Introduction
Retroperitoneal vascular lesions, such as
hemangiomas, are very rare and are confirmed in only 1–3% of all
retroperitoneal tumors, which comprise approximately 0.2–0.5% of
all malignancies (1). It is
sometimes difficult to diagnose them without pathological specimens
(2). Of these, anastomosing
hemangioma (AH) is extremely rare and a newly recognized variant of
capillary hemangioma that is most found in the genitourinary tract
(3–5). It is difficult to diagnose
preoperatively due to the lack of specific clinical and radiologic
appearance. Herein, we report the case of a 67-year-old woman who
presented without any symptoms and was incidentally found to have a
retroperitoneal hemangioma by imaging examination. Although we
could confirm a retroperitoneal cavernous hemangioma
preoperatively, the tumor was pathologically diagnosed as AH after
surgical resection. We describe the imaging features from a
radiological perspective and outline the clinicopathologic features
and treatment options.
Case report
Case presentation
A 67-year-old woman was referred to our hospital for
a retroperitoneal tumor that was identified by abdominal ultrasound
at a medical checkup four years prior. She had no symptoms, no
abnormal physical signs and no past medical or specific family
history. Routine blood tests, biochemical function, coagulation
panel, and tumor markers were all within the normal ranges. A
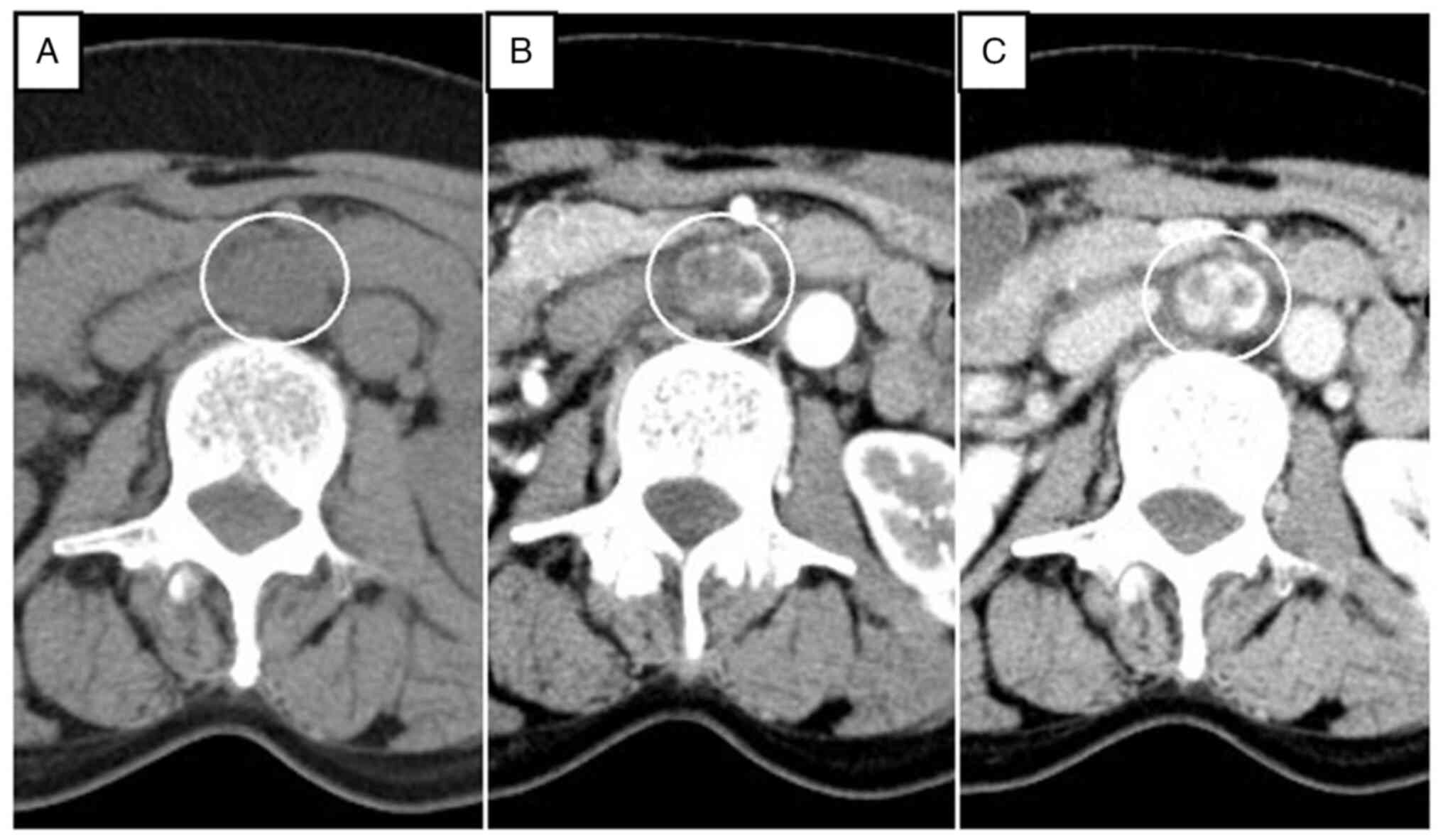
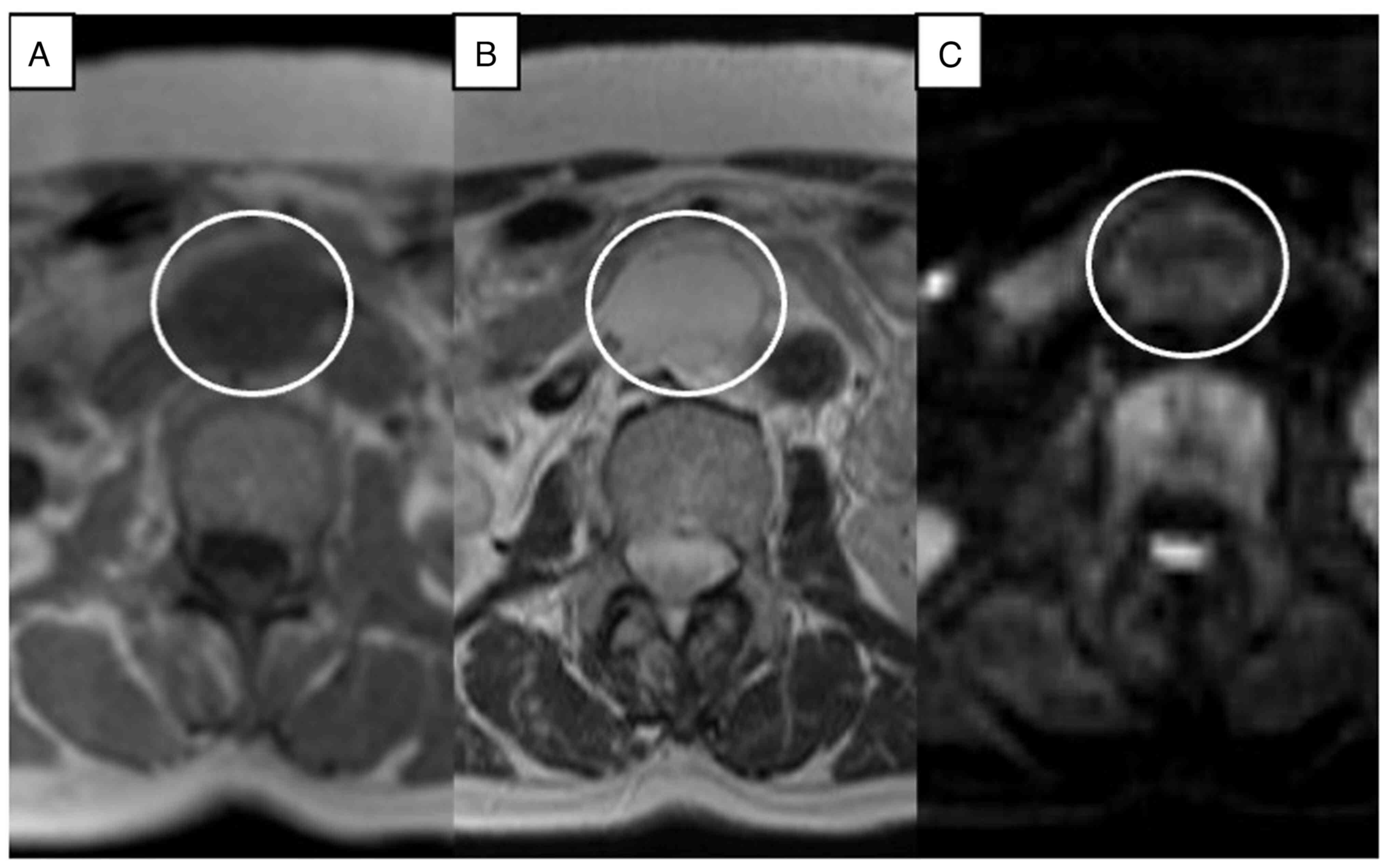
nonenhanced axial CT scan showed a circular, homogenous,
well-circumscribed retroperitoneal tumor that was approximately
32×23 mm in size, between the abdominal aorta and the inferior vena
cava, and just below the left renal vein. On contrast-enhanced
multidetector CT scan, the tumor showed heterogeneous septal
enhancement in the arterial phase and continuous enhancement in the
portal phase (Fig. 1). To clarify
the differential diagnosis, we also performed contrast-enhanced
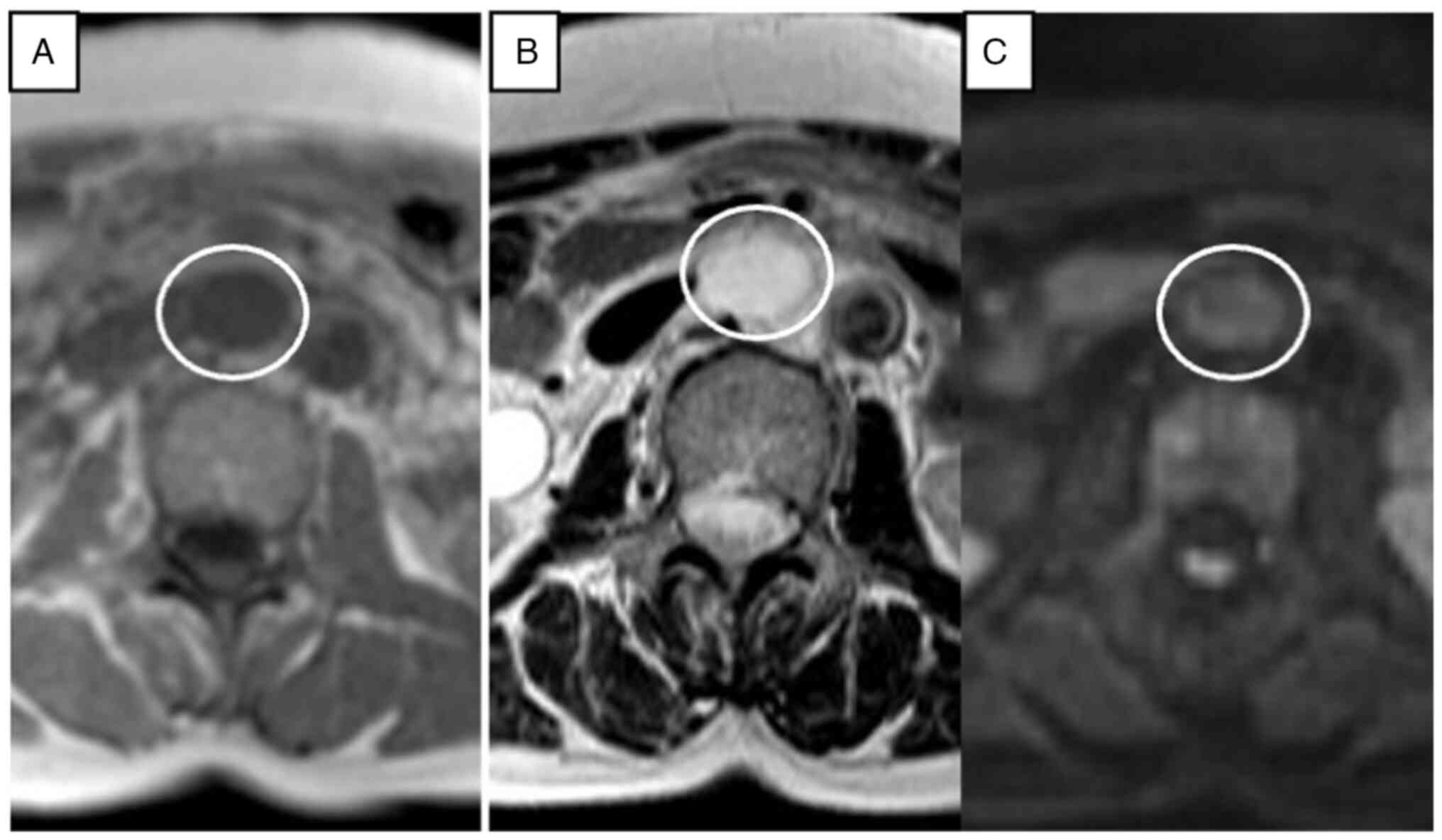
MRI. On the pre-contrast T1-weighted image (WI), the tumor showed a
circular homogenous low-density area. On T2-weighted imaging, the
tumor showed linear and curvilinear low-signal-intensity areas
within the circular high-density area, and diffusion WI showed
iso-intensity (Fig. 2). On
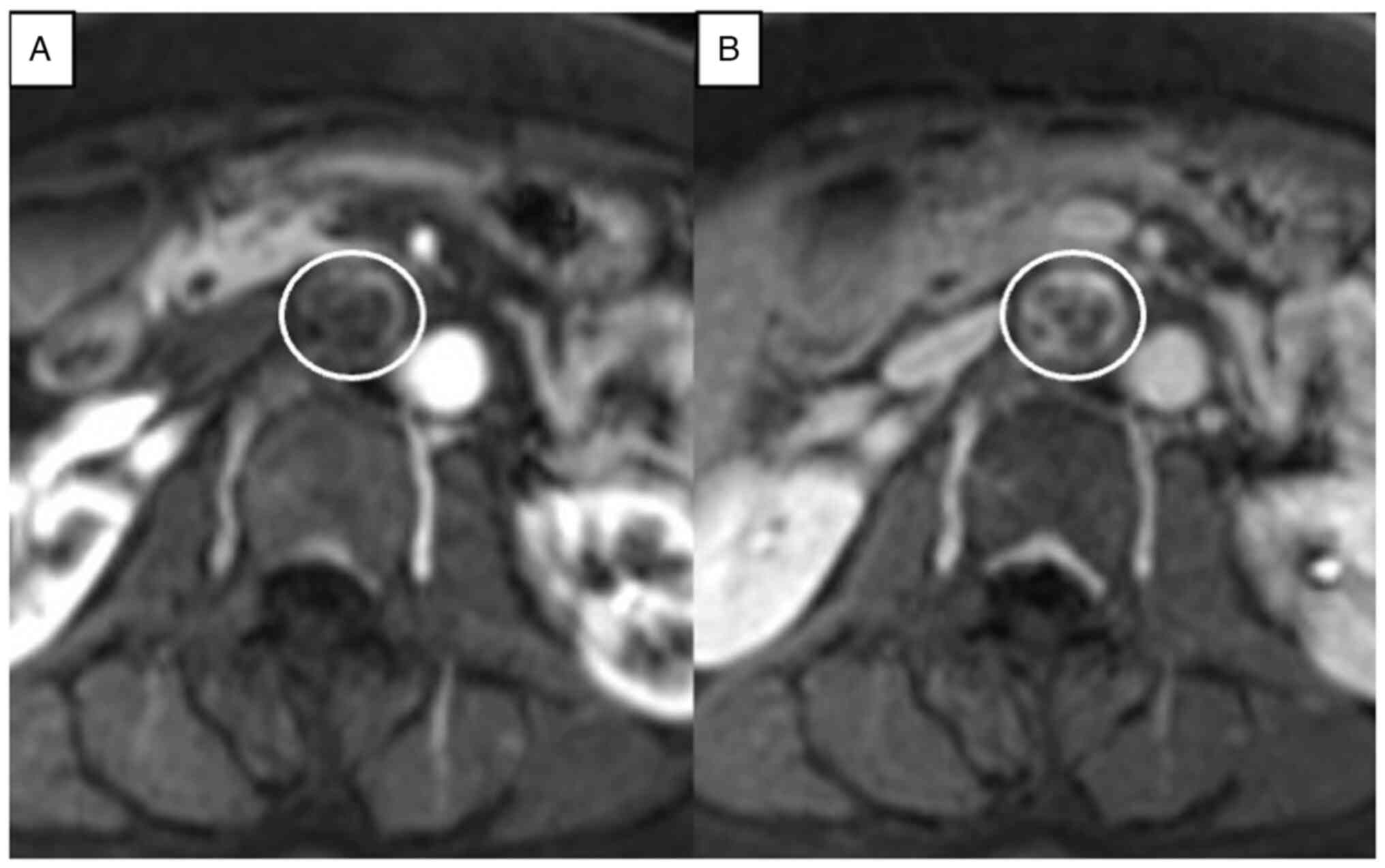
postcontrast T1-weighted imaging, the tumor was heterogeneously
contrasted and showed persistent enhancement peripherally and
without centrally (Fig. 3). From
those findings, we diagnosed it as a benign neurogenic tumor or a
retroperitoneal cavernous hemangioma at the time, we planned to
follow her at the outpatient clinic. Annual follow-up CT scan and
MRI were performed, and the tumor was not compressed and had not
invaded the duodenum, inferior vena cava, bilateral renal veins, or
urinary tracts and did not involve retroperitoneal lymphadenopathy.
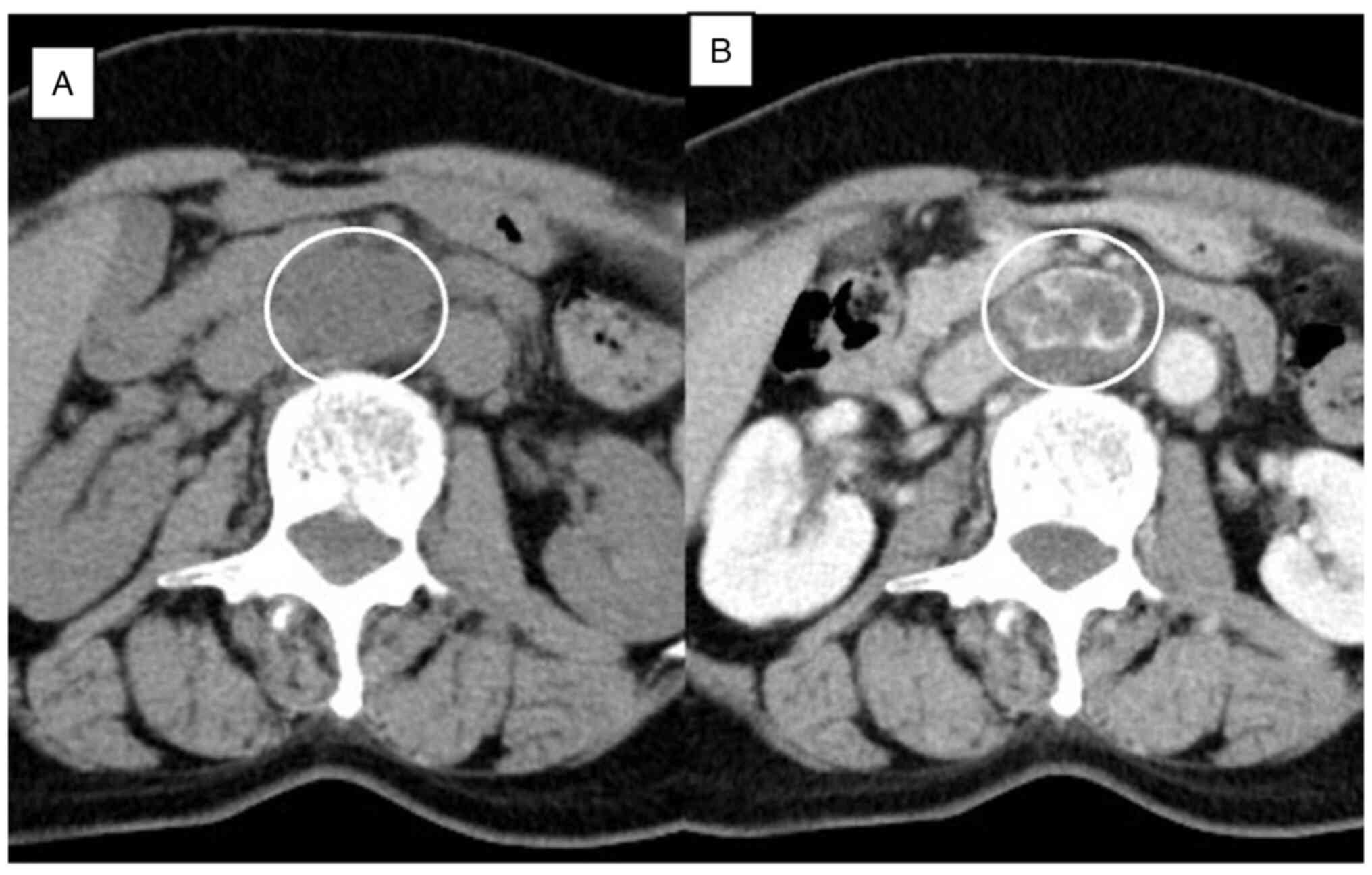
However, it gradually increased to a maximum diameter of 35 mm over
4 years (Figs. 4 and 5). The patient was evaluated again, the
image pattern was the same as before, and there were no findings
suggesting malignancy. However, the definite nature of the lesion
could not be established preoperatively, and we finally decided to
perform laparotomy to prevent its spontaneous rupture and rule out
malignancy.
Surgical treatment
At first, laparoscopic or robot-assisted surgical
resection was considered because of the benefit of being less
invasive to the patient. However, we finally performed open
laparotomy because we evaluated the surgical difficulty and tried
to avoid postoperative complications. A vertical midline incision
and Kocher's maneuver were performed for the surgery. The tumor was
located between the abdominal aorta and the inferior vena cava,
just below the left renal vein, according to the preoperative
radiological findings. It was elastic, hard and easy to mobilize
around tissues. No metastatic lesions were found in the peritoneum,
abdominal organs, or pelvic organs. The feeding arteries of the
tumor were found to originate from retroperitoneal tissue, and each
vessel was ligated before the tumor was removed. After these
procedures, the size and elasticity of the tumor were obviously
decreased. Based on these operative findings, we confirmed that the
tumor could be a hemangioma. There was no evidence intraoperatively
of invasion into the inferior vena cava, ureter, renal capsule,
pancreas, duodenum, or other surrounding organs.
Radical resection of the tumor was completed in 3.5
h with an estimated blood loss of 75 ml. After the surgery, she had
asymptomatic pancreatic hyperenzymemia that was characterized by
temporary elevation of serum amylase above the upper normal limits
in the absence of pancreatic symptoms. However, she was discharged
on postoperative Day 10 without other surgical complications and
was in good health without recurrence more than 15 months after the
operation.
Pathological findings
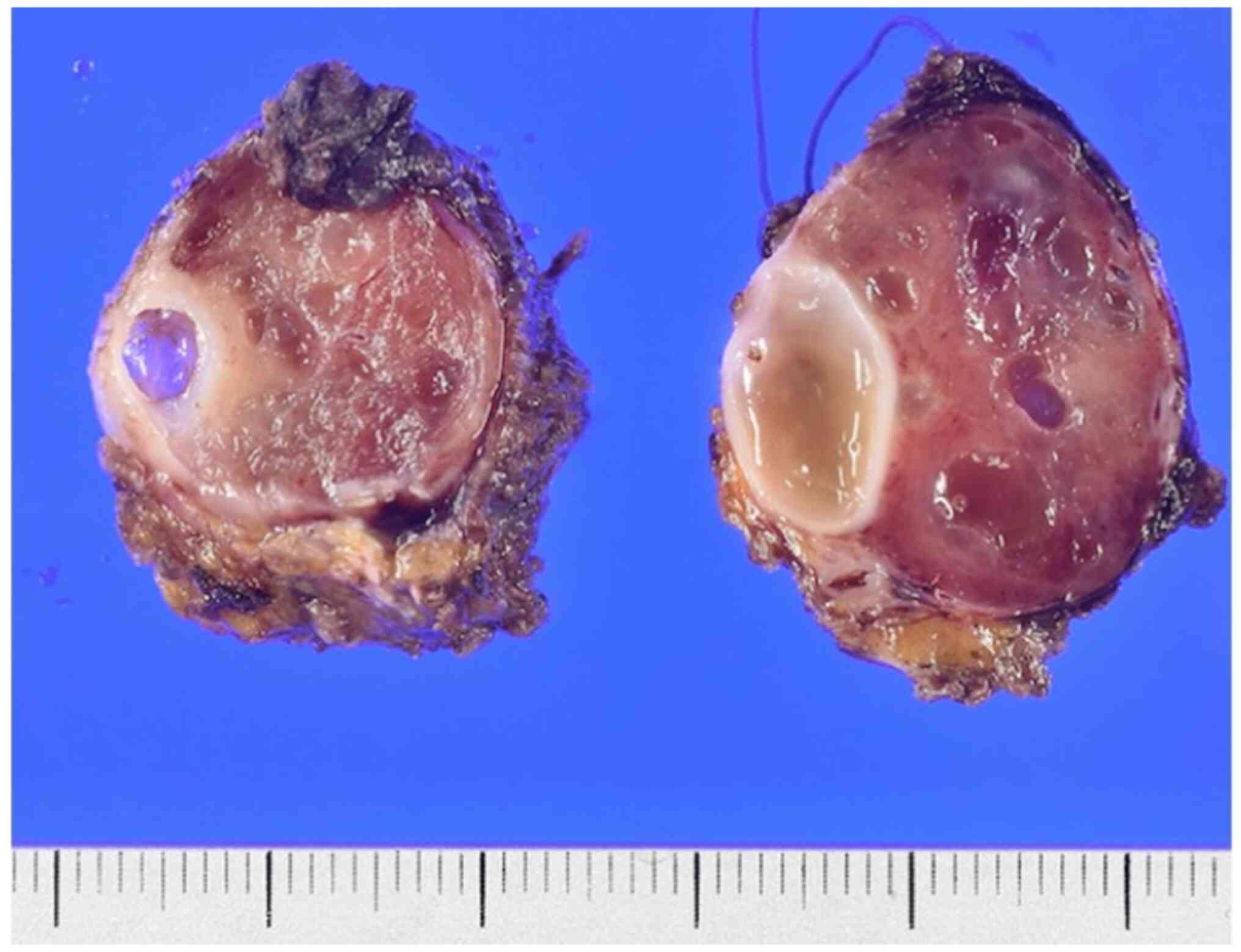
In the macroscopic examination, the tumor was a
brownish-colored solid mass and was 35×30×23 mm in diameter
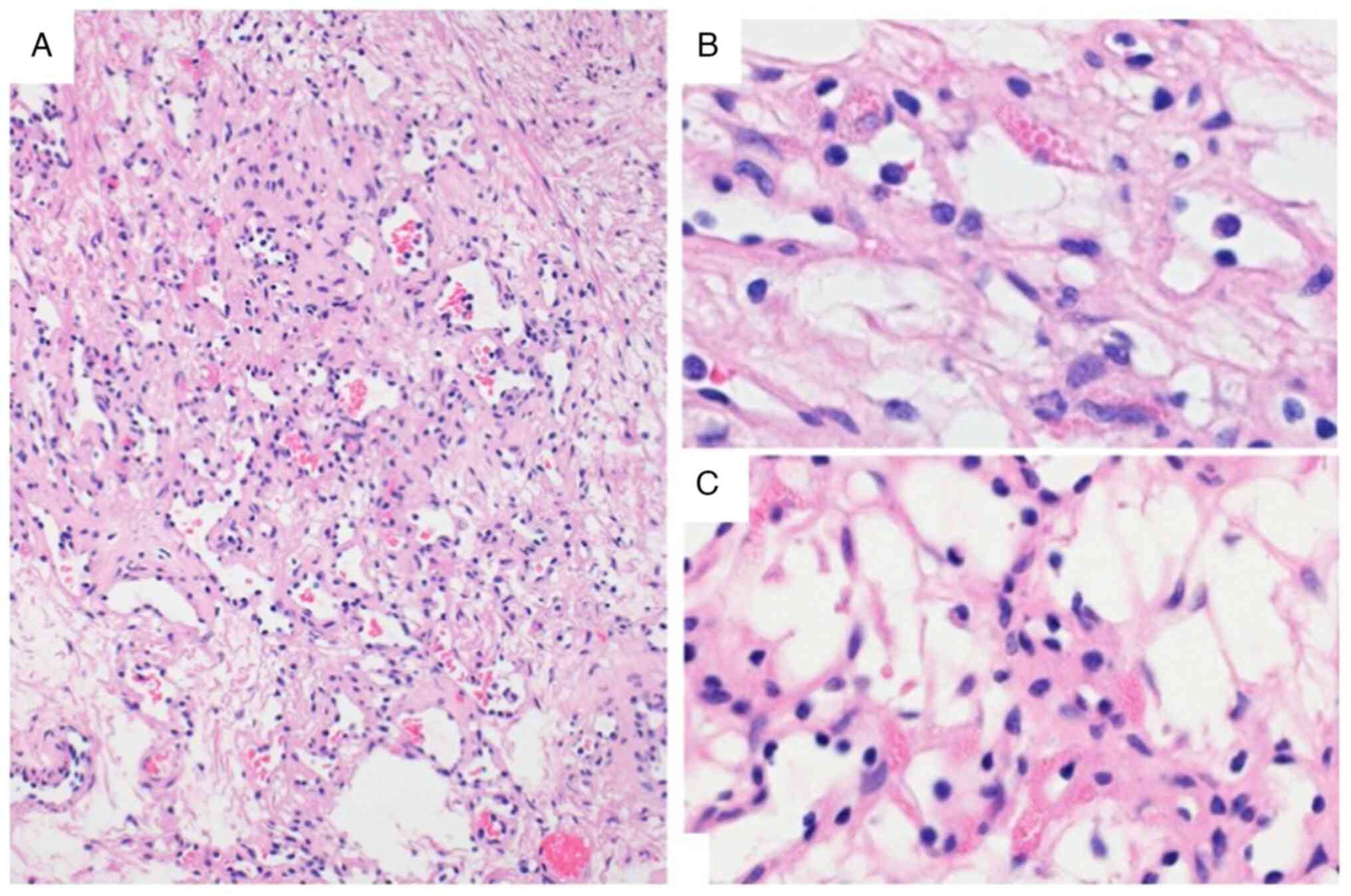
(Fig. 6). Microscopically, the
tumor was edematous and covered by a fibrous capsule and was
composed of an anastomosing proliferation of various-sized
capillary vessels that were lined with hobnail endothelial cells
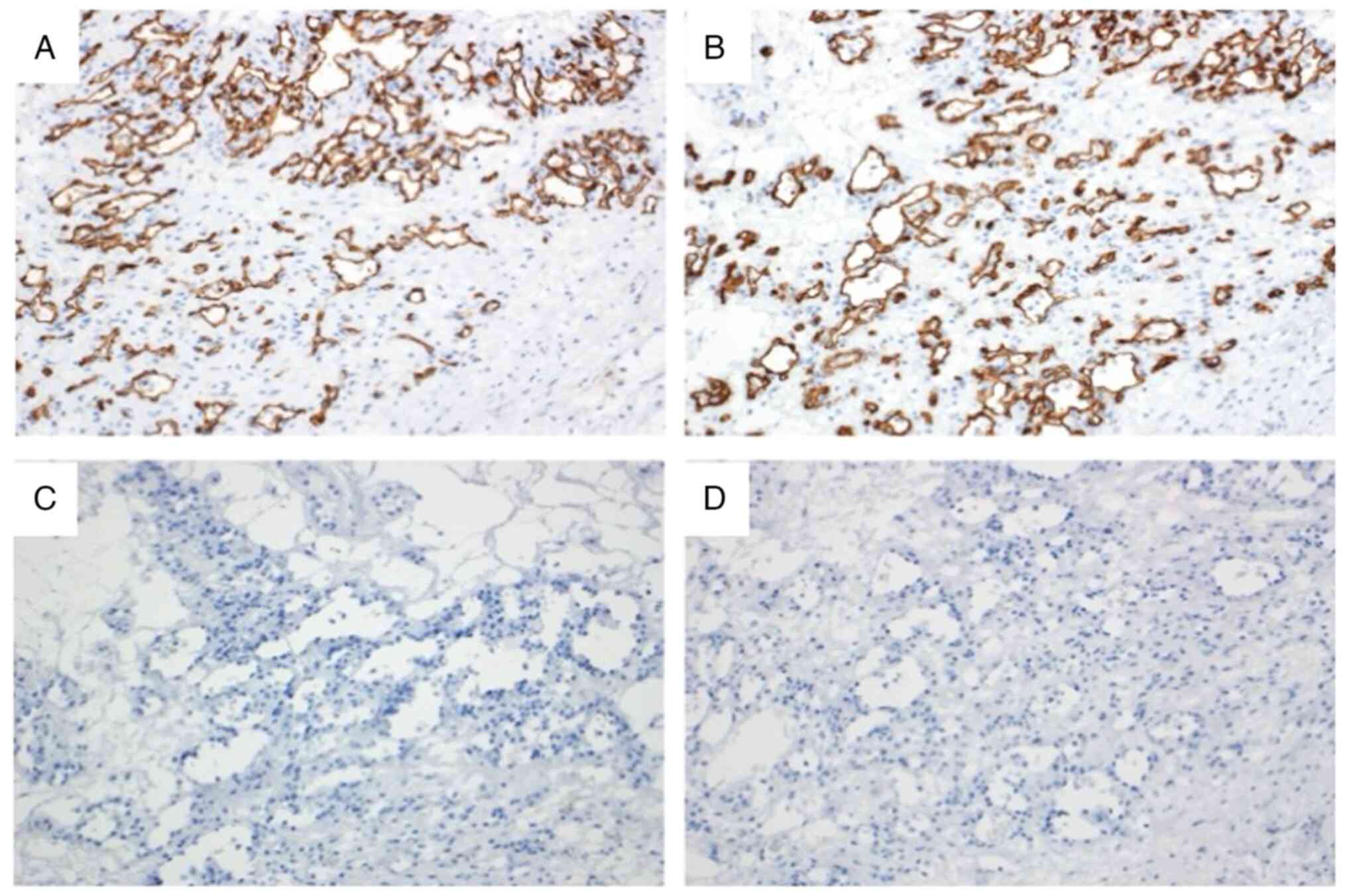
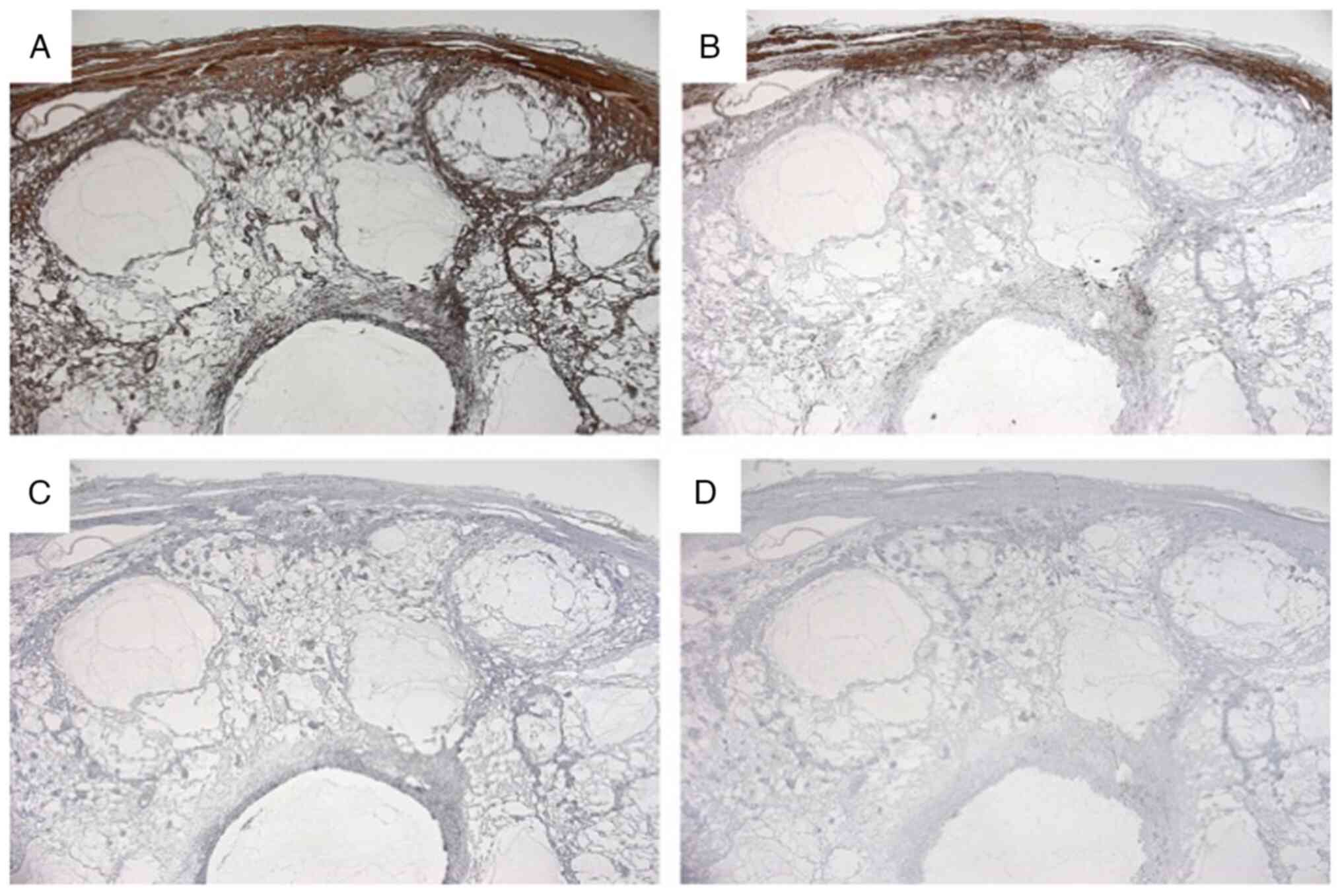
(Fig. 7). On immunohistochemical
examination, the cells that covered the capsule were positive for
CD31 and CD34 and negative for D2-40 (Fig. 8). The walls of the capsule and
stroma were positive for anti-alpha Smooth Muscle Actin, partially
positive for desmin. S100, and human melanoma black-45 and
Epithelial Membrane Antigen were negative (Fig. 9). Atypia and mitosis were not noted.
The histopathologic appearance and immunophenotypic features of the
tumor were indicative of a vascular tumor, and these
characteristics were consistent with a diagnosis of AH.
Discussion
Hemangiomas are conventionally classified into two
histological subtypes: cavernous and capillary. Most hemangiomas of
the liver, kidney, ad ovary reported to date have been classified
as benign hemangiomas of the cavernous type (6). Primary retroperitoneal tumors are
relatively rare, accounting for only 0.1 to 0.2% of all malignant
tumors in the body. However, 70 to 80% of these tumors are
malignant in nature (1,2). Among these, retroperitoneal
hemangiomas are extremely rare in adults, being identified in only
1 to 3% of all retroperitoneal tumors (7). The most common type of previously
reported retroperitoneal hemangioma is the cavernous type (8), and it was described as a round shaped
solid mass with minor or poor enhancement on enhanced CT scan
(7).
On the other hand, AH was first described by
Montgomery and Epstein in 2009 (3),
and the tumor is a new variant of capillary renal hemangioma, a
rare benign hemangioma that overlaps features of both sinusoidal
and hobnail hemangiomas of the skin and soft tissues. According to
the WHO Classification of tumors 5th edition for soft tissue and
bone tumors, AH is classified as a soft tissue vascular tumor
(4–6). Most cases of AH occur in the
retroperitoneum, especially the genitourinary tract. AH has also
been reported in the ovary, adrenal gland, liver, and
gastrointestinal tract (3,4). However, some AH arises in unusual
regions, such as the breast, skin, paravertebral region, and
para-aorta. To the best of our knowledge, only 6 cases of
para-aortic AHs have been reported (3,4)
AH is more common in middle-aged and slightly more
common in males. (9). Generally,
its diameter ranges from 0.1 to 6.0 cm (10). Zhang et al (11) reported a case of AH that progressed
slowly over a four-year observation period. In the present case,
the tumor also exhibited slow growth over a four-year period. AH
has no special clinical symptoms or laboratory findings, and it is
often found incidentally on imaging examination. However, the
imaging findings of AH are not specific and are similar to most
benign lesions. On noncontrast-enhanced CT, the AH showed lobular
lesions with soft-tissue attenuation, and on contrast-enhanced CT
showed heterogeneous solid lesions with persistent enhancement
(10,12). On noncontrast-enhanced MRI, the AH
presented as a round, well demarcated T1-hypointense and
T2-hyperintense lesion, while on contrast-enhanced MRI, it
presented with strong peripheral enhancement in the arterial phase,
which persisted in the delayed phase without central enhancement
(13). On the other hand, a
previous report (14) also showed
the different characteristics of their AH on MRI. In this report,
the lesions showed uniform enhancement both peripherally and
centrally in the arterial phase, which persisted in the delayed
phase. Either way, the enhancement pattern of MRI was similar to
that of CT, with clear heterogeneous enhancement in the arterial
phase and persistent hyperenhancement in the portal and delayed
phases (12,14,15).
In our case, the lesion showed heterogeneous septal enhancement in
the arterial phase and persisted peripherally and centrally in the
portal phase.
Most AHs are incidentally found and likely diagnosed
after surgical resection because of the difficulty of differential
diagnosis. To avoid surgery, biopsy was proposed (5). In our case, performing biopsy was
difficult because the tumor was located on the para-aorta and in
front of the vertebra. Therefore, we had no choice but to diagnose
using images and to operate if we wanted to confirm the
pathological features. The diagnosis of AH is basically based on
histopathological examination. Macroscopically, AH usually shows a
spongy neoplasm without capsule but with a clear boundary and a
mahogany-brown color (10,11,16).
Microscopically, AH is characterized by dense capillary vessels
lined with hobnailed endothelial cells, which resembles the red
pulp of the spleen in appearance, have extramedullary
hematopoiesis, and lack endothelial atypia (10,17).
Immunohistochemical staining was strongly and diffusely positive
for CD31, CD34, and EGR (5,17). It is important to note that mitotic
activity was absent, cellular atypia was no or only slight, and the
Ki-67 index was low (5,9,10).
It is important to differentiate AH from
angiosarcoma (18). Angiosarcoma is
a rare, invasive, malignant tumor, and it cannot be differentiated
from AH using radiological examinations. Histologically, it also
presents with hobnailed endothelial cells and can mimic AH
(9). However, angiosarcoma is
characterized by high-grade cell atypia, multiple layers of
endothelial cells, and obvious mitotic activity, none of which were
present in our case. Therefore, the present case was diagnosed as
AH from those characteristic histopathological findings. It is
difficult to make a definitive diagnosis as AH from preoperative
radiologic examinations, so it is controversial how to treat
AH.
When biopsy results are obtained, different
treatment modalities such as follow-up, embolization, or
radiofrequency ablation may be used depending on the location of
the lesion, size of the lesion, and presence of symptoms, and local
or radical resection may be performed to avoid overtreatment.
Previous studies have shown no tendency for disease recurrence
(7,9). However, we must be concerned about the
risk of bleeding and safety. Patients should not be disadvantaged
by biopsies.
There are limited imaging data available for AH, and
when available, it is typically described as having nonspecific
features. In addition, imaging may vary according to the location
and size of the tumor (6). However,
we should rule it out in the differential diagnosis of
retroperitoneal vascular tumors.
Robot-assisted (RA) surgery is becoming a popular
and effective approach in the treatment of retroperitoneal tumors
(19). In a previous report,
conventional surgery had the shortest operation time but the
greatest amount of blood loss. The median duration of postoperative
drainage, morbidity rate, and postoperative length of stay were
lower after RA approaches. The RA significantly reduces risks in
cases when the tumor is in hard-to-reach small spaces and/or
attached to the main vessels and when the size of the tumor is less
than 10 cm. In the future, we will try RA approaches for resecting
retroperitoneal tumors.
We describe a rare hemangioma variant in the
para-aortic region that showed an anastomosing pattern of vascular
channels on pathological examination. However, AH may be included
in the differential diagnosis when a slowly progressing
heterogeneous mass appears in the para-aortic region that exhibits
a CT-enhanced pattern similar to a typical cavernous
hemangioma.
Acknowledgements
The authors would like to thank Dr Taizen Urahashi
(Saitama Medical Center, Kawagoe, Saitama, Japan) for their advice
on this article.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HI, HT, SM, MT, TT, KK, TO and HY participated in
the conception, design and data acquisition of the study. TO and HY
performed data analysis and interpretation. HI wrote the
manuscript, and completed the follow-up. YO and SB performed the
pathological assessment of the anastomosing hemangioma and wrote
the manuscript. HT revised the manuscript. HT, TO and HY confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from this
patient in accordance with the ethical principles of the 1964
Declaration of Helsinki and its subsequent amendments.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this report and its accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AH
|
anastomosing hemangioma
|
|
RA
|
robot-assisted
|
References
|
1
|
Xu YH, Guo KJ, Guo RX, Ge CL, Tian YL and
He SG: Surgical management of 143 patients with adult primary
retroperitoneal tumor. World J Gastroenterol. 13:2619–2621. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishino M, Hayakawa K, Minami M, Yamamoto
A, Ueda H and Takasu K: Primary retroperitoneal neoplasms: CT and
MR imaging findings with anatomic and pathologic diagnostic clues.
Radiographics. 23:45–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montgomery E and Epstein JI: Anastomosing
hemangioma of the genitourinary tract: A lesion mimicking
angiosarcoma. Am J Surg Pathol. 33:1364–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
John I and Folpe AL: Anastomosing
hemangiomas arising in unusual locations: A clinicopathologic study
of 17 soft tissue cases showing a predilection for the paraspinal
region. Am J Surg Pathol. 40:1084–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Neill AC, Craig JW, Silverman SG and
Alencar RO: Anastomosing hemangiomas: Locations of occurrence,
imaging features, and diagnosis with percutaneous biopsy. Abdom
Radiol (NY). 41:1325–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kryvenko ON, Gupta NS, Meier FA, Lee MW
and Epstein JI: Anastomosing hemangioma of the genitourinary
system: Eight cases in the kidney and ovary with
immunohistochemical and ultrastructural analysis. Am J Clin Pathol.
136:450–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanaoka M, Hashimoto M, Sasaki K, Matsuda
M, Fujii T, Ohashi K and Watanabe G: Retroperitoneal cavernous
hemangioma resected by a pylorus preserving
pancreaticoduodenectomy. World J Gastroenterol. 19:4624–4629. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godar M, Yuan Q, Shakya R, Xia Y and Zhang
P: Mixed capillary venous retroperitoneal hemangioma. Case Rep
Radiol. 2013:2583522013.PubMed/NCBI
|
|
9
|
Omiyale AO: Anastomosing hemangioma of the
kidney: A literature review of a rare morphological variant of
hemangioma. Ann Transl Med. 3:1512015.PubMed/NCBI
|
|
10
|
Tao LL, Dai Y, Yin W and Chen J: A case
report of a renal anastomosing hemangioma and a literature review:
An unusual variant histologically mimicking angiosarcoma. Diagn
Pathol. 9:1592014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Wang Q, Liu YL, Yu WJ, Liu Y,
Zhao H, Zhuang J, Jiang YX and Li YJ: Anastomosing hemangioma
arising from the kidney: A case of slow progression in four years
and review of literature. Int J Clin Exp Pathol. 8:2208–2213.
2015.PubMed/NCBI
|
|
12
|
Silva MA, Fonseca EKUN, Yamauchi FI and
Baroni RH: Anastomosing hemangioma simulating renal cell carcinoma.
Int Braz J Urol. 43:987–989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng X, Li J and Liang Z: Anastomosing
haemangioma of liver: A case report. Mol Clin Oncol. 7:507–509.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Merritt B, Behr S, Umetsu SE, Roberts J
and Kolli KP: Anastomosing hemangioma of liver. J Radiol Case Rep.
13:32–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue X, Song M, Xiao W, Chen F and Huang Q:
Imaging findings of retroperitoneal anastomosing hemangioma: A case
report and literature review. BMC Urol. 22:77–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Maghrabi HA and Al Rashed AS:
Challenging pitfalls and mimickers in diagnosing anastomosing
capillary hemangioma of the kidney: Case report and literature
review. Am J Case Rep. 18:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheon PM, Rebello R, Naqvi A, Popovic S,
Bonert M and Kapoor A: Anastomosing hemangioma of the kidney:
Radiologic and pathologic distinctions of a kidney cancer mimic.
Curr Oncol. 25:e220–e223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heidegger I, Pichler R, Schäfer G, Zelger
B, Zelger B, Aigner F, Bektic J and Horninger W: Long-term follow
up of renal anastomosing hemangioma mimicking renal angiosarcoma.
Int J Urol. 21:836–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berelavichus S, Kriger A, Kaldarov A,
Panteleev V and Raevskaya M: Robotic surgery in treatment of
retroperitoneal tumors. Comparative single center study. J Robot
Surg. 15:363–367. 2021. View Article : Google Scholar : PubMed/NCBI
|