compared clinical outcomes in patients
who received either daratumumab before elotuzumab (dara-first) or
elotzumab before daratumumab (elo-first) and demonstrated that the
response rate to elotuzumab was diminished in the dara-first group,
with a significantly longer cumulative progression free survival in
the elo-first group. In randomized phase III studies, daratumumab
was more effective against relapsed/refractory MM when administered
with fewer prior lines of therapy (10,16–18).
Furthermore, in transplant-ineligible newly diagnosed MM, the
ALCYONE and MAIA studies showed that treatment with daratumumab as
a front-line therapy produced superior outcomes (19–22).
Based on these results, daratumumab is likely to be administered
before elotuzumab, and an approach that allows retention of
elotuzumab's efficacy is required.
Disease burden, NK cell count, and SLAMF7 expression
in myeloma cells have been suggested as biomarkers of elotuzumab
response (23). However, these
markers are not readily measurable in clinical settings. Recently,
a simpler clinical index has been reported to predict response to
elotuzumab treatment. This report showed that the median time to
next treatment was significantly longer in patients with higher
lymphocyte counts (>1,400/µl), non-deviated κ/λ ratio (0.1–10),
lower β2 microglobulin (<5.5 mg/l), and no prior
daratumumab use (7). In the present
case, although elotuzumab was administered immediately after
daratumumab treatment, the patient responded well. Interestingly,
in the present case, the lymphocyte counts during DBd gradually
increased to a level at which elotuzumab was sufficiently effective
(Fig. 2). Although the NK cell
fraction was not measured in the present case, the increase in
lymphocytes during DBd may reflect the existence of abundant NK
cells. Furthermore, in a mouse model, DBd treatment enhanced the
cytotoxicity of expanded NK cells by upregulating the expression of
NK cell-activating ligands, downregulating the expression of NK
cell-inhibitory ligands, and promoting ADCC (24). The augmentation of NK cell
cytotoxicity by DBd may also contribute to the enhanced therapeutic
effect of EPd. On the other hand, elotuzumab has been shown to
inhibit myeloma cell growth in vivo through
antibody-dependent cellular phagocytosis by macrophages (25,26).
This mechanism of action of elotuzumab may have contributed to the
dramatic response seen in our case.
Overall, the findings in our case suggest that EPd
might be a promising strategy for the treatment of patients with
relapsed extramedullary MM while on daratumumab treatment. Further
studies are required to select a more appropriate sequence of
treatment for these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS, NS and SKo conceived and designed the study. MS,
NS, JA, TM, SKo, SKi, TF and NO acquired, analyzed and interpreted
the data. MS and NS drafted and revised the manuscript, and confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of case details and associated
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
multiple myeloma
|
|
NK
|
natural killer
|
|
mAbs
|
monoclonal antibodies
|
|
SLAMF7
|
signaling lymphocytic activation
molecule family member 7
|
|
IgG
|
immunoglobulin G
|
|
CT
|
computed tomography
|
|
ADCC
|
antigen-dependent cellular
cytotoxicity
|
|
EPd
|
elotuzumab, pomalidomide and
dexamethasone
|
|
DBd
|
daratumumab, bortezomib and
dexamethasone
|
References
|
1
|
Bladé J, Beksac M, Caers J, Jurczyszyn A,
von Lilienfeld-Toal M, Moreau P, Rasche L, Rosiñol L, Usmani SZ,
Zamagni E and Richardson P: Extramedullary disease in multiple
myeloma: A systematic literature review. Blood Cancer J. 12:452022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laubach JP, van de Donk N, Davies FE and
Mikhael J: Practical considerations for antibodies in myeloma. Am
Soc Clin Oncol Educ Book. 38:667–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Casneuf T, Xu XS, Adams HC III, Axel AE,
Chiu C, Khan I, Ahmadi T, Yan X, Lonial S, Plesner T, et al:
Effects of daratumumab on natural killer cells and impact on
clinical outcomes in relapsed or refractory multiple myeloma. Blood
Adv. 24:2105–2114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
His ED, Steinle R, Balasa B, Szmania S,
Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y,
et al: CS1, a potential new therapeutic antibody target for the
treatment of multiple myeloma. Clin Cancer Res. 14:2775–2784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tai YT, Dillon M, Song W, Leiba M, Li XF,
Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al: Anti-CS1
humanized monoclonal antibody HuLuc63 inhibits myeloma cell
adhesion and induces antibody-dependent cellular cytotoxicity in
the bone marrow milieu. Blood. 112:1329–1337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoylman E, Brown A, Perissinotti AJ,
Marini BL, Pianko M, Ye JC, Campagnaro E and Nachar VR: Optimal
sequence of daratumumab and elotuzumab in relapsed and refractory
multiple myeloma. Leuk Lymphoma. 61:691–698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimazu Y, Kanda J, Kosugi S, Ito T,
Kaneko H, Imada K, Shimura Y, Fuchida SI, Fukushima K, Tanaka H, et
al: Efficacy of elotuzumab for multiple myeloma in reference to
lymphocyte counts and kappa/lambda ratio or B2 microglobulin. Sci
Rep. 13:51592023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised International Staging System
for multiple myeloma: A report from International Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et
al: International Myeloma Working Group consensus criteria for
response and minimal residual disease assessment in multiple
myeloma. Lancet Oncol. 17:e328–e346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palumbo A, Chanan-Khan A, Weisel K, Nooka
AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV,
et al: Daratumumab, bortezomib, and dexamethasone for multiple
myeloma. N Engl J Med. 375:754–766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimopoulos MA, Dytfeld D, Grosicki S,
Moreau P, Takezako N, Hori M, Leleu X, LeBlanc R, Suzuki K, Raab
MS, et al: Elotuzumab plus pomalidomide and dexamethasone for
multiple myeloma. N Engl J Med. 379:1811–1822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badar T, Srour S, Bashir Q, Shah N,
Al-Atrash G, Hosing C, Popat U, Nieto Y, Orlowski RZ, Champlin R
and Qazilbash MH: Predictors of inferior clinical outcome in
patients with standard-risk multiple myeloma. Eur J Haematol.
98:263–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasche L, Bernard C, Topp MS, Kapp M,
Duell J, Wesemeier C, Haralambieva E, Maeder U, Einsele H and Knop
S: Features of extramedullary myeloma relapse: High proliferation,
minimal marrow involvement, adverse cytogenetics: A retrospective
single-center study of 24 cases. Ann Hematol. 91:1031–1037. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danhof S, Rasche L, Mottok A, Steinmüller
T, Zhou X, Schreder M, Kilian T, Strifler S, Rosenwald A, Hudecek
M, et al: Elotuzumab for the treatment of extramedullary myeloma: A
retrospective analysis of clinical efficacy and SLAMF7 expression
patterns. Ann Hematol. 100:1537–1546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krejcik J, Casneuf T, Nijhof IS, Verbist
B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, et
al: Daratumumab depletes CD38+ immune regulatory cells, promotes
T-cell expansion, and skews T-cell repertoire in multiple myeloma.
Blood. 128:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dimopoulos MA, Oriol A, Nahi H, San-Miguel
J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki
K, et al: Daratumumab, lenalidomide, and dexamethasone for multiple
myeloma. N Engl J Med. 375:1319–1331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dimopoulos MA, Oriol A, Nahi H, San-Miguel
J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Suzuki K, Plesner T,
et al: Overall survival with daratumumab, lenalidomide, and
dexamethasone in previously treated multiple myeloma (POLLUX): A
randomized, open-label, phase III trial. J Clin Oncol.
41:1590–1599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sonneveld P, Chanan-Khan A, Weisel K,
Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos
MV, et al: Overall survival with daratumumab, bortezomib, and
dexamethasone in previously treated multiple myeloma (CASTOR): A
randomized, open-label, phase III trial. J Clin Oncol.
41:1600–1609. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mateos MV, Dimopoulos MA, Cavo M, Suzuki
K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, et al:
Daratumumab plus bortezomib, melphalan, and prednisone for
untreated myeloma. N Engl J Med. 378:518–528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mateos MV, Cavo M, Blade J, Dimopoulos MA,
Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, et al:
Overall survival with daratumumab, bortezomib, melphalan, and
prednisone in newly diagnosed multiple myeloma (ALCYONE): A
randomised, open-label, phase 3 trial. Lancet. 395:132–141. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Facon T, Kumar S, Plesner T, Orlowski RZ,
Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al:
Daratumumab plus lenalidomide and dexamethasone for untreated
myeloma. N Engl J Med. 380:2104–2115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Facon T, Kumar SK, Plesner T, Orlowski RZ,
Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, et al:
Daratumumab, lenalidomide, and dexamethasone versus lenalidomide
and dexamethasone alone in newly diagnosed multiple myeloma (MAIA):
Overall survival results from a randomised, open-label, phase 3
trial. Lancet Oncol. 22:1582–1596. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danhof S, Strifler S, Hose D, Kortüm M,
Bittrich M, Hefner J, Einsele H, Knop S and Schreder M: Clinical
and biological characteristics of myeloma patients influence
response to elotuzumab combination therapy. J Cancer Res Clin
Oncol. 145:561–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thangaraj JL, Ahn SY, Jung SH, Vo MC, Chu
TH, Thi Phan MT, Kwon M, Lee KH, Kim M, Song GY, et al: Expanded
natural killer cells augment the antimyeloma effect of daratumumab,
bortezomib, and dexamethasone in a mouse model. Cell Mol Immunol.
18:1652–1661. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurdi AT, Glavey SV, Bezman NA, Jhatakia
A, Guerriero JL, Manier S, Moschetta M, Mishima Y, Roccaro A,
Detappe A, et al: Antibody-dependent cellular phagocytosis by
macrophages is a novel mechanism of action of elotuzumab. Mol
Cancer Ther. 17:1454–1463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dimopoulos MA, Dytfeld D, Grosicki S,
Moreau P, Takezako N, Hori M, Leleu X, LeBlanc R, Suzuki K, Raab
MS, et al: Elotuzumab plus pomalidomide and dexamethasone for
relapsed/refractory multiple myeloma: Final overall survival
analysis from the randomized phase II ELOQUENT-3 trial. J Clin
Oncol. 41:568–578. 2023. View Article : Google Scholar : PubMed/NCBI
|