|
1
|
Alawyia B and Constantinou C:
Hepatocellular carcinoma: A narrative review on current knowledge
and future prospects. Curr Treat Options Oncol. 24:711–724. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
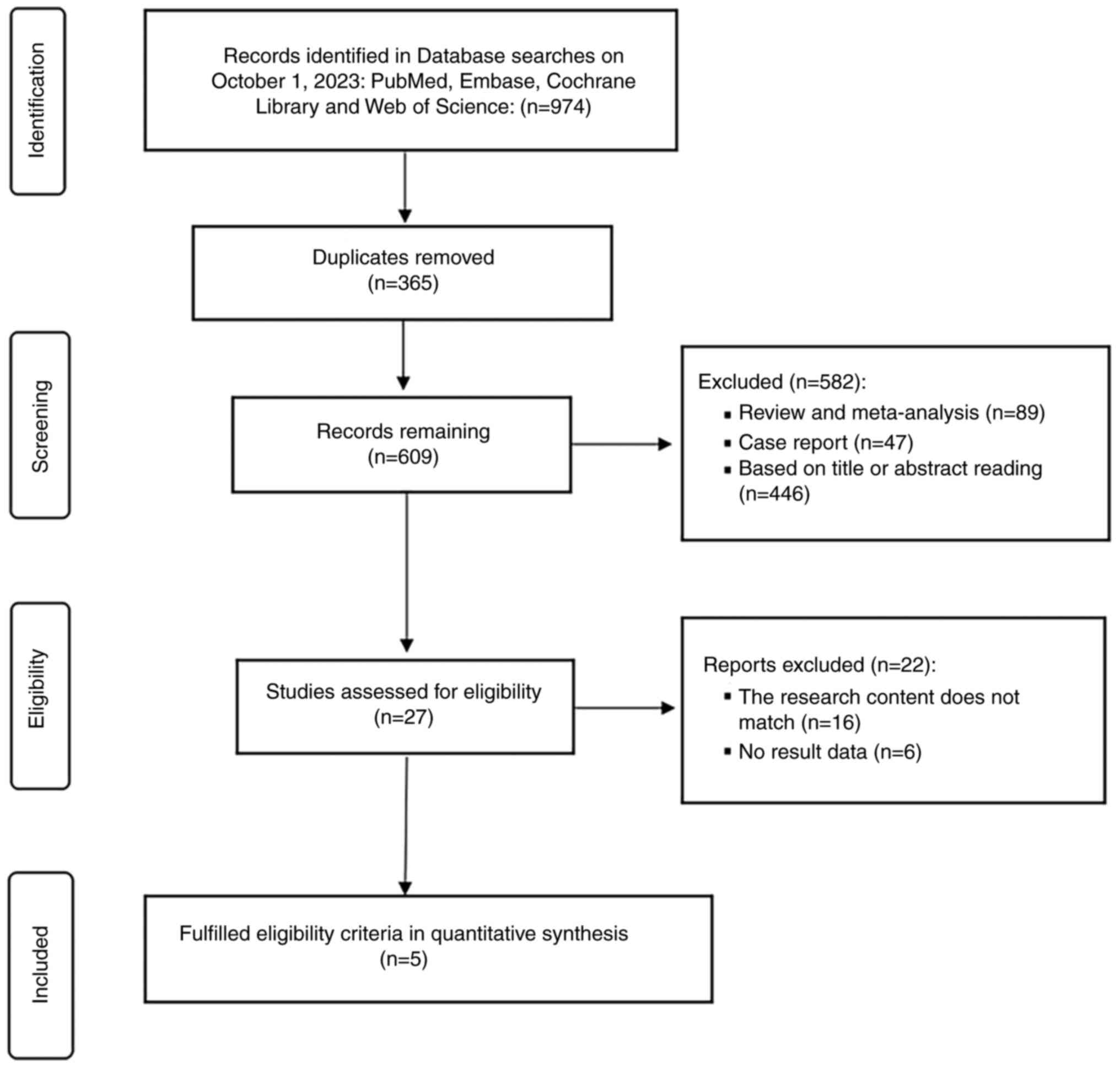
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX,
Kim TY, Kudo M, Breder V, Merle P, Kaseb A, et al: Patient-reported
outcomes with atezolizumab plus bevacizumab versus sorafenib in
patients with unresectable hepatocellular carcinoma (IMbrave150):
An open-label, randomised, phase 3 trial. Lancet Oncol.
22:991–1001. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia J, Gelfond J and Arora SP: Second-line
treatment with nivolumab, cabozantinib, regorafenib, or best
supportive care in patients with advanced hepatocellular carcinoma:
Analysis at a Hispanic-majority NCI-designated cancer center. J
Gastrointest Oncol. 12:2943–2951. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73–4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naruto K, Kawaoka T, Amioka K, Ogawa Y,
Chihiro K, Yoshikawa Y, Ando Y, Suehiro Y, Kosaka Y, Uchikawa S, et
al: Clinical outcomes of 2nd- and 3rd-Line regorafenib for advanced
hepatocellular carcinoma. Oncology. 99:491–498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo M: Recent trends in the management of
hepatocellular carcinoma with special emphasis on treatment with
Regorafenib and immune checkpoint inhibitors. Dig Dis. 34:714–730.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Liu M, Cai M, Huang W, Guo Y, Liang
L, Cai W, Liu J, Liang W, Tan Y, et al: Regorafenib enhances
anti-tumor efficacy of immune checkpoint inhibitor by regulating
IFN-γ/NSDHL/SREBP1/TGF-β1 axis in hepatocellular carcinoma. Biomed
Pharmacother. 159:1142542023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shigeta K, Matsui A, Kikuchi H, Klein S,
Mamessier E, Chen IX, Aoki S, Kitahara S, Inoue K, Shigeta A, et
al: Regorafenib combined with PD1 blockade increases CD8 T-cell
infiltration by inducing CXCL10 expression in hepatocellular
carcinoma. J Immunother Cancer. 8:e0014352020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solimando AG, Susca N, Argentiero A,
Brunetti O, Leone P, De Re V, Fasano R, Krebs M, Petracci E, Azzali
I, et al: Second-line treatments for advanced hepatocellular
carcinoma: A systematic review and Bayesian network meta-analysis.
Clin Exp Med. 22:65–74. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
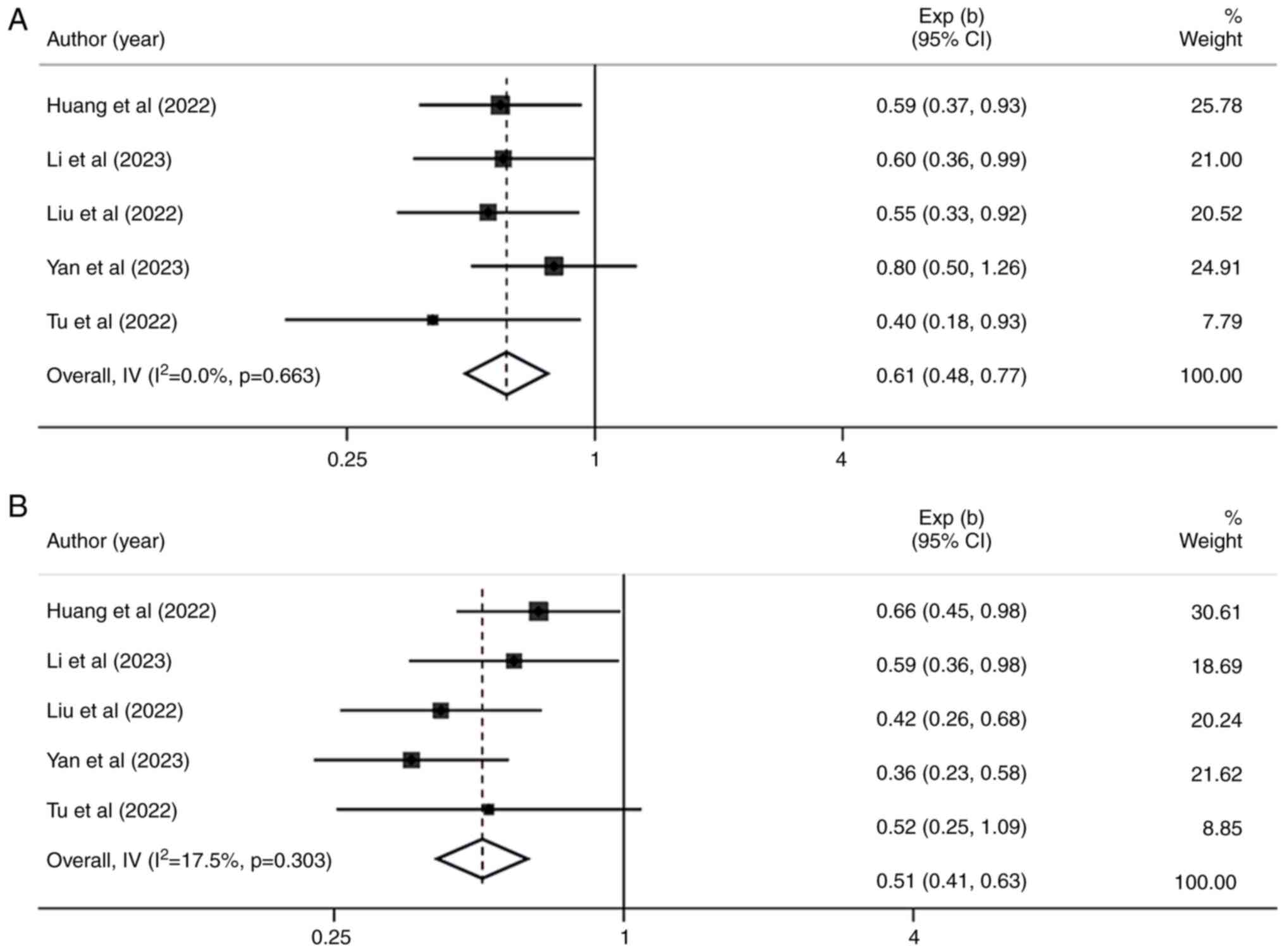
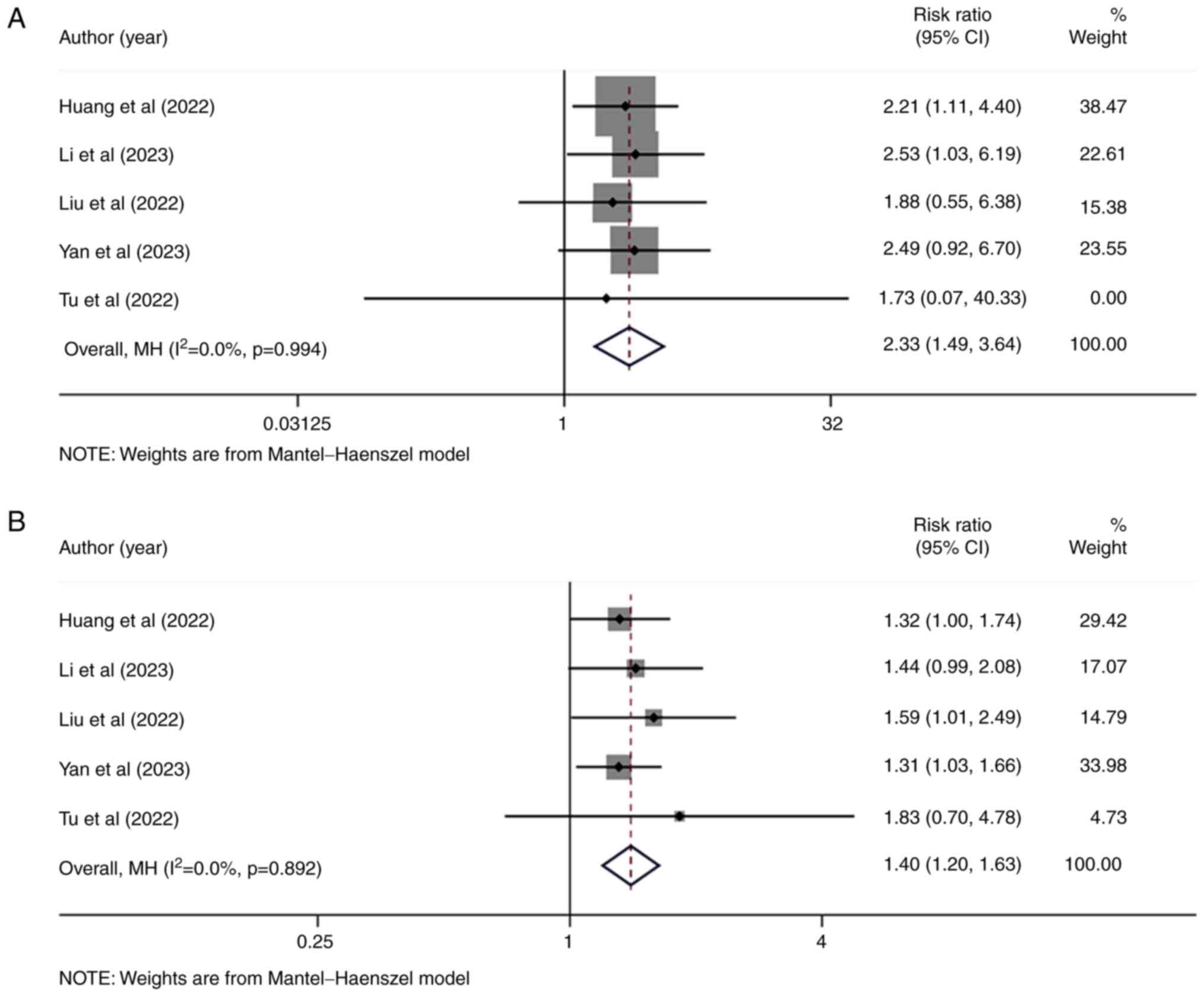
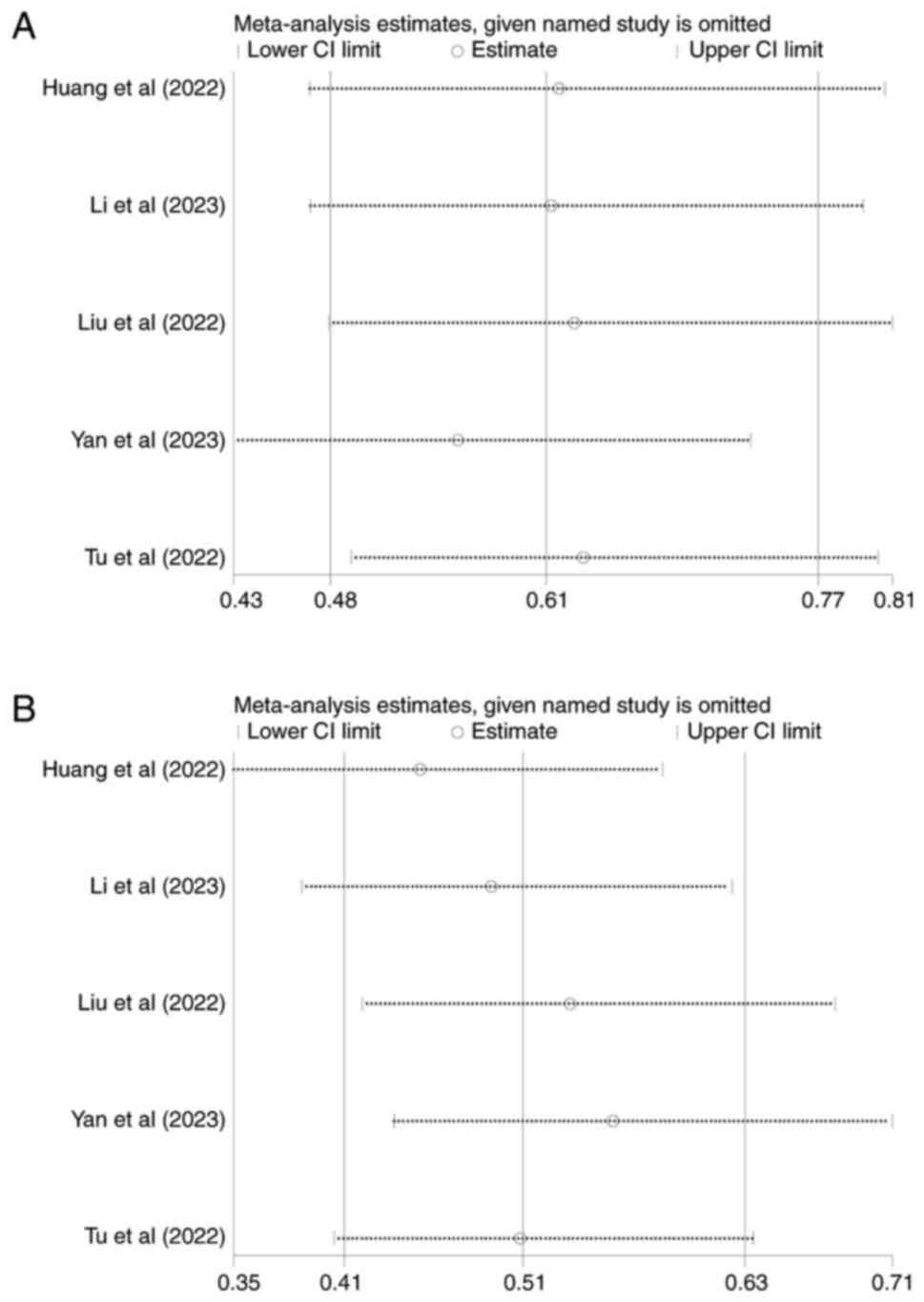
Huang J, Guo Y, Huang W, Hong X, Quan Y,
Lin L, Zhou J, Liang L, Zhang Y, Zhou J, et al: Regorafenib
combined with PD-1 blockade immunotherapy versus Regorafenib as
second-line treatment for advanced hepatocellular carcinoma: A
multicenter retrospective study. J Hepatocell Carcinoma. 9:157–170.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Jia Y, Shao C, Li Y and Song J:
Clinical efficacy and safety of an immune checkpoint inhibitor in
combination with regorafenib therapy as second-line regimen for
patients with unresectable hepatocellular carcinoma. Ther Clin Risk
Manag. 19:329–339. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu K, Wu J, Xu Y, Li D, Huang S and Mao
Y: Efficacy and safety of Regorafenib with or without PD-1
inhibitors as second-line therapy for advanced hepatocellular
carcinoma in real-world clinical practice. Onco Targets Ther.
15:1079–1094. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan T, Huang C, Peng C, Duan X, Ji D, Duan
Y, Zhang W, Zhao H, Gao K, Yang X, et al: A multi-center
retrospective study on the efficacy and safety of regorafenib vs.
regorafenib combined with PD-1 inhibitors as a second-line therapy
in patients with advanced hepatocellular carcinoma. Ann Transl Med.
11:1092023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tu X, Yang J and Zheng Y, Liang C, Tao Q,
Tang X, Liu Z, Jiang L, He Z, Xie F and Zheng Y: Immunotherapy
combination with regorafenib for refractory hepatocellular
carcinoma: A real-world study. Int Immunopharmacol. 113:1094012022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grothey A, Blay JY, Pavlakis N, Yoshino T
and Bruix J: Evolving role of regorafenib for the treatment of
advanced cancers. Cancer Treat Rev. 86:1019932020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heo YA and Syed YY: Regorafenib: A review
in hepatocellular carcinoma. Drugs. 78:951–958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Granito A, Forgione A, Marinelli S,
Renzulli M, Ielasi L, Sansone V, Benevento F, Piscaglia F and
Tovoli F: Experience with regorafenib in the treatment of
hepatocellular carcinoma. Therap Adv Gastroenterol.
14:175628482110169592021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu RY, Kong PF, Xia LP, Huang Y, Li ZL,
Tang YY, Chen YH, Li X, Senthilkumar R, Zhang HL, et al:
Regorafenib promotes antitumor immunity via inhibiting PD-L1 and
IDO1 expression in melanoma. Clin Cancer Res. 25:4530–4541. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stefanini B, Ielasi L, Chen R, Abbati C,
Tonnini M, Tovoli F and Granito A: TKIs in combination with
immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer
Ther. 23:279–291. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HD, Jung S, Lim HY, Ryoo BY, Ryu MH,
Chuah S, Chon HJ, Kang B, Hong JY, Lee HC, et al: Regorafenib plus
nivolumab in unresectable hepatocellular carcinoma: The phase 2
RENOBATE trial. Nat Med. 30:699–707. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Huang L, Ge C, Zhu X, Qiu M, Chen C,
Wei S and Yan Y: Simultaneous and sequential use of molecular
targeted agents plus immune checkpoint inhibitors for advanced
hepatocellular carcinoma: A real-world practice in China. J
Hepatocell Carcinoma. 10:949–958. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu X, Wei C, Cui R and Jiang O: Lenvatinib
plus immune checkpoint inhibitors versus Lenvatinib monotherapy as
treatment for advanced hepatocellular carcinoma: A meta-analysis.
Int J Clin Exp Pathol. 16:321–331. 2023.PubMed/NCBI
|
|
33
|
Zhao J, Guo Y, Feng T, Rong D, Kong X,
Huang T, Lopez-Lopez V, Yarmohammadi H, Sakamoto Y, Zhu D, et al:
Efficacy and safety of regorafenib in combination with immune
checkpoint inhibitor therapy as second-line and third-line regimen
for patients with advanced hepatocellular carcinoma: A
retrospective study. J Gastrointest Oncol. 14:2549–2558. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nct, . Regorafenib Plus Sintilimab vs.
Regorafenib as the Second-line Treatment for HCC. https://clinicaltrials.gov/show/NCT047189092021
|
|
35
|
Yoo C, Park JW, Kim YJ, Kim DY, Yu SJ, Lim
TS, Lee SJ, Ryoo BY and Lim HY: Multicenter retrospective analysis
of the safety and efficacy of regorafenib after progression on
sorafenib in Korean patients with hepatocellular carcinoma. Invest
New Drugs. 37:567–572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022. View Article : Google Scholar : PubMed/NCBI
|