Introduction
Breast cancer is the most common type of cancer in
women; one in 9–12 women will develop breast cancer in their
lifetime in developed countries. Up to 10% of all breast cancer
cases are inherited, and BRCA1 and BRCA2 gene
mutations account for the majority of families with inherited
breast cancer (1).
Individuals with a mutation in either BRCA1
or BRCA2 genes have a significantly increased lifetime risk
of breast and ovarian cancer (2–4).
Olaparib, a poly ADP-ribose polymerase (PARP) inhibitor, was
approved by the US Food and Drug Administration in 2014 for the
treatment of BRCA-positive advanced ovarian cancer and by
the National Medical Products Administration in 2018. There are few
reports of germline mutations in both BRCA1 and BRCA2
(5–8). Carriers with inherited deleterious
mutations in both BRCA1 and BRCA2 only account for
0.3% of all female BRCA1/2 mutation carriers (5) and for 2.2% of breast cancer BRCA1/2
mutation carriers (6). Previous
case reports have described such breast cancer patients but there
is a lack of reports on the efficacy of olaparib on them (7,8).
Similarly, clinical trials of olaparib have recruited such patients
with cancer but lacked specific description of efficacy (9,10). A
previous study reported that a patient with BRCA1 and
BRCA2 double-germline mutant gastric cancer was resistant to
olaparib treatment (11). To the
best of our knowledge, however, there are no reports on the
efficacy of olaparib in Chinese patients with breast cancer with
mutations in both BRCA1 and BRCA2.
Case report
The proband, a 56-year-old woman, underwent a
modified radical mastectomy for left breast cancer in May 2004. The
pathological diagnosis was medullary carcinoma, pT2N0M0, stage IIA,
estrogen receptor (ER)−, progesterone receptor
(PR)− and human epidermal growth factor receptor
(HER2)1+. The patient received four cycles of
adriamycin/cyclophosphamide (dose unknown) regimen. Right breast
cancer was diagnosed during the routine follow-up examination and a
modified radical mastectomy was performed in May 2016. The
pathological diagnosis was invasive ductal carcinoma, pT1N2M0,
stage IIIA, ER+++, PR−, HER21+ and
Ki-67 (30%). Germline or somatic mutations were not identified at
this time. Based on clinical pathology, chemotherapy with
epirubicin (90 mg/m2)/cyclophosphamide (600
mg/m2) for four cycles with weekly paclitaxel (80
mg/m2) for 12 weeks was performed from August 2016 to
July 2017, followed by 5,000 cGy/25f radiotherapy targeting the
chest wall and supraclavicular region. Letrozole (2.5 mg QD) was
administered until multiple tumor metastases were detected in bone,
liver and lymph nodes (left cervical lymph nodes, clavicular area
and internal mammary, right hilar lymph nodes and mediastinal, lung
and liver hilar and retroperitoneal lymph nodes) by PET/CT in
October 2018. The proband refused biopsy of the metastatic sites.
The proband had multiple liver metastases with a maximum metastatic
focus 3.1×2.6 cm. The tumor burden was high with multiple liver,
lymph node and bone metastases. Due to primary adjuvant endocrine
resistance and palbociclib, the only available CDK4/6 inhibitor in
China at that time (October 2018), was not affordable for the
patient, the patient received combination chemotherapy.
Chemotherapy with abraxane (125 mg/m2 d1,d8 q21d)
combined with capecitabine (1 g/m2 d1-14 q21d) was
initiated but the disease continued to progress in the liver after
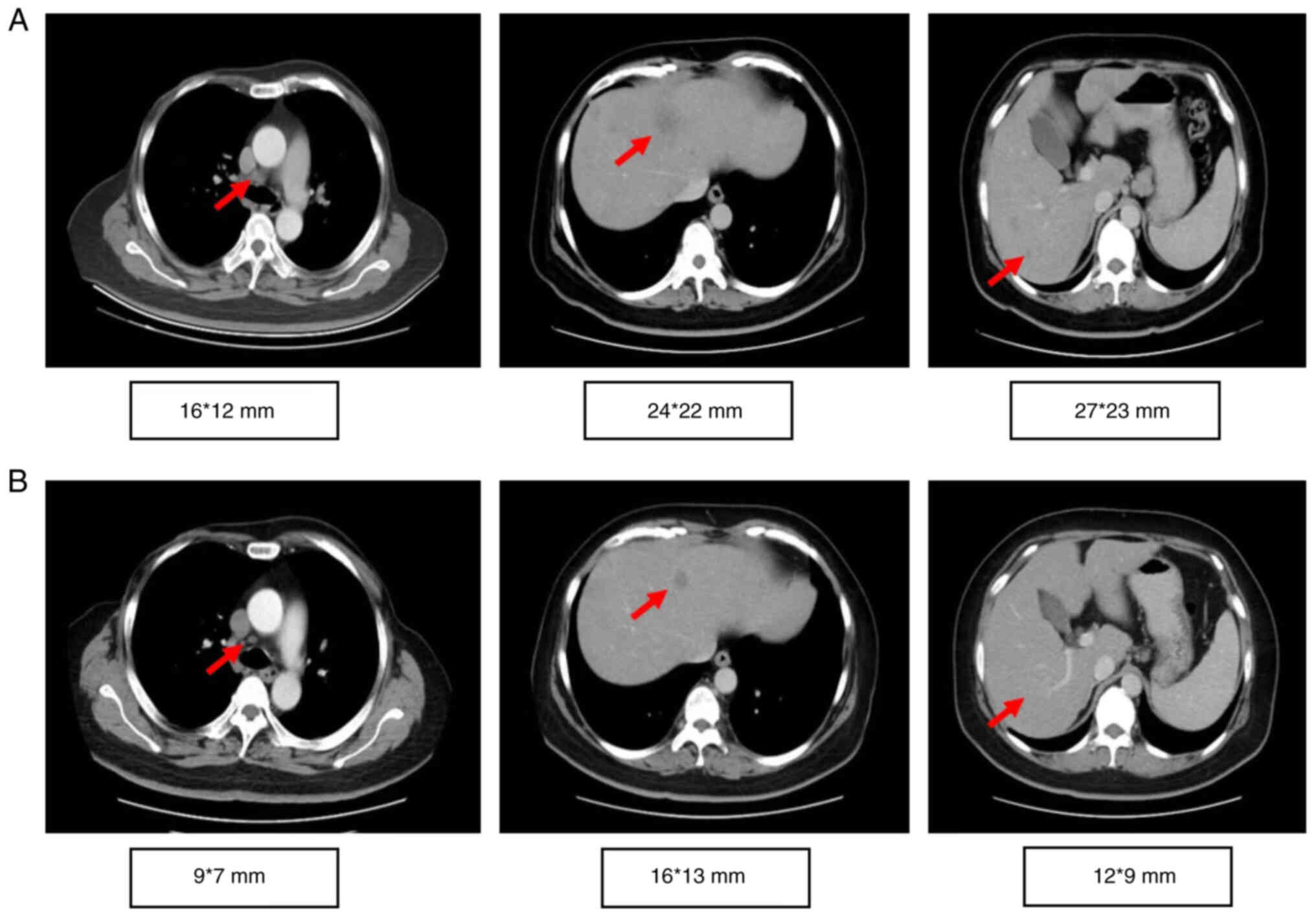
two cycles (Fig. 1). The timeline
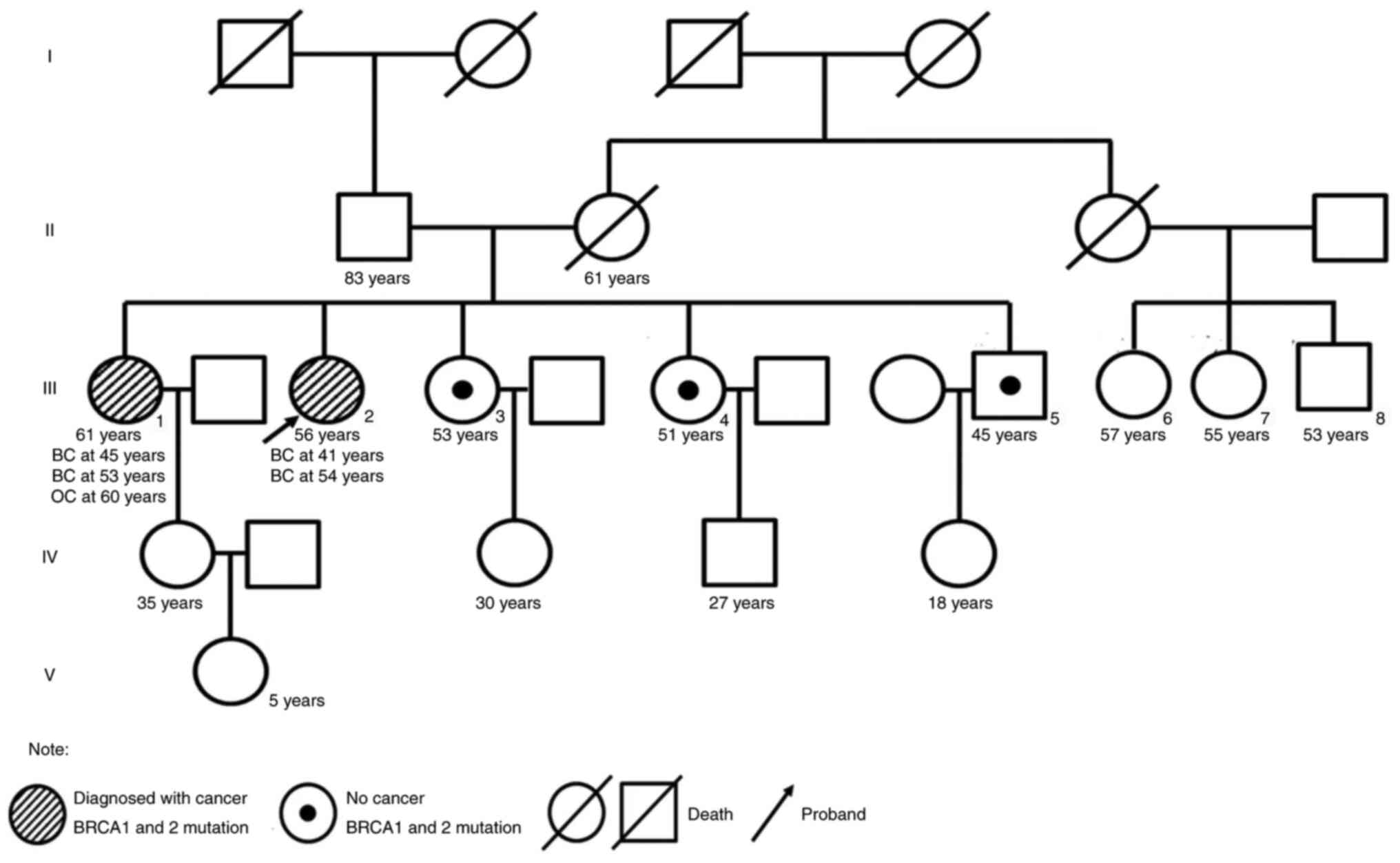
of her past medical history is outlined in Table I. The patient had a family history
of breast and ovarian cancer (Fig.
2). Targeted next-generation sequencing testing with a panel of
18 cancer genetic susceptibility genes was performed. Genomic DNA
(gDNA) was extracted from paraffin-embedded section of the primary
tumor with AllPrep DNA/RNA mini Kit (Qiagen 80204). The libraries
of gDNA were constructed with a KAPA Hyper Prep kit (Kapa
Biosystems, USA) according to the operation manual. gDNA libraries
were enriched through a panel of 18 cancer genetic susceptibility
genes with its custom-designed capture probes were manufactured by
Agilent, USA. P5/P7 primers were adopted to amplify the enriched
gDNA libraries. The amplified libraries were qualified by the 2200
Bioanalyzer (Agilent Technologies, Palo Alto, Calif) and quantified
by the qBittorrent (version 3). Paired-end sequencing with reads
length of 250 bp on the Hiseq X Ten platform (Illumina, San Diego,
CA) using MiSeq Reagent Kit v3 (MS-102-3001, Illumina, USA) and the
loading concentration was 4 pM. The NGS raw data was initially
analyzed with trimmomatic-0.36. Reads were then aligned against
human reference genome (version GRCh37/hg19) with bwa (version
0.7.10). Candidate somatic mutations were determined using Samtools
(version 1.3.1) and pindel (version 0.2.5b8). Finally, filter
alignment and sequencing artifacts were conducted using IGV
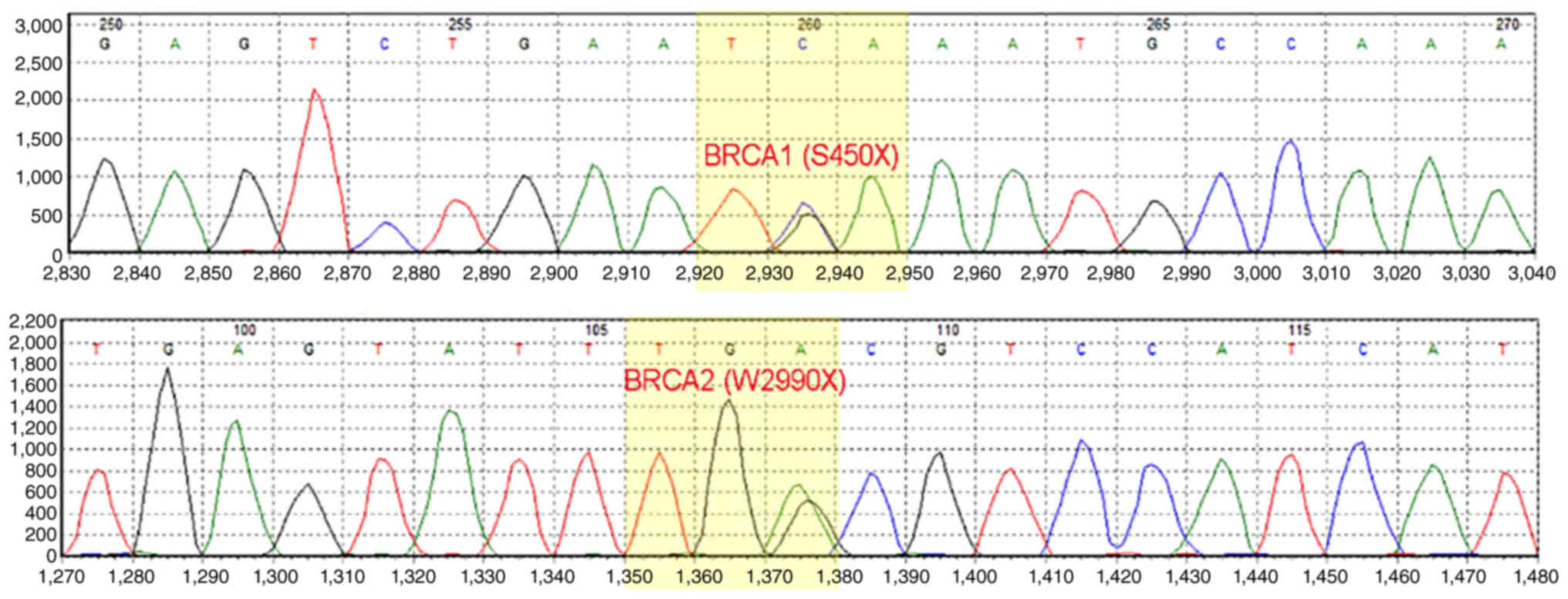
(Integrative Genomics Viewer). Double deleterious germline
mutations in both BRCA1 (S405X) and BRCA2 (W2990X)
were identified (Fig. 3). On the
basis of these mutations, the proband started olaparib treatment
(600 mg BID) in January 2019. The most severe adverse event was
anemia (grade 3), which occurred 4 months after the start of the
treatment. Other side effects, including leukopenia,
thrombocytopenia, loss of appetite and nausea were mild and well
tolerated. To alleviate symptoms of anemia, olaparib treatment was
suspended and the proband received erythropoietin (10,000 IU 3
times/week) and ferrous succinate tablets (0.1 g 3 times/day).
Olaparib treatment was resumed at a decreased dosage (450 mg/day)
when anemia was reduced to grade 2 after 1 week of the treatment.
Grade 3 anemia reoccurred after 2 weeks of decreased dosage
olaparib, thus olaparib was suspended, and erythropoietin and
ferrous succinate tablets at the aforementioned dosage were
administered to relieve the anemia. Subsequently, olaparib
treatment was resumed with further decreased dosage (300 mg/day).
Grade 3 anemia reoccurred and olaparib treatment was terminated in
July 2019. The olaparib treatment intermittently lasted for a total
of 6 months and CT imaging showed a partial response (35%
reduction; Fig. 1A and B). The
proband did not receive chemotherapy or palbociclib because of
persistent grade 3 anemia and the prohibitive cost of palbociclib.
The proband received oral exemestane (25 mg qd). The patient
continued treatment elsewhere and did not disclose her condition
during a follow-up telephone conversation 3 months later.
 | Table I.Timeline of the patient's medical
history. |
Table I.
Timeline of the patient's medical
history.
| Date | Therapy | Regimen | Response |
|---|
| May 2004 | Modified radical
mastectomy for left breast cancer |
|
|
| June-September
2004 | Adjuvant
chemotherapy | Adriamycin 60
mg/m2, cyclophosphamide 750 mg/m2) | NA |
| May 2016 | Modified radical
mastectomy of the right breast cancer |
|
|
| August 2016-July
2017 | Adjuvant
chemotherapy | Epirubicin (90
mg/m2)/cyclophosphamide (600 mg/m2) 4 cycles
followed by weekly paclitaxel (80 mg/m2) for 12
weeks |
|
| July-August
2017 | Adjuvant
radiotherapy of the chest wall and supraclavicular region | 5,000 cGy/25
fractions |
|
| August 2017-October
2018 | Adjuvant endocrine
therapy | Letrozole |
|
| October-December
2018 | First-line
chemotherapy | Albumin-bound
paclitaxel + capecitabine | Progressive
disease |
| January-June
2019 | Second-line
therapy | Olaparib | Partial
response |
The family members of the proband received genetic
counseling and underwent NGS of BRCA genes. The same
germline mutations in both BRCA1 and BRCA2 were
identified in the proband's elder sister who was diagnosed with
bilateral breast cancer at the age of 43 in 2005. Clinical
information was not available. The proband's two younger sisters
were carriers of the same BRCA1 mutation and her younger
brother was a carrier of the same BRCA2 mutation. The
offspring of the proband and their siblings declined genetic
testing. To the best of our knowledge, no other family member has
reported cancer symptoms to date.
Discussion
Hereditary breast/ovarian cancer syndrome,
accounting for ~10% of breast and 15% of ovarian cancer cases, is
often associated with germline mutations in BRCA1 or
BRCA2 (3,12), which are present in 0.1–2.17% of the
population worldwide (13–18). Due to the high risk of developing
breast (up to 87%) and/or ovarian cancer (up to 63%),
identification of carriers and surveillance is key to successful
clinical management (19).
The frequency of double germline mutations in both
BRCA1 and BRCA2 is only 0.3% in female BRCA1/2
mutation carriers (5), which
accounts for 0.64–1.80% of BRCA1 (20) and 0.53–0.87% of BRCA2
deleterious mutations (6,21–26).
Palmirotta et al (27)
summarized worldwide literature on double mutations in BRCA1
and BRCA2 from 1998 to 2017, in which Jewish Ashkenazi
patients were deliberately excluded due to high mutation frequency
of BRCA1 and BRCA2. Only 34 families with 56 subjects (34 probands
and 22 relatives) carrying double mutations of BRCA1 and
BRCA2 in 20 articles were reported (27). It is unclear whether double
mutations increase cancer risk. In the present study, two
individuals (the proband and their elder sister) with the double
mutations exhibited cancer symptoms while the single mutation
carriers remained healthy, indicating a potential higher risk of
cancer in double mutation carriers.
The characteristics of individuals with clinically
significant double heterozygosity for BRCA1 and BRCA2
are poorly understood (28).
Bilateral breast cancer, a characteristic of familial breast
cancer, was present in both the proband and their elder sister in
the present case report. Furthermore, previous studies have
suggested that patients with both BRCA1 and BRCA2 are
more susceptible to gastric cancer, pancreatic cancer, uterine
cancer and prostate cancer in addition to breast cancer and ovarian
cancer (29,30). Palmirotta et al (27) reported that the phenotype of 56
cases with double heterozygosity varied from unilateral breast
cancer at age 26 to asymptomatic at age 72. A total of 42 cases
(75%) had a primary tumor, including 35 patients with breast, two
each with ovarian and prostate, and one each with cervix, caecum
and stomach cancer; 14 of these cases suffered secondary neoplasia
(27).
Notably, two pathologically different breast tumors
occurred in the present proband (left triple negative medullary
carcinoma, and right invasive ductal breast cancer with
ER+++, PR− and HER21+). More
extensive sequencing on tumor somatic genetic alterations may
reveal differences in tumorigenesis as well as additional potential
therapeutic targets.
Treatment of BRCA-mutated metastatic breast
cancer with olaparib is recommended by the Chinese Society of
Clinical Oncology (CSCO) Breast Cancer Guidelines (31) based on the clinical data showing
slower spread of hereditary breast cancer with olaparib treatment
(32). To the best of our
knowledge, however, the response of metastatic breast cancer with
double heterozygous BRCA1 and BRCA2 mutations to
olaparib has not been described previously. To the best of our
knowledge, the present study is the first report demonstrating a
partial response to olaparib in such patients. Platinum is also an
effective treatment for metastatic breast cancer with BRCA
mutations (33). There are also
other potential options for patients with hormone
receptor-positive, HER2 negative breast cancer including
CDK4/6 inhibitors and immunotherapy (34).
Since BRCA1 and BRCA2 genes are
located on 13q and 17q respectively, each gene has the same
probability (50%) of transmitting to the next generation (35). Genetic counseling is necessary in
the medical care of familial breast cancer.
Family history and genetic counseling are key for
the clinical management of patients with germline BRCA1/2 mutations
(36). With the rapid development
of treatment options, genetic screening for both germline and
somatic mutations of BRCA is key for making clinical decisions,
including type of surgery, the consideration of radiotherapy, and
the value of systemic therapy in neoadjuvant and advanced settings
(including response to platinum-based chemotherapy and PARP
inhibitors) (37,38). The phase III trial OlympiAD has
shown that compared with standard therapy, median progression-free
survival (PFS) is 2.8 months longer and risk of disease progression
or death is 42% lower with olaparib monotherapy in
BRCA-mutated metastatic breast cancer (32). Thus, olaparib is a treatment option
for metastatic breast cancer with germline mutations of
BRCA1 and/or BRCA2 (38).
The present case described a 56-year-old female
patient with rare BRCA1 and BRCA2 double germline-mutant metastatic
breast cancer. The patient had a family history of breast and
ovarian cancer. The patient exhibited primary resistance to both
letrozole and albumin-bound paclitaxel combined with capecitabine.
NGS showed germline mutations of both BRCA1 and
BRCA2, and the patient received olaparib monotherapy. The
patient achieved a partial response and PFS was 6 months, although
they discontinued olaparib treatment due to continuous grade 3
anemia. Genetic testing is key to determine optimal treatment for
breast cancer. In conclusion, olaparib exhibited therapeutic
potential for a patient with BRCA1 and BRCA2 double germline-mutant
metastatic breast cancer.
Acknowledgements
The authors would like thank Dr Yue Zhang (Genetron
Health Beijing, China) for their constructive suggestions regarding
the writing and revision of the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the GSA-human repository, https://ngdc.cncb.ac.cn/gsa-human/s/30mKWWj6.
Authors' contributions
BS and LD confirm the authenticity of all the raw
data. BS and LD conceived and designed the study. BS collected
cases, analyzed data analysis. BS wrote the manuscript. BS and LD
revised the content. LD was responsible for treating the patient.
Both authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The patient and her relatives took part in the
program ‘Genetic test of solid tumor, circulating tumor DNA and
genetic diseases’, which was approved by the ethics committee of
Beijing Cancer Hospital (Beijing, China; approval no.
2016XJS01-ZY01). All participants provided written informed consent
to participate.
Patient consent for publication
Written informed consent was obtained from the
patient and her family members for publication of this case report
and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
PARP
|
poly ADP-ribose polymerase
|
|
PFS
|
progression-free survival
|
References
|
1
|
Stenehjem DD, Telford C, Unni SK, Bauer H,
Sainski A, Deka R, Schauerhamer MB, Ye X, Tak CR, Ma J, et al: BRCA
testing and outcomes in women with breast cancer. Breast Cancer Res
Treat. 186:839–850. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ford D, Easton DF, Bishop DT, Narod SA and
Goldgar DE: Risks of cancer in BRCA1-mutation carriers. Breast
cancer linkage consortium. Lancet. 343:692–695. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miki Y, Swensen J, Shattuck-Eidens D,
Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM,
Ding W, et al: A strong candidate for the breast and ovarian cancer
susceptibility gene BRCA1. Science. 266:66–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wooster R, Neuhausen SL, Mangion J, Quirk
Y, Ford D, Collins N, Nguyen K, Seal S, Tran T, Averill D, et al:
Localization of a breast cancer susceptibility gene, BRCA2, to
chromosome 13q12-13. Science. 265:2088–2090. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rebbeck TR, Friebel TM, Mitra N, Wan F,
Chen S, Andrulis IL, Apostolou P, Arnold N, Arun BK, Barrowdale D,
et al: Inheritance of deleterious mutations at both BRCA1 and BRCA2
in an international sample of 32,295 women. Breast Cancer Res.
18:1122016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavie O, Narod S, Lejbkowicz F, Dishon S,
Goldberg Y, Gemer O and Rennert G: Double heterozygosity in the
BRCA1 and BRCA2 genes in the Jewish population. Ann Oncol.
22:964–966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meynard G, Mansi L, Lebahar P, Villanueva
C, Klajer E, Calcagno F, Vivalta A, Chaix M, Collonge-Rame MA,
Populaire C, et al: First description of a double heterozygosity
for BRCA1 and BRCA2 pathogenic variants in a French metastatic
breast cancer patient: A case report. Oncol Rep. 37:1573–1578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedman E, Bar-Sade Bruchim R, Kruglikova
A, Risel S, Levy-Lahad E, Halle D, Bar-On E, Gershoni-Baruch R,
Dagan E, Kepten I, et al: Double heterozygotes for the Ashkenazi
founder mutations in BRCA1 and BRCA2 genes. Am J Hum Genet.
63:1224–1227. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed. Olaparib for metastatic
breast cancer in patients with a germline BRCA mutation. N Engl J
Med. 377:17002017. View Article : Google Scholar
|
|
10
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen L, Li X, Shi J, Zhang S, Wang R, Yao M
and Guo J: Allele-specific expression mediates primary resistance
to poly (ADP-ribose) polymerase inhibitor therapy in a case of
BRCA1/2 double-germline mutant gastric cancer. J Int Med Res.
48:3000605198862262020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wooster R, Bignell G, Lancaster J, Swift
S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C and Micklem G:
Identification of the breast cancer susceptibility gene BRCA2.
Nature. 378:789–792. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei H, Zhang M, Zhang L, Hemminki K, Wang
XJ and Chen T: Overview on population screening for carriers with
germline BRCA mutation in China. Front Oncol. 12:10023602022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernández-Lopez JC, Romero-Córdoba S,
Rebollar-Vega R, Alfaro-Ruiz LA, Jiménez-Morales S, Beltrán-Anaya
F, Arellano-Llamas R, Cedro-Tanda A, Rios-Romero M,
Ramirez-Florencio M, et al: Population and breast cancer patients'
analysis reveals the diversity of genomic variation of the BRCA
genes in the Mexican population. Hum Genomics. 13:32019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gabai-Kapara E, Lahad A, Kaufman B,
Friedman E, Segev S, Renbaum P, Beeri R, Gal M, Grinshpun-Cohen J,
Djemal K, et al: Population-based screening for breast and ovarian
cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA.
111:14205–14210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manickam K, Buchanan AH, Schwartz MLB,
Hallquist MLG, Williams JL, Rahm AK, Rocha H, Savatt JM, Evans AE,
Butry LM, et al: Exome sequencing-based screening for BRCA1/2
expected pathogenic variants among adult biobank participants. JAMA
Netw Open. 1:e1821402018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Momozawa Y, Iwasaki Y, Parsons MT,
Kamatani Y, Takahashi A, Tamura C, Katagiri T, Yoshida T, Nakamura
S, Sugano K, et al: Germline pathogenic variants of 11 breast
cancer genes in 7,051 Japanese patients and 11,241 controls. Nat
Commun. 9:40832018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maxwell KN, Domchek SM, Nathanson KL and
Robson ME: Population frequency of germline BRCA1/2 mutations. J
Clin Oncol. 34:4183–4185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith A, Moran A, Boyd MC, Bulman M,
Shenton A, Smith L, Iddenden R, Woodward ER, Lalloo F, Maher ER and
Evans DGR: Phenocopies in BRCA1 and BRCA2 families: Evidence for
modifier genes and implications for screening. J Med Genet.
44:10–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frank TS, Deffenbaugh AM, Reid JE, Hulick
M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV,
Pruss DR and Critchfield GC: Clinical characteristics of
individuals with germline mutations in BRCA1 and BRCA2: Analysis of
10,000 individuals. J Clin Oncol. 20:1480–1490. 2022. View Article : Google Scholar
|
|
21
|
Ramus SJ, Friedman LS, Gayther SA, Ponder
BA, Bobrow L, van der Looji M, Papp J and Olah E: A breast/ovarian
cancer patient with germline mutations in both BRCA1 and BRCA2. Nat
Genet. 15:14–15. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liede A, Rehal P, Vesprini D, Jack E,
Abrahamson J and Narod SA: A breast cancer patient of Scottish
descent with germ-line mutations in BRCA1 and BRCA2. Am J Hum
Genet. 62:1543–1544. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caldes T, de la Hoya M, Tosar A, Sulleiro
S, Godino J, Ibañez D, Martin M, Perez-Segura P and Diaz-Rubio E: A
breast cancer family from Spain with germline mutations in both the
BRCA1 and BRCA2 genes. J Med Genet. 39:e442002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bell DW, Erban J, Sgroi DC and Haber DA:
Selective loss of heterozygosity in multiple breast cancers from a
carrier of mutations in both BRCA1 and BRCA2. Cancer Res.
62:2741–2743. 2002.PubMed/NCBI
|
|
25
|
Claus EB, Petruzella S, Matloff E and
Carter D: Prevalence of BRCA1 and BRCA2 mutations in women
diagnosed with ductal carcinoma in situ. JAMA. 293:964–969. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuradelli M, Peissel B, Manoukian S,
Zaffaroni D, Barile M, Pensotti V, Cavallari U, Masci G, Mariette
F, Benski AC, et al: Four new cases of double heterozygosity for
BRCA1 and BRCA2 gene mutations: Clinical, pathological, and family
characteristics. Breast Cancer Res Treat. 124:251–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palmirotta R, Lovero D, Stucci LS,
Silvestris E, Quaresmini D, Cardascia A and Silvestris F: Double
heterozygosity for BRCA1 pathogenic variant and BRCA2 polymorphic
stop codon K3326X: A case report in a Southern Italian family. Int
J Mol Sci. 19:2852018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugano K, Nakamura S, Ando J, Takayama S,
Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, et
al: Cross-sectional analysis of germline BRCA1 and BRCA2 mutations
in Japanese patients suspected to have hereditary breast/ovarian
cancer. Cancer Sci. 99:1967–1976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nomizu T, Sakuma T, Yamada M, Matsuzaki M,
Katagata N, Watanabe F, Nihei M, Ishioka C, Takenoshita S and Abe
R: Three cases of kindred with familial breast cancer in which
carrier detection by BRCA gene testing was performed on family
members. Breast Cancer. 19:270–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tobalina L, Armenia J, Irving E, O'Connor
MJ and Forment JV: A meta-analysis of reversion mutations in BRCA
genes identifies signatures of DNA end-joining repair mechanisms
driving therapy resistance. Ann Oncol. 32:103–112. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Z, Li J, Chen J, Liu Y, Wang K, Nie
J, Wang X, Hao C, Yin Y, Wang S, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer
Res. 3:132022. View Article : Google Scholar
|
|
32
|
Robson ME, Tung N, Conte P, Im SA, Senkus
E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al: OlympiAD
final overall survival and tolerability results: Olaparib versus
chemotherapy treatment of physician's choice in patients with a
germline BRCA mutation and HER2-negative metastatic breast cancer.
Ann Oncol. 30:558–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tutt A, Tovey H, Cheang MCU, Kernaghan S,
Kilburn L, Gazinska P, Owen J, Abraham J, Barrett S, Barrett-Lee P,
et al: Carboplatin in BRCA1/2-mutated and triple-negative breast
cancer BRCAness subgroups: The TNT trial. Nat Med. 24:628–637.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spring LM, Wander SA, Andre F, Moy B,
Turner NC and Bardia A: Cyclin-dependent kinase 4 and 6 inhibitors
for hormone receptor-positive breast cancer: past, present, and
future. Lancet. 395:817–827. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heidemann S, Fischer C, Engel C, Fischer
B, Harder L, Schlegelberger B, Niederacher D, Goecke TO, Doelken
SC, Dikow N, et al: Double heterozygosity for mutations in BRCA1
and BRCA2 in German breast cancer patients: Implications on test
strategies and clinical management. Breast Cancer Res Treat.
134:1229–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samadder NJ, Giridhar KV, Baffy N,
Riegert-Johnson D and Couch FJ: Hereditary cancer syndromes-A
primer on diagnosis and management: Part 1: Breast-ovarian cancer
syndromes. Mayo Clin Proc. 94:1084–1098. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tung NM and Garber JE: BRCA1/2 testing:
Therapeutic implications for breast cancer management. Br J Cancer.
119:141–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daly MB, Pilarski R, Berry M, Buys SS,
Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, et al:
NCCN guidelines insights: Genetic/familial high-risk assessment:
breast and ovarian, version 2.2017. J Natl Compr Canc Netw.
15:9–20.38. 2017.Robert M, Frenel JS, Gourmelon C, Patsouris A,
Augereau P and Campone M: Olaparib for the treatment of breast
cancer. Expert Opin Investig Drugs 26: 751–759, 2017. View Article : Google Scholar : PubMed/NCBI
|