Introduction
The prognosis of patients with pancreatic ductal adenocarcinoma (PDAC) is generally poor even in resectable cases. The postoperative recurrence rate exceeds 70% (1) Systemic chemotherapy is the treatment strategy for postoperative recurrence; however, the average survival period after recurrence is 6.8 to 11.1 months (2,3). Since 1997, Gemcitabine (GEM) monotherapy has historically been the standard primary treatment for unresectable or recurrent PDAC (4); however, recently, GEM plus nab-paclitaxel or FOLFIRINOX was shown to be superior to GEM monotherapy in phase III trials (2,3).
GEM is a key drug for pancreatic cancer. This nucleoside analog drug exerts its antiproliferative action after tumoral conversion into active triphosphorylated nucleotides by interfering with deoxyribonucleic acid (DNA) synthesis and targeting ribonucleotide reductase. GEM is a hydrophilic molecule that cannot cross the plasma membrane by passive diffusion; therefore, specialized plasma membrane nucleoside transporters are required for the uptake of GEM into tumor cells (5). hENT1 is a transmembrane protein that acts as a nucleoside transporter and has been reported to be the main mediator of GEM uptake into human cells (5). High expression of hENT1 is considered as a potential predictive biomarker for the response to GEM in several kinds of cancers including pancreatic, biliary, and urinary bladder cancer (6).
We encountered a case of PDAC with postoperative multiple liver metastases that underwent GEM monotherapy and achieved clinical complete remission (cCR) without disease regression for over 7 years. This case prompted our investigation into the factors influencing the therapeutic efficacy of GEM-based chemotherapy focusing on hENT1 protein expression in PDAC post-treated with GEM monotherapy.
Case report
Clinical course of treatment
A 63-year-old woman with epigastric pain was diagnosed with resectable PDAC in January 2011 at National Defense Medical College (Saitama, Japan). Serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) was 1.2 ng/ml (normal value: <5.3) and 10.0 U/ml (normal value: <37), respectively (Table I). A scheduled pancreaticoduodenectomy was performed in March 2011. Postoperative course was uneventful, and the patient was discharged on post operative day 17. Pathological findings were compatible with the diagnosis of PDAC (Fig. 1). The tumor size was 3.2 cm, and lymph node metastasis was positive in one of 24 dissected nodes. Pathologic stage according to 8th UICC (7) was IIB (pT2 pN1 pM0).
 |
Figure 1.
Pancreaticoduodenectomy was performed on this patient. The tumor was 3.2 cm in diameter, and pathological findings were consistent with the diagnosis of PDAC.
|
 |
Table I.
Preoperative blood tests.
|
Table I.
Preoperative blood tests.
| A, Blood count |
|
| Variable |
Result |
Normal values |
| WBC, ×103/µl |
4.3 |
3.3–8.6 |
| RBC, ×106/µl |
4.53 |
3.85–4.92 |
| Hb, g/dl |
13.6 |
11.6–14.8 |
| Hct, % |
41.7 |
35.1–44.4 |
| Plt, ×104/µl |
27.3 |
15-40 |
|
| B, Biochemistry |
|
| T-bil, mg/dl |
0.5 |
0.2–1.2 |
| AST, IU/l |
18 |
8-30 |
| ALT, IU/l |
15 |
5-35 |
| ALP, IU/l |
169 |
100-340 |
| TP, g/dl |
6.3 |
6.5–8.2 |
| Alb, g/dl |
3.7 |
3.8–5.2 |
| Amy, IU/l |
74 |
29-132 |
| HbA1c, % |
5.6 |
4.3–5.8 |
| BUN, mg/dl |
14 |
8-20 |
| Cr, mg/dl |
0.85 |
0.44–0.78 |
| CRP, mg/dl |
0.3 |
≤0.3 |
|
| C, Tumor markers |
|
| CA19-9, U/ml |
9 |
≤37 |
| CEA, ng/ml |
1.1 |
≤5.3 |
| DUPAN-2, U/ml |
25 |
≤150 |
| Span-1, U/ml |
7 |
≤30 |
We administered GEM plus S-1 as postoperative adjuvant chemotherapy for 6 months. We checked serum CEA and CA19-9 values every 3 months. CT was performed every 6 months after surgery. We detected multiple liver metastases 15 months after surgery (Fig. 2). The carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) in the serum at this time were 4.2 ng/ml and 7.6 U/ml, respectively (Table II). There were no signs suggesting the diagnosis of liver abscess such as pyrexia.
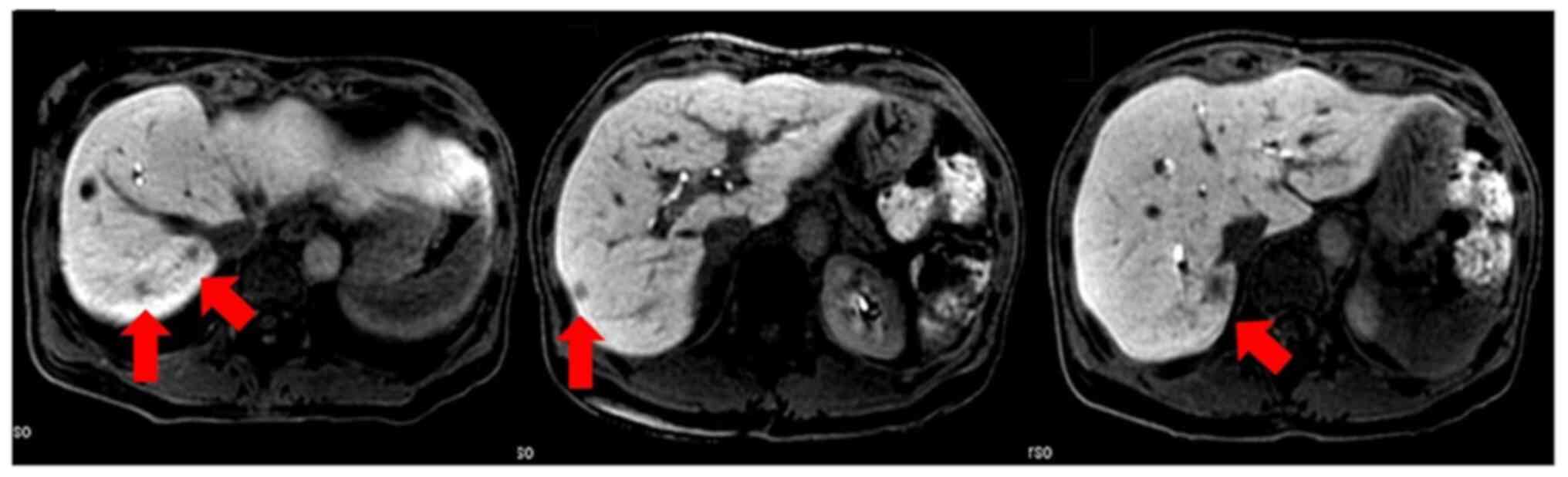 |
Figure 2.
Postoperative CT at 15 months after surgery demonstrating multiple liver nodules (red arrow).
|
 |
Table II.
Blood tests at relapse.
|
Table II.
Blood tests at relapse.
| A, Blood count |
|
| Variable |
Result |
Normal values |
| WBC, ×103/µl |
4.7 |
3.3–8.6 |
| RBC, ×106/µl |
4.52 |
3.85–4.92 |
| Hb, g/dl |
13.4 |
11.6–14.8 |
| Hct, % |
41.3 |
35.1–44.4 |
| Plt, ×104/µl |
28.9 |
15-40 |
|
| B, Biochemistry |
|
| T-bil, mg/dl |
0.5 |
0.2–1.2 |
| AST, IU/l |
26 |
8-30 |
| ALT, IU/l |
20 |
5-35 |
| ALP, IU/l |
309 |
100-340 |
| TP, g/dl |
6.4 |
6.5–8.2 |
| Alb, g/dl |
3.9 |
3.8–5.2 |
| BUN, mg/dl |
13 |
8-20 |
| Cr, mg/dl |
0.84 |
0.44–0.78 |
| CRP, mg/dl |
0.3 |
≤0.3 |
|
| C, Tumor markers |
|
| CA19-9, U/ml |
7.6 |
≤37 |
| CEA, ng/ml |
4.2 |
≤5.3 |
The patient did not wish to receive intensive chemotherapy; therefore, GEM monotherapy was initiated (1,000 mg/m2 a week) on days 1, 8, and 15, and the course was repeated every 28 days. The patient was followed up weekly. CT at 6 months after the start of chemotherapy revealed remarkable reduction in the size of all metastatic lesions. CT after 12 cycles showed cCR (Fig. 3). We stopped GEM monotherapy after 15 cycles. The last follow-up was in February 2021, the patient has been followed up with no signs or symptoms of recurrence for more than 7 years after the initial recurrence (Fig. 4).
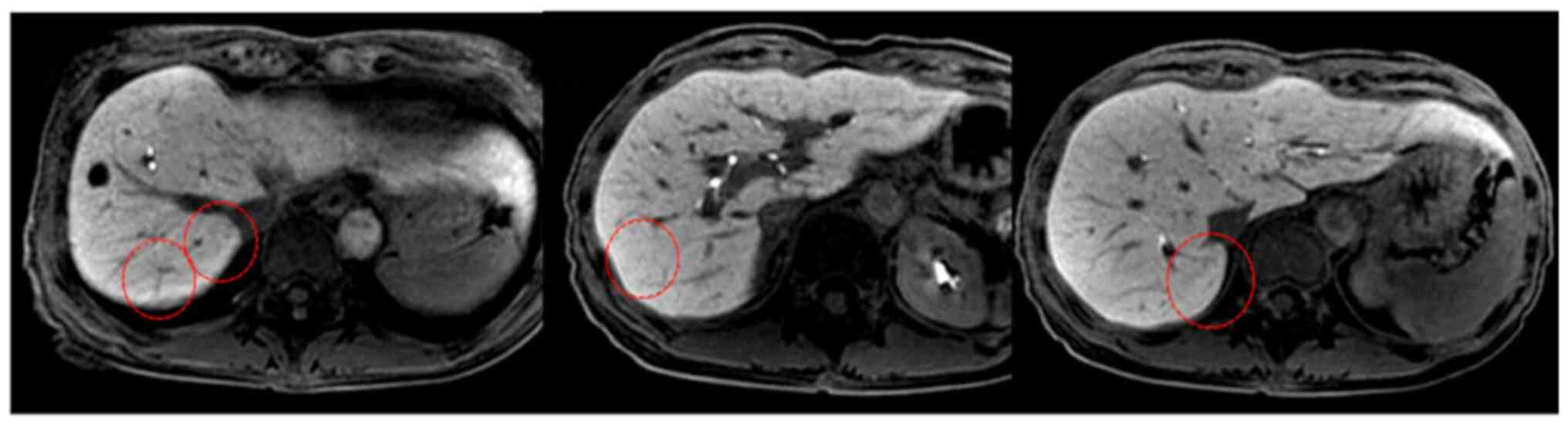 |
Figure 3.
Liver metastases disappeared 12 months after chemotherapy (red circle).
|
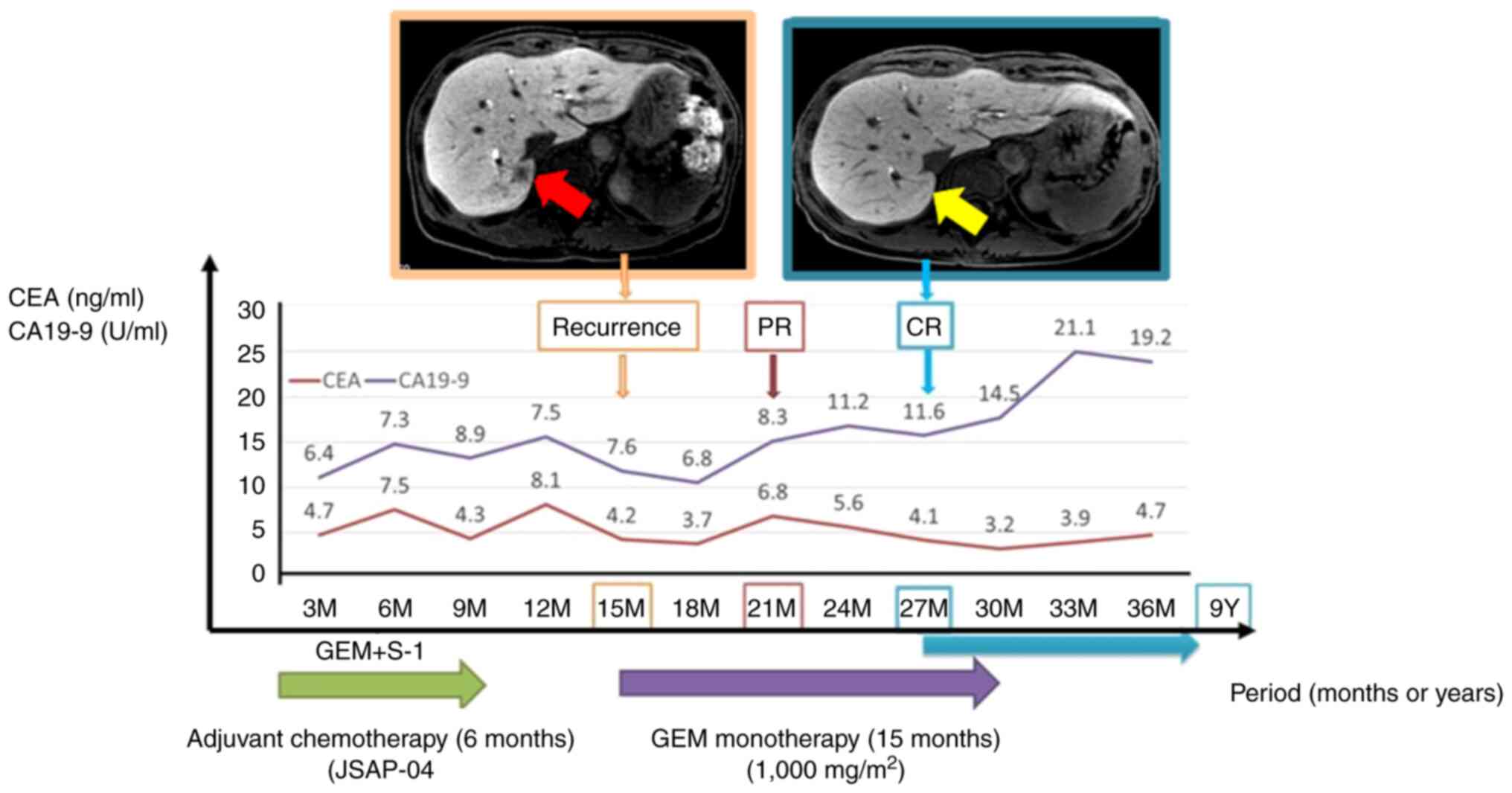 |
Figure 4.
Changes in CEA and CA19-9 levels following the clinical course are summarized. Liver metastasis was found 15 months after surgery. CT shows the complete disappearance of hepatic tumors after GEM monotherapy at 27 months after surgery. Red arrow indicates areas of liver metastasis. Yellow arrow indicates complete disappearance of liver tumor. CEA, carcinoembryonic antigen; CA199, carbohydrate antigen; GEM, gemcitabine; PR, partial response; CR, complete response.
|
Intratumoral expression levels of hENT1
We analyzed surgically resected PDAC specimens from four patients, including the present case, to determine the intratumor protein expression level of hENT1, and compared two responders (including the present case) and two non-responders. All patients received GEM-based chemotherapy following postoperative recurrence. We defined responders as patients who maintained partial or complete response for more than 1 year after receiving GEM-based chemotherapy, and non-responders as patients who did not achieve a response (Table III). Tissue sections and slides were stained with an automated immunostainer (DISCOVERY XT; Ventana Medical Systems, Tucson, AZ, USA) using heat-induced epitope retrieval and a standard diaminobenzidine detection kit. The primary antibody used was anti-hENT1 (1:50; ab182023, Abcam, Cambridge, UK) and the secondary antibody was the universal secondary antibody (#760-4205, Ventana Medical Systems). Incubation times for anti-hENT1D and the secondary antibody were 12 and 1 h, respectively. All tissue sections were counterstained with hematoxylin.
 |
Table III.
hENT1 expression in samples from gemcitabine responders and non-responders.
|
Table III.
hENT1 expression in samples from gemcitabine responders and non-responders.
| Case |
Preoperative chemotherapy |
Surgical procedure |
Evans classification |
Adjuvant chemotherapy |
Recurrence after surgery |
Treatments |
Date of operation |
| Present case |
No |
SSPPD |
- |
GEM + S-1 |
15 months |
cCR |
March 2011 |
| Responder |
GEM (6 months) + GEM + nab-PTX (6 months) |
SSPPD |
Grade III |
S-1 |
24 months |
Tumor shrunk with chemotherapy; after chemotherapy, conversion surgery was performed |
December 2018 |
| Non-responder case 1 |
GEM + S-1 (2 cycle) |
SSPPD |
Grade Ib |
S-1 |
6 months |
GEM + S-1, and GEM + nab-PTX performed after recurrence but no response |
February 2020 |
| Non-responder case 2 |
GEM + S-1 (2 cycle) |
SSPPD |
Grade Ia |
GEM |
5 months |
Cancer recurred during adjuvant chemotherapy |
March 2021 |
In the present case, hENT1 staining exhibited low frequency and weak positivity in the central region, whereas a strong positive reaction was observed in nearly all cell membranes at the invasive front of the cancer (Fig. 5A-D). In the responders, hENT1 expression was highly and strongly detected in both the central and invasive front cancer areas (Fig. 6A and B). In the nonresponders, hENT1 expression had a low-frequency and was weakly positive in both the central and advanced cancer areas (Fig. 6C and D) and mixed findings of strong and weak positives were found in the invasive front areas of the cancer (Fig. 6E and F).
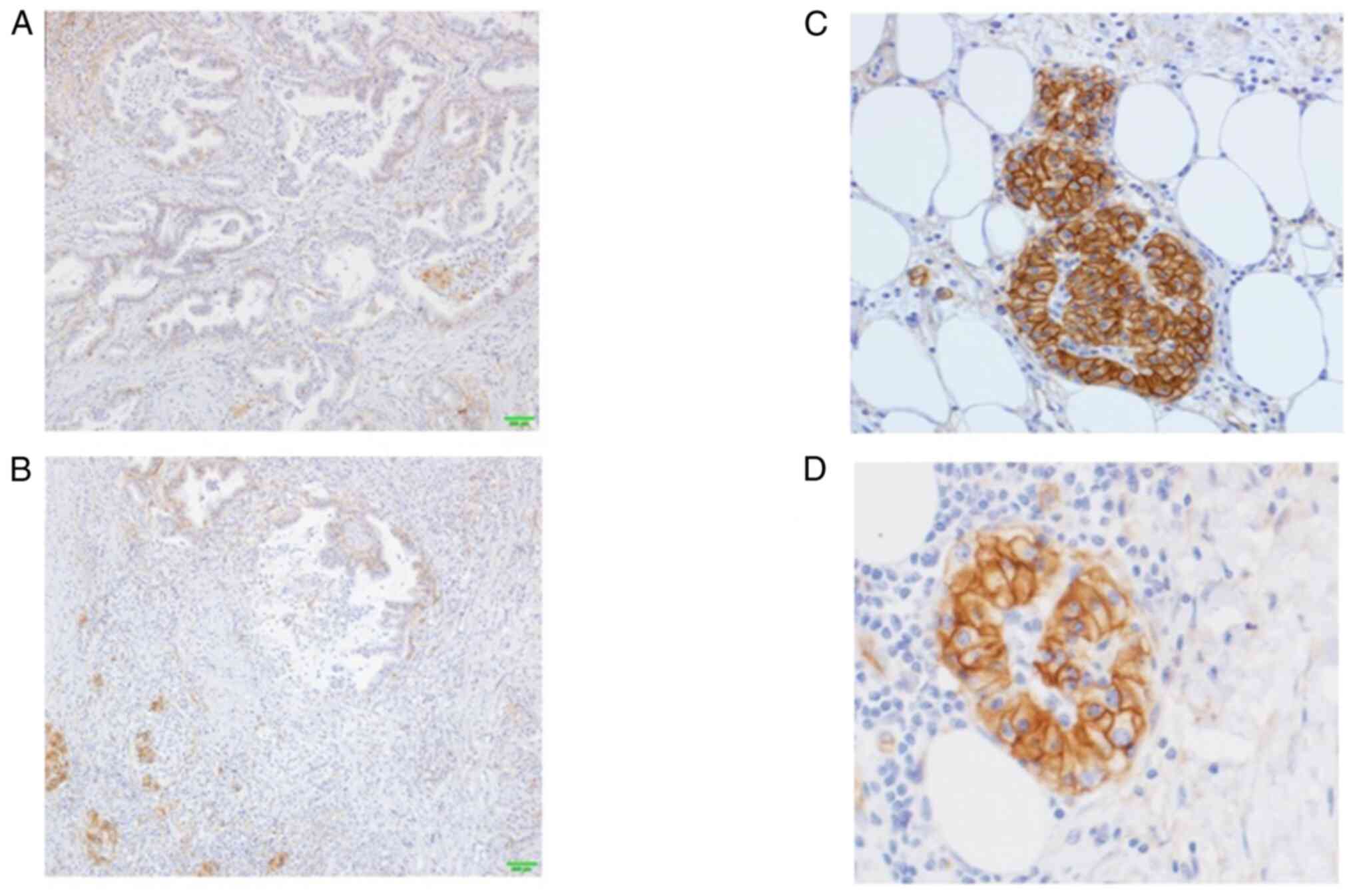 |
Figure 5.
Results of hENT1 immunostaining. (A) hENT1 staining showedlow-frequency and weak positivity in the central area, (B) whereas it exhibited strong staining in the invasive front of the cancer. (C) Staining of almost all cellmembranes was found in the invasive front of the cancer. (D) In the other invasive front cancer cells, strong staining of the cell membrane was found.
|
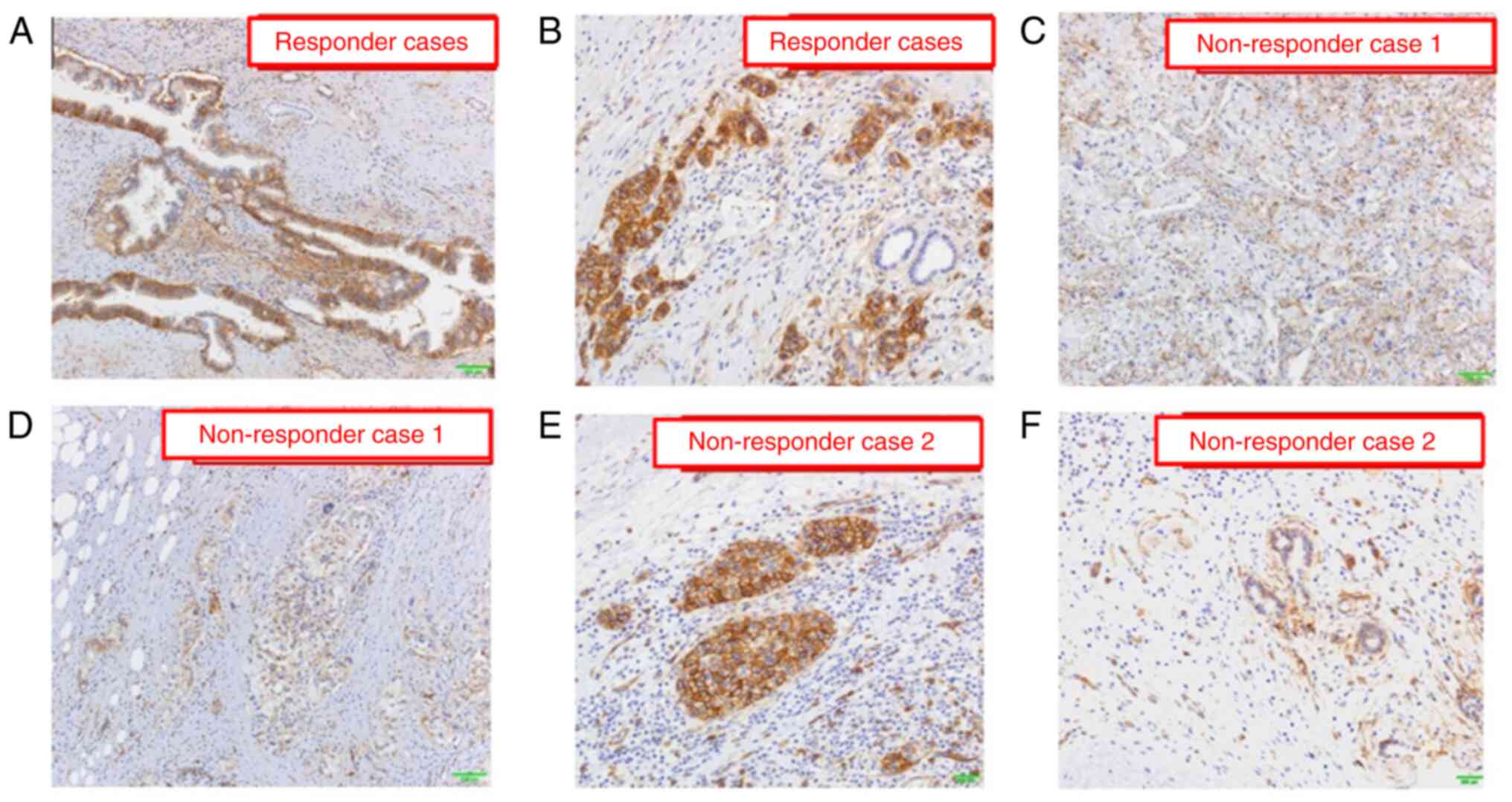 |
Figure 6.
Results of hENT1 immunostaining in gemcitabine response and non-response cases. hENT1 expression is frequently detected in both the (A) central area and (B) invasive front cancer area in response cases. The frequency of hENT1 expression is low and weakly positive in both the (C) central area and (D) advanced cancer area in non-response case 1. Mixed findings of (E) strongly and positive (F) weakly hENT1 expression are found in the invasive front areas of the cancer in non-response case 2.
|
Discussion
The response rates of various chemotherapy drugs for pancreatic cancer have been reported: FOLFIRINOX=31.6%, GEM + Nab-PTX=23% (2,3), GEM + S-1=29.3%, GEM alone=13.3%, and S-1 alone=21.0% (8). The response rates for single agents were low. To the best of our knowledge, this is the first report of a patient with metastatic pancreatic cancer who achieved cCR for 7 years using GEM monotherapy.
Recently, cCR after chemotherapy in PDAC has been sporadically but increasingly reported. These patients were mostly treated with combination chemotherapy such as FOLFIRINOX, GEM + nab-paclitaxel, GEM + S-1, or GEM + erlotinib (9–11). cCR with GEM monotherapy is extremely rare, and remission was gained after only 3 months of GEM administration in the present case.
hENT1 protein expression has been reported to be a predictor of the therapeutic efficacy of GEM-based chemotherapy (12). Immunohistochemically detected hENT1 expression was significantly associated with prolonged disease-free survival and overall survival in patients receiving surgical resection followed by GEM-based adjuvant chemotherapy for PDAC (13). Previous studies reported the frequency of hENT1 expression and that the cytoplasm and membrane were stained (14); however, there have been no reports on the frequency of staining in the tumor center or limbus. In the present case, hENT1 staining had a low frequency and was weakly positive in the central area of the cancer but was very strongly positive in almost all cell membranes in the invasive front of the cancer. As hENT1 is a transporter located on the cell membrane, immunostaining may show that hENT1 expression is strongly expressed on the cell membrane. Our results suggest that the origin of the liver metastases in the present case was the tumor cells in the invasive front area of the cancer that had strong hENT1 staining, not the cells in the central area that had weak hENT1 staining. However, further studies are needed to confirm this hypothesis because we did not perform hENT1 staining with the tumors of the hepatic metastases. A previous study investigated the predictive role of hENT-1 localization in tumor cells of cholangiocarcinoma patients receiving adjuvant GEM (15). Moreover, Brandi et al reported that membrane hENT-1 positive patients had longer disease-free survival than those who were negative or positive only in the cytoplasm of tumor cells (14). Their findings support our hypothesis, as we found the invasive front was strongly positive for hENT1 membrane staining.
As described above, chemotherapy options are now being expanded. Predicting the efficacy of GEM prior to treatment by investigating the expression of hENT1 may improve prognosis and lead to personalized medicine.
There remains the possibility that the multiple liver metastases found at 15 months postoperatively might be liver abscesses (Fig. 2). Although we have not confirmed the diagnosis pathologically, we clinically consider these lesions as liver metastases. The reasons were that these lesions were not seen on preoperative or postoperative images, and that this patient did not present any signs of cholangitis such as high fever, and that these lesions shrank gradually after the treatment (Fig. 3).
In conclusion, we report a rare case of liver metastasis of pancreatic cancer that achieved cCR with GEM monotherapy and remained in remission for 9 years after discontinuation of treatment. Our findings suggest a relationship between hENT1 expression and GEM efficacy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
MO and TT were involved in drafting the manuscript, revising it critically for important intellectual content, and have made substantial contributions to data acquisition. TE and YKi made substantial contributions to the conception and design. KI, FK, KK, NY, YT, MT, AN, YKa and HU contributed to data acquisition, conception, and reviewed and edited the manuscript. Yoshiki Kajiwara and HU confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of the National Defense Medical College (approval no. 4610), and was conducted following the human and ethical principles of the Declaration of Helsinki. We have obtained written informed consent from each patient, including this case, to participate in this study.
Patient consent for publication
Written informed consent was obtained from four patients for the publication of detailed clinical information.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Einama T, Takihata Y, Aosasa S, Konno F, Kobayashi K, Yonamine N, Fujinuma I, Tsunenari T, Nakazawa A, Shinto E, et al: Prognosis of pancreatic cancer based on resectability: A single center experience. Cancers (Basel). 15:11012023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al: Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al: Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prudner BC, Rathore R, Robinson AM, Godec A, Chang SF, Hawkins WG, Hirbe AC and Van Tine BA: Arginine starvation and docetaxel induce c-Myc-Driven hENT1 surface expression to overcome gemcitabine resistance in ASS1-negative tumors. Clin Cancer Res. 25:5122–5134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orlandi A, Calegari MA, Martini M, Cocomazzi A, Bagalà C, Indellicati G, Zurlo V, Basso M, Cassano A, Larocca LM and Barone C: Gemcitabine versus FOLFIRINOX in patients with advanced pancreatic adenocarcinoma hENT1-positive: Everything was not too bad back when everything seemed worse. Clin Transl Oncol. 18:988–995. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brierley JD, Gospodarowicz MK and Wittekind C: TNM classification of malignant tumours. 8th edition. Oxford; pp. 83–90. 2017
|
|
8
|
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al: Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukui H, Kou C and Fujioka M: Effective multidisciplinary therapy mainly using S-1+ gemcitabine (GEM) for a case of pancreatic body cancer with multiple liver metastases. Gan To Kagaku Ryoho. 39:1727–1731. 2012.(In Japanese). PubMed/NCBI
|
|
10
|
Ohkawa S, Sakamoto Y and Ueno M: A case of pancreatic body cancer with multiple liver metastases experiencing long-term response by gemcitabine plus erlotinib therapy. Gan To Kagaku Ryoho. 40:785–788. 2013.(In Japanese). PubMed/NCBI
|
|
11
|
Shelemey PT, Amaro CP, Ng D, Falck V and Tam VC: Metastatic pancreatic cancer with complete response to FOLFIRINOX treatment. BMJ Case Rep. 14:e2383952021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Wang X, Sun W, Cheng K, Qin H, Han X, Lin Y, Wang Y, Lang J, Zhao R, et al: Precision design of nanomedicines to restore gemcitabine chemosensitivity for personalized pancreatic ductal adenocarcinoma treatment. Biomaterials. 158:44–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bird NTE, Elmasry M, Jones R, Psarelli E, Dodd J, Malik H, Greenhalf W, Kitteringham N, Ghaneh P, Neoptolemos JP and Palmer D: Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br J Surg. 104:328–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brandi G, Deserti M, Vasuri F, Farioli A, Degiovanni A, Palloni A, Frega G, Barbera MA, de Lorenzo S, Garajova I, et al: Membrane localization of human equilibrative nucleoside transporter 1 in tumor cells may predict response to adjuvant gemcitabine in resected cholangiocarcinoma patients. Oncologist. 21:600–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada R, Mizuno S, Uchida K, Yoneda M, Kanayama K, Inoue H, Murata Y, Kuriyama N, Kishiwada M, Usui M, et al: Human equilibrative nucleoside transporter 1 expression in endoscopic ultrasonography-guided fine-needle aspiration biopsy samples is a strong predictor of clinical response and survival in the patients with pancreatic ductal adenocarcinoma undergoing gemcitabine-based chemoradiotherapy. Pancreas. 45:761–771. 2016. View Article : Google Scholar : PubMed/NCBI
|




















