Introduction
At present, a variety of factors, including a
significant rise in the incidence of cancer, an aging population
worldwide and improvements in detection methods, are together
leading to cancer being the second most common cause of death
globally (1). However, the
incidence of lung adenosquamous carcinoma (ASC) is relatively low
globally, representing only 0.4–4% of all diagnosed cases of lung
cancer (2). The rarity of this
disease limits research into its diagnosis and prognosis,
contributing to the significant challenges of a poor prognosis and
limited treatment options faced by patients with lung ASC (3). The pathological characteristics of ASC
include squamous cell carcinoma components and adenocarcinoma
components (4), and ASC exhibits
pathological heterogeneity, so is therefore widely regarded as a
subtype of non-small cell lung cancer (NSCLC). Compared with
classical lung adenocarcinoma and squamous cell carcinoma, it is
known for its higher treatment difficulty (5,6).
Immunotherapy for lung cancer has received increasing attention and
several clinical studies have emerged. Specifically, the CheckMate
816 trial demonstrated that neoadjuvant immunotherapy, in
combination with platinum-doublet chemotherapy, provided marked
survival benefits for patients with resectable NSCLC, endorsing the
use of nivolumab alongside platinum-doublet chemotherapy as the
preferred neoadjuvant treatment approach for resectable NSCLC
(7). This regimen not only aims to
enhance surgical outcomes but also to minimize the risk of
recurrence.
For the current case, when considering the patient's
economic circumstances and the existing clinical trial support for
sintilimab, a comprehensive evaluation led to the selection of a
novel neoadjuvant treatment regimen, namely, sintilimab injection
combined with pemetrexed and carboplatin. Sintilimab, a highly
selective, recombinant humanized monoclonal antibody, inhibits the
interaction between programmed cell death protein 1 (PD-1) and its
ligands, proving effective in the treatment of squamous cell lung
cancer by enhancing the antitumor response (8). Based on clinical research findings on
neoadjuvant therapy for NSCLC (9),
the goal of the present study was to explore a novel neoadjuvant
treatment strategy for stage III lung adenocarcinoma by
synergistically combining chemotherapy with sintilimab.
Case report
In July 2023, a 65-year-old male patient presented
to Shandong Second Medical University (Weifang, China) for a
detailed evaluation of the upper lobe of the left lung, following
an incidental discovery during a health screening at Shouguang City
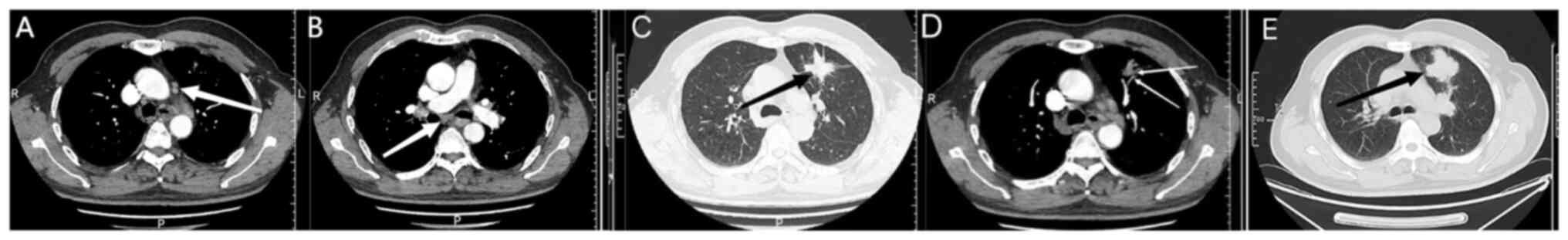
People's Hospital (Shougang, China). A lesion with soft-tissue
density, measuring ~4.9×4 cm at its widest point, was detected in
the upper lobe of the left lung through a chest CT scan conducted
in July 2023. Tiny nodules were also observed in both lungs, along
with enlarged lymph nodes in the left pulmonary hilum and
mediastinum (Fig. 1A and B). The
patient had no systemic symptoms such as a cough, hemoptysis or
chest pain. However, the patient had a 2-year history of
hypertension and a 30-year history of smoking. To clarify the
pathological features, a biopsy was immediately performed, which
revealed the coexistence of adenocarcinoma and squamous carcinoma
with a predominantly adenocarcinomatous component (Fig. 2A). Tissues were fixed in 10% neutral
buffered formalin at room temperature for 24 h, sectioned to 5-µm
thick and stained with hematoxylin and eosin (H&E) at room
temperature (hematoxylin for 5 min and eosin for 3 min). Results
were assessed using a light microscope. For immunohistochemical
staining, paraffin-embedded tissues were fixed with 10% neutral
buffered formalin at room temperature for 24 h and sectioned to a
thickness of 5 µm. The sections were blocked with 5% normal goat
serum at room temperature for 1 h, before incubating with the
following primary antibodies overnight at 4°C: p40 antibody (cat.
no. RMA-1006) and TTF-1 antibody (car. no. MAB-0266) (both diluted
1:200; Fuzhou Maixin Biotech Co., Ltd.). The secondary antibodies,
including avidin and D-biotin, from the endogenous biotin blocking
kit (both Fuzhou Maixin Biotech Co., Ltd.) were diluted at 1:500
and incubated with the sections at room temperature for 20 min.
Immunohistochemical staining for p40 and TTF1 was positive
(Fig. 2B and C) and the Ki-67
positivity rate was ~60% in adenocarcinoma (Fig. 2D) and ~5% in squamous carcinoma
(Fig. 2E). Programmed death-ligand
1 expression had a positivity rate of 15% (Fig. 2F). Genetic testing identified
mutations exclusively in KRAS G12A/V/R/C and G13C, whereas no
distant metastasis was detected through other diagnostic
examinations. Testing was performed internally using the PCR
method: DNA was extracted from tissue samples. Taq DNA polymerase
from Thermo Fisher Scientific was used for PCR amplification, with
the following primer sequences: Forward, 5′-ACTTGTGGTAGTTGGAGCT-3′
and reverse, 5′-CTGTATCAAAGAATGGTCCTGCACC-3′. PCR was conducted
with the following conditions: Initial denaturation at 95°C for 3
min, followed by 35 cycles of denaturation at 95°C for 30 sec,
annealing at 55°C for 30 sec and extension at 72°C for 1 min, with
a final extension step at 72°C for 5 min. Agarose gel
electrophoresis using a 1.5% gel and ethidium bromide staining
under ultraviolet light were employed for visualization. Utilizing
chest CT within the confines of available diagnostic modalities,
and guided by the AJCC 8th edition Tumor-Node-Metastasis staging
criteria (10), the patient's
condition was ascertained to be cT2N2M0 (stage IIIA). This staging
was predicated on the presence of N2 positive mediastinal lymph
nodes (Fig. 1A and B).
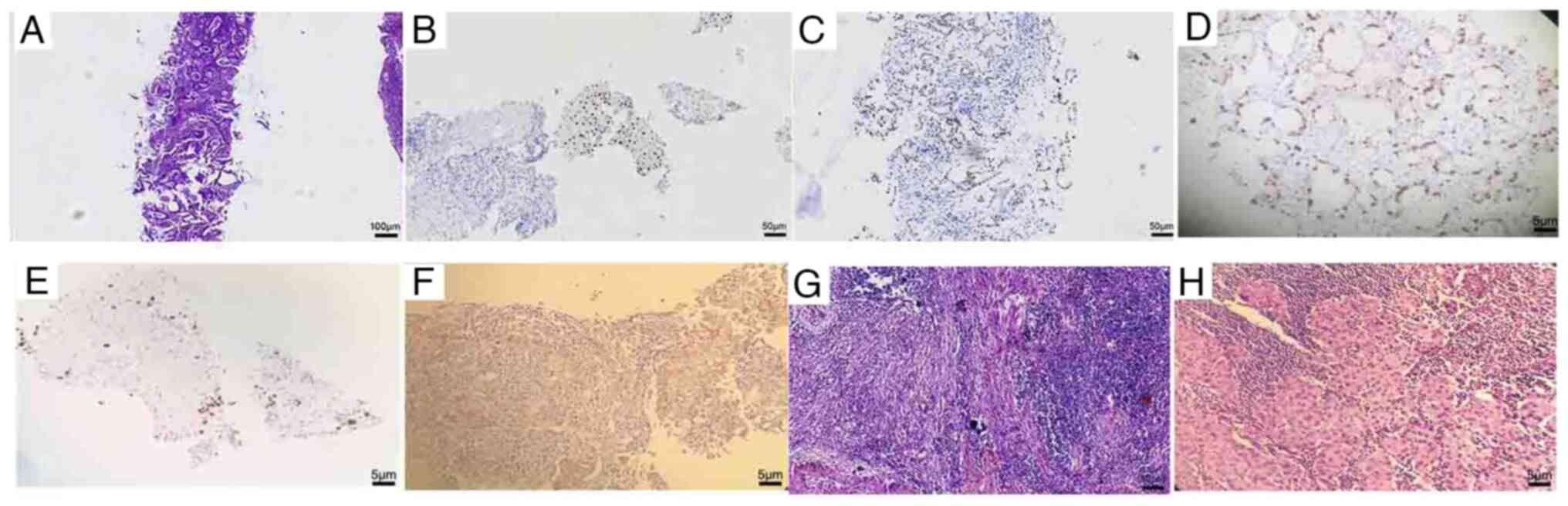 | Figure 2.Pathological images. (A) H&E
staining was used to detect surviving tumor cells before the
administration of adjuvant therapy (magnification, ×100). (B)
Immunohistochemical staining of p40 (magnification, ×200). (C)
Immunohistochemical staining of TTF-1 (magnification, ×200). (D)
The Ki-67 positivity rate was ~60% in adenocarcinoma
(magnification, ×200). (E) The Ki-67 positivity rate was ~5% in
squamous carcinoma (magnification, ×200). (F) Programmed
death-ligand 1 expression had a positivity rate of 15%
(magnification, ×200). (G) After adjuvant therapy, the primary
tumor bed was stained with H&E, showing no surviving tumor
cells (magnification, ×200). (H) After adjuvant therapy, the 10th
group of lymph nodes was immunohistochemically characterized as
poorly differentiated adenocarcinoma (magnification, ×200).
H&E, hematoxylin and eosin; TTF-1, thyroid transcription factor
1. |
During the initial comprehensive treatment
evaluation, it was concluded that the tumor was located near the
pulmonary artery (Fig. 1C), and
that surgical resection presented significant challenges and risks.
The enhanced inflammatory response and potential scar tissue
formation around the tumor due to neoadjuvant therapy increased the
difficulty and risks associated with the surgical procedure.
Although synchrotron radiation therapy remains the primary
treatment for advanced NSCLC (11),
in order to perform surgery and preserve as much lung tissue as
possible, an immunotherapy plus chemotherapy regimen was adopted as
neoadjuvant therapy based on the CheckMate 816 trial (12). Before surgery in September 2023, the
patient received two cycles of treatment, each lasting 21 days,
including 200 mg sindilizumab on day 0, 1,000 mg pemetrexed on day
1 and 600 mg carboplatin on day 2. No significant adverse effects
were observed in the patient throughout the course of neoadjuvant
therapy. The imaging findings indicated a slight reduction of the
primary tumor size (4.7×4.0 cm), indicating a stable response to
treatment (Fig. 1D and E). In
addition, the mediastinal lymph node size was also reduced compared
with previous measurements. According to the Response Evaluation
Criteria in Solid Tumors, the response of the patient to treatment
was classified as stable disease (13). The patient underwent thoracoscopic
surgery 2 weeks after the last neoadjuvant therapy, for effective
removal of the left upper lung tumor and lymph node examination.
Analysis of the pathological findings revealed a complete absence
of residual cancer cells within the primary left lung tumor.
Instead, it demonstrated a degenerative change characterized by a
lack of active tumor cells, accompanied by extensive fibrous and
lymphoid tissue proliferation, indicating a pathological complete
response (Fig. 2G). However,
metastatic malignant cells were detected in the lymph nodes in the
mediastinal and parabronchial regions (Fig. 2H). The following tumor markers were
evaluated in October 2023: Carcinoembryonic antigen, 4.29 ng/ml
(normal, <5 ng/ml); neuron-specific enolase, 10.9 ng/ml (normal,
<12.5 ng/ml); and cytokeratin 19 fragment antigen 21-1, 1.83
ng/ml (normal, <3.3 ng/ml). The patient did not experience any
postoperative complications during hospitalization. Subsequently,
between October and December, the patient received four cycles of
adjuvant chemoimmunotherapy, with each cycle spanning 21 days. The
regimen included administration of 200 mg sindilizumab on day 0,
1,000 mg pemetrexed on day 1 and 600 mg carboplatin on day 2. Since
October 2023, the patient has been undergoing evaluations every 3
months, including chest CT scans and tumor biomarker assessments,
all of which have shown no signs of recurrence. Currently, the
patient remains on regular tumor surveillance to closely monitor
for possible instances of recurrence. To comprehensively understand
the treatment process and clinical response of the patient, a
detailed timeline has been provided in Table I that outlines each critical stage
from initial diagnosis to current monitoring.
 | Table I.Comprehensive treatment timeline of
the patient. |
Table I.
Comprehensive treatment timeline of
the patient.
| Date | Event | Category |
|---|
| July 2023 | Chest CT scan
revealed a lesion in the upper lobe of the left lung, along with
tiny nodules and enlarged lymph nodes in both the hilum and
mediastinum. | Initial
diagnosis |
| July 2023 | Biopsy confirmed
adenocarcinoma and squamous carcinoma. Immunohistochemical staining
was positive for p40 and TTF-1. PD-L1 expression showed a
positivity rate of 15%. | Diagnostic
confirmation |
| September 2023 | Initiation of two
cycles of neoadjuvant therapy with sindilizumab combined with
pemetrexed and carboplatin. | Treatment
initiation |
| September 2023 | Thoracoscopic surgery
for the removal of the left upper lung tumor. The pathological
report indicated a pCR, with no residual cancer cells found.
Metastatic malignant cells were detected in the mediastinal and
parabronchial lymph nodes. | Surgical
intervention |
| October 2023 | Tumor marker
evaluation [CEA, 4.29 ng/ml (normal, <5 ng/ml); NSE, 10.9 ng/ml
(normal, <12.5 ng/ml); and CYFRA21-1, 1.83 ng/ml (normal,
<3.3 ng/ml)] indicated a response to treatment. | Treatment
monitoring |
| October to December
2023 | Administration of
four cycles of adjuvant chemoimmunotherapy post-surgery. | Continued
treatment |
| From October 2023 to
the present | Every 3 months,
routine follow-up assessments, including chest CT scans and
evaluations of tumor biomarkers, have shown no signs of
recurrence. | Long-term
monitoring |
Discussion
Lung cancer is a prevalent malignancy worldwide and
a leading cause of cancer-related mortality (14). Compared with other types of lung
cancer, lung ASC is a rare variant with an unfavorable prognosis.
As adenosquamous lung cancer is a specific subtype of NSCLC, its
management should be similar to that of NSCLC. At present,
concurrent chemoradiotherapy is the primary approach for managing
locally advanced NSCLC (15);
however, a significant proportion of patients cannot tolerate the
treatment. Neoadjuvant therapy is currently in its nascent stages
of development (16). The study by
Kang et al (17) indicated
that the utilization of neoadjuvant anti-PD-1 immunotherapy
exhibits promising long-term efficacy in individuals diagnosed with
resectable stage I–IIIA NSCLC. A phase 1b clinical trial
(ChiCTR-OIC-17013726) involving patients with stage IIIB NSCLC
evaluated the safety and efficacy of neoadjuvant therapy using PD-1
inhibitors, specifically sintilimab (18). Patients received two cycles of
sintilimab (200 mg; intravenous injection on day 22), followed by
surgical intervention between days 29 and 43. The results showed
that out of the 37 participants who underwent curative resection,
15 patients (40.5%) achieved a major pathological response, with
complete tumor regression in 6 patients (16.2%) and lymph node
regression in 3 patients (8.1%) (19). These findings highlight the
significant benefits of sintilimab as a neoadjuvant therapy in the
treatment of lung cancer.
Recent research findings have suggested the
feasibility of combining neoadjuvant sintilimab with chemotherapy
for stage III NSCLC (20). However,
further clinical investigations are warranted to explore the
potential of sintilimab as neoadjuvant immunotherapy for lung
adenocarcinoma and squamous cell carcinoma. The present study
demonstrated a favorable outcome of chemoimmunotherapy implemented
as neoadjuvant therapy for lung adenocarcinoma, and the success of
the treatment can be attributed to two potential factors: Firstly,
the pathological examination revealed the coexistence of both
adenocarcinoma and squamous cell carcinoma components in this
tumor, which exhibit distinct immune microenvironments (21). Furthermore, the augmented
presentation of surface antigens due to increased tumor volume can
potentiate the response of the immune system to tumor eradication,
thereby facilitating enhanced therapeutic outcomes (22). Currently, there is a lack of
standardized guidelines for assessing the efficacy of adjuvant
therapy in lung adenocarcinoma. However, several scholars have
argued that pathological assessment can effectively reflect
clinical efficacy (23). After two
cycles of treatment, the patient's response was assessed as stable
disease, while postoperative pathology results confirmed the
success of complete resection (R0). However, in this case, it is
noteworthy that the primary lesions of the patients showed a higher
response rate than the lymph nodes, which was possibly attributable
to differences in the molecular composition of the tumors (24). It is also possible that Ki-67
expression in primary tumors is higher than the expression in the
lymph nodes (25). Enhanced blood
supply in primary tumors improves the delivery and efficacy of
chemotherapy and targeted therapy drugs, while poorer blood flow to
the lymph nodes may restrict drug access, serving as another
possible reason for this discrepancy (26).
In conclusion, the present case study highlights a
promising approach for the neoadjuvant treatment of operable lung
adenosquamous carcinoma. Preliminary evidence suggests the
potential efficacy of neoadjuvant chemoimmunotherapy using
sintilimab for treating lung adenosquamous carcinoma. A limitation
of the present study was the absence of pre-treatment
histopathological or positron emission tomography-CT confirmation
of lymph node status, increasing the potential for false-positives
from CT imaging alone. This methodology was chosen to minimize
patient burden and costs before the planned surgical evaluation and
lymphadenectomy. In comparison with a previous study utilizing
dacomitinib alone, the neoadjuvant treatment regimen used in the
present study resulted in a a pathological complete response, while
the comparative case still presented with adenosquamous carcinoma
(15). In terms of adverse
reactions, the good tolerability of the present regimen contrasts
with the comparative literature, where a grade 1 rash was reported
(27). This highlights the
advantages of the present treatment with regard to safety and
reducing adverse effects. The present regimen had a notable impact
by reducing tumor size and enhancing treatment safety, reinforcing
the importance of further exploring and validating different
neoadjuvant treatment approaches. The future direction in lung
adenosquamous carcinoma research requires comprehensive clinical
trials to be conducted that not only benchmark the efficacy of
various neoadjuvant regimens against each other, but also
rigorously assess the predictive utility of biomarkers such as
programmed death-ligand 1 expression levels, tumor mutational
burden and EGFR mutations. This approach aims to personalize
therapy based on individual patient profiles. Essential to this
research are longitudinal studies that track long-term outcomes,
highlighting the importance of sustained patient welfare
post-treatment. By adopting a multidisciplinary and patient-centric
methodology, we are dedicated to refining treatment strategies that
promise to significantly improve patient prognosis and elevate
quality of life.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS drafted the initial manuscript and was involved
in the conception and design of the study. YN critically revised
the manuscript for important intellectual content and contributed
to the analysis and interpretation of data. TL, XP, JL and ZZ were
involved in the preparation of images, contributing to the analysis
and interpretation of data. YH contributed to the project's
conception and design, and provided final approval for the version
to be published. YN and YH confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from family
members of the patient to publish the present case report with the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang C, Xu J and Li Z: Research Progress
of Cancer-associated Fibroblasts in Lung Cancer]. Zhongguo Fei Ai
Za Zhi. 23:267–273. 2020.(In Chinese). PubMed/NCBI
|
|
2
|
Marx A, Chan JK, Coindre JM, Detterbeck F,
Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, et
al: The 2015 World health organization classification of tumors of
the thymus: Continuity and changes. J Thorac Oncol. 10:1383–1395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Zhu Y, Bai L, Chen F, Wang J and
Guo Y: Adenocarcinomatous-predominant subtype associated with a
better prognosis in adenosquamous lung carcinoma. BMC Cancer.
20:5202020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to The 2015 World health
organization classification of tumors of the lung, pleura, thymus,
and heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filosso PL, Ruffini E, Asioli S, Giobbe R,
Macri L, Bruna MC, Sandri A and Oliaro A: Adenosquamous lung
carcinomas: A histologic subtype with poor prognosis. Lung Cancer.
74:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maeda H, Matsumura A, Kawabata T, Suito T,
Kawashima O, Watanabe T, Okabayashi K and Kubota I; Japan National
Hospital Organization Study Group for Lung Cancer, : Adenosquamous
carcinoma of the lung: Surgical results as compared with squamous
cell and adenocarcinoma cases. Eur J Cardiothorac Surg. 41:357–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Wang Y, Gao Y, Sugimura H,
Minervini F, Uchino J, Halmos B, Yendamuri S, Velotta JB and Li M:
Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell
lung cancer: A systematic review and meta-analysis. Transl Lung
Cancer Res. 11:277–294. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The Eighth Edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber RM, Kauffmann-Guerrero D, Hoffmann H
and Flentje M: New developments in locally advanced nonsmall cell
lung cancer. Eur Respir Rev. 30:2002272021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun C, Liu Y, Zhang P, Wang X, Xu Y, Lin
X, Ma X, Guo Y, Qiu S, Shao G, et al: Interim analysis of the
efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab)
combined with chemotherapy (nab-paclitaxel and carboplatin) in
potentially resectable stage IIIA/IIIB non-small cell lung cancer:
A single-arm, phase 2 trial. J Cancer Res Clin Oncol. 149:819–831.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang N, Chen S, Guo L and Chen X: A case
report of right upper lung adenosquamous carcinoma resection
following neoadjuvant targeted therapy. Ann Palliat Med.
10:4987–4993. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brody R, Zhang Y, Ballas M, Siddiqui MK,
Gupta P, Barker C, Midha A and Walker J: PD-L1 expression in
advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer.
112:200–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang J, Zhang C and Zhong WZ: Neoadjuvant
immunotherapy for non-small cell lung cancer: State of the art.
Cancer Commun (Lond). 41:287–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie J, Wu X, Wu J, Huang F and Xu L:
Meta-analysis of the efficacy and safety of sintilimab for treating
advanced non-small cell lung cancer. Oncol Lett. 24:4252022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao M, Yao J, Wang Y, Zhao L, Li B, Li L,
Wu Z, Chen Z, Fan J and Qiu F: Two vs three cycles of neoadjuvant
sintilimab plus chemotherapy for resectable non-small-cell lung
cancer: NeoSCORE trial. Signal Transduct Target Ther. 8:1462023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus Chemotherapy in Metastatic
Non-Small-Cell Lung Cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Expert Committee on Quality Control of
Lung Cancer, National Quality Control Center for Cancer: Expert
consensus on the pathological evaluation of neoadjuvant therapy
efficacy for non-small cell lung cancer. Zhonghua Bing Li Xue Za
Zhi. 50:1002–1007. 2021.(In Chinese). PubMed/NCBI
|
|
23
|
Song P, Zhang J, Shang C and Zhang L:
Curative effect assessment of immunotherapy for non-small cell lung
cancer: The ‘blind area’ of Immune Response Evaluation Criteria in
Solid Tumors (iRECIST). Thorac Cancer. 10:587–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleming CA, McCarthy K, Ryan C, McCarthy
A, O'Reilly S, O'Mahony D, Browne TJ, Redmond P and Corrigan MA:
Evaluation of discordance in primary tumor and lymph node response
after neoadjuvant therapy in breast cancer. Clin Breast Cancer.
18:e255–e261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei J, Xiang J, Hao Y, Si J, Gu X, Xu M
and Song Z: Clinical outcomes of immune checkpoint inhibitor
therapy for advanced lung adenosquamous carcinoma. J Thorac Dis.
15:260–269. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li C and Lu H: Adenosquamous carcinoma of
the lung. Onco Targets Ther. 11:4829–4835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Gao Z, Zhang C, Liu X, Liu Z, Lin
X, Qian B, Jin F, Shao G and Yang Z: Asynchrony of primary tumor
and mediastinal lymph nodes response after neoadjuvant
immunotherapy plus chemotherapy in a patient with stage IIIA
non-small-cell lung cancer: A case report. Anticancer Drugs.
33:e784–e788. 2022. View Article : Google Scholar : PubMed/NCBI
|