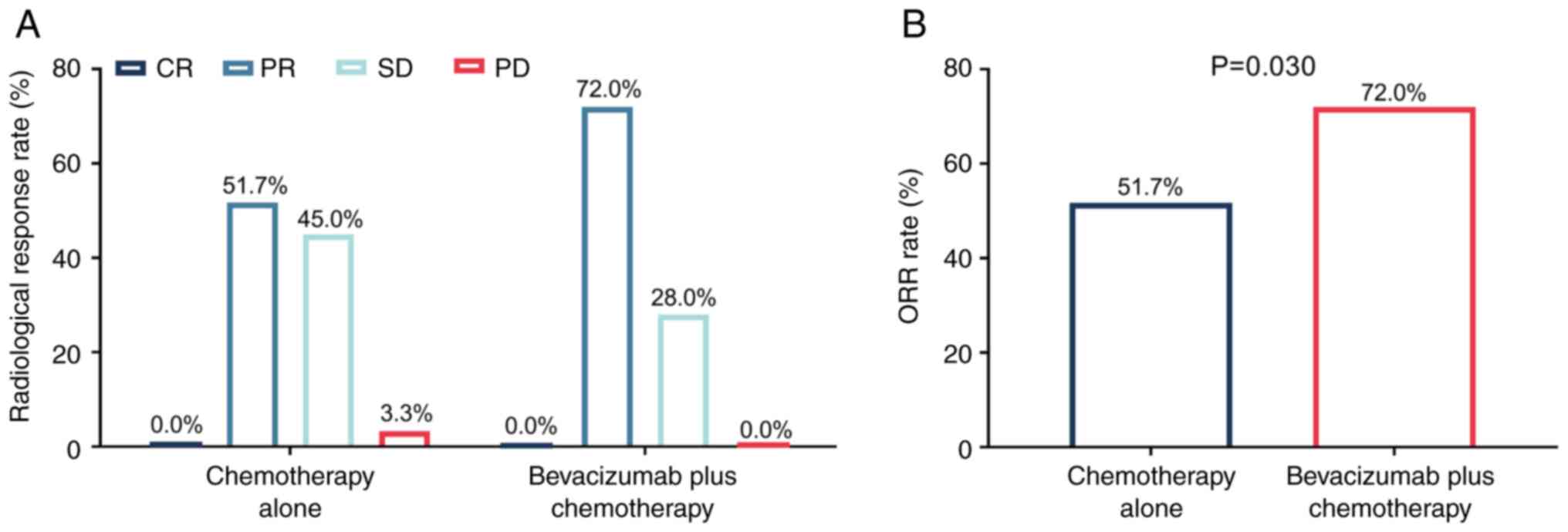
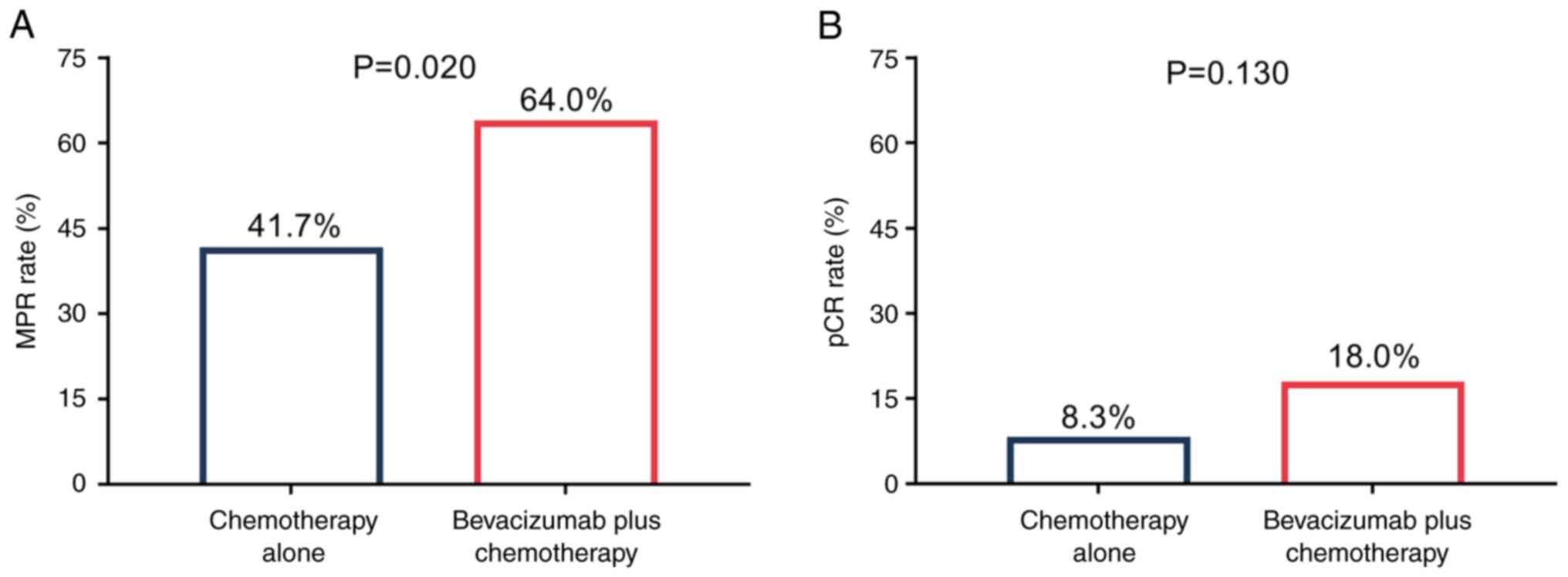
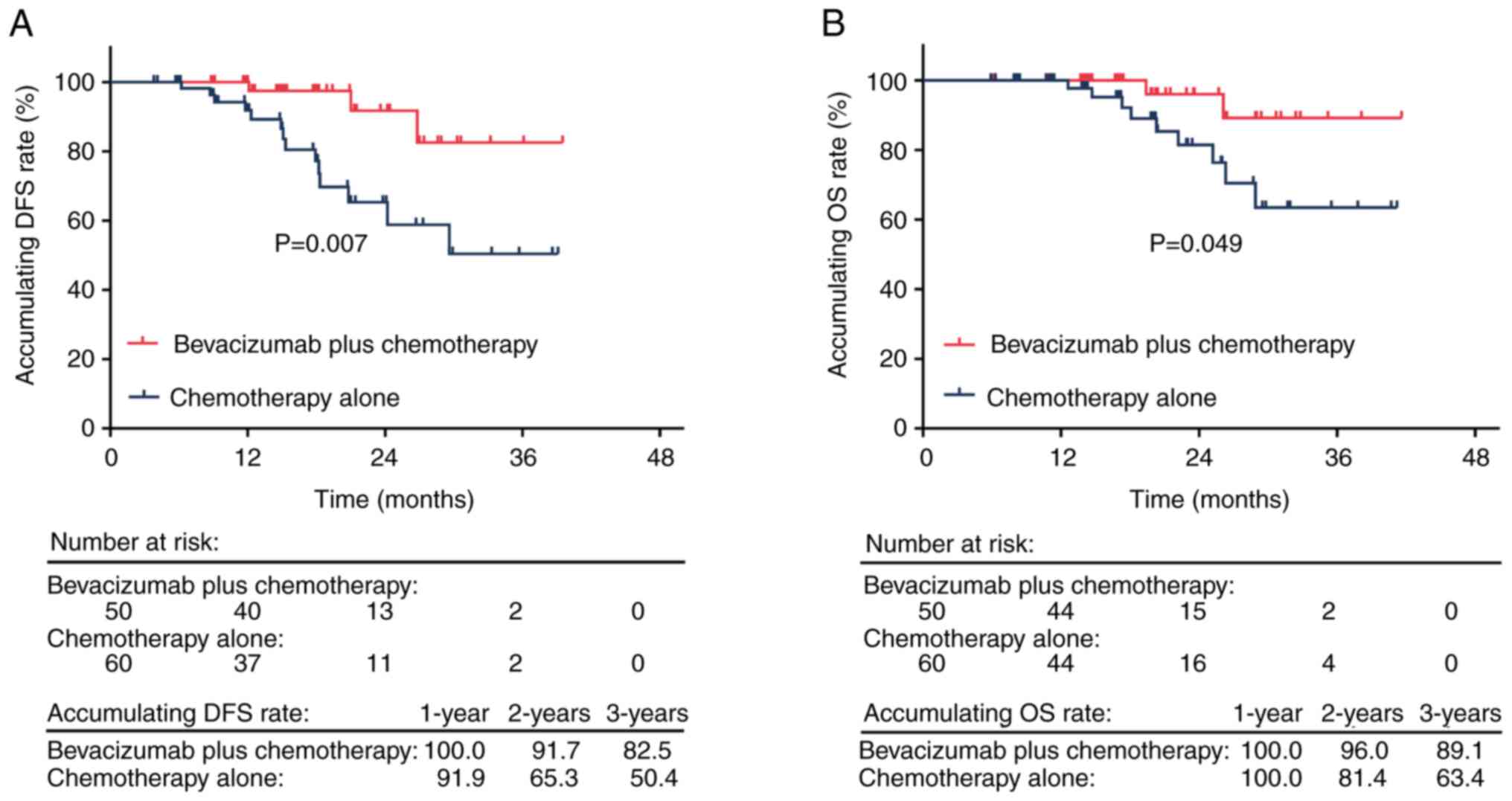
|
1
|
Zhu D, Shi X, Nicholas S, Ma Y and He P:
Estimated annual prevalence, medical service utilization and direct
costs of lung cancer in urban China. Cancer Med. 10:2914–2923.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daly ME, Singh N, Ismaila N, Antonoff MB,
Arenberg DA, Bradley J, David E, Detterbeck F, Fruh M, Gubens MA,
et al: Management of stage III non-small-cell lung cancer: ASCO
guideline. J Clin Oncol. 40:1356–1384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SM, Shaikh T and Hallman M:
Therapeutic management options for stage III non-small cell lung
cancer. World J Clin Oncol. 8:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagasaka M and Gadgeel SM: Role of
chemotherapy and targeted therapy in early-stage non-small cell
lung cancer. Expert Rev Anticancer Ther. 18:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai R, Li L, Chen X, Chen N, Song W and
Cui J: Neoadjuvant and adjuvant immunotherapy: Opening new horizons
for patients with early-stage non-small cell lung cancer. Front
Oncol. 10:5754722020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai J, Lu J, Zhang Z, Wang Y, Li X, Zhang
S, Mu S, Zhi X, Ge X, Lu D, et al: First-line PD-1/PD-L1 inhibitors
plus chemotherapy versus bevacizumab plus chemotherapy for advanced
non-squamous non-small cell lung cancer: A Bayesian network
meta-analysis of randomized controlled trials. Cancer Med.
11:2043–2055. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varlotto JM, Wang Y, Sun Z, Wakelee HA,
Ramalingam S and Schiller J: Bevacizumab's association with a
decreased risk of brain metastases in ECOG-ACRIN E1505, a phase 3
randomized trial of adjuvant chemotherapy with or without
bevacizumab in surgically resected NSCLC. JTO Clin Res Rep.
3:1002742022.PubMed/NCBI
|
|
12
|
Wakelee HA, Dahlberg SE, Keller SM, Tester
WJ, Gandara DR, Graziano SL, Adjei AA, Leighl NB, Aisner SC,
Rothman JM, et al: Adjuvant chemotherapy with or without
bevacizumab in patients with resected non-small-cell lung cancer
(E1505): An open-label, multicentre, randomised, phase 3 trial.
Lancet Oncol. 18:1610–1623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaft JE, Rusch V, Ginsberg MS, Paik PK,
Finley DJ, Kris MG, Price KAR, Azzoli CG, Fury MG, Riely GJ, et al:
Phase II trial of neoadjuvant bevacizumab plus chemotherapy and
adjuvant bevacizumab in patients with resectable nonsquamous
non-small-cell lung cancers. J Thorac Oncol. 8:1084–1090. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ou W, Li N, Wang SY, Li J, Liu QW, Huang
QA and Wang BX: Phase 2 trial of neoadjuvant bevacizumab plus
pemetrexed and carboplatin in patients with unresectable stage III
lung adenocarcinoma (GASTO 1001). Cancer. 122:740–747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie N, Qu YZ, Jiang HP and Yang XJ: Effect
of preoperative of bevacizumab combined with platinum-containing
neoadjuvant chemotherapy on the prognosis of patients with stage
IIIA-N2 stage lung cancer surgery. Chin J Surg Oncol. 14:371–375.
2022.
|
|
16
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pataer A, Weissferdt A, Correa AM,
Vaporciyan AA, Sepesi B, Heymach JV, Berezowska S, Cascone T and
Swisher SG: Major pathologic response and prognostic score predict
survival in patients with lung cancer receiving neoadjuvant
chemotherapy. JTO Clin Res Rep. 3:1004202022.PubMed/NCBI
|
|
20
|
Mabeta P and Steenkamp V: The VEGF/VEGFR
axis revisited: Implications for cancer therapy. Int J Mol Sci.
23:155852022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le X, Nilsson M, Goldman J, Reck M,
Nakagawa K, Kato T, Ares LP, Frimodt-Moller B, Wolff K,
Visseren-Grul C, et al: Dual EGFR-VEGF pathway inhibition: A
promising strategy for patients with EGFR-mutant NSCLC. J Thorac
Oncol. 16:205–215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dell'Amore A, Lomangino I, Tamburini N,
Bongiolatti S, Parri NSF, Grossi W, Catelli C, Lorenzoni G, Gregori
D, Nicotra S, et al: Video-assisted thoracoscopic lobectomy after
neoadjuvant chemotherapy for non-small cell lung cancer: A
multicenter propensity-matched study. Surg Endosc. 36:1466–1475.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Zhang L, Gong F and Xu J:
Thoracoscopic radical resection in the treatment of NSCLC patients
(stage IIIA) after neoadjuvant chemotherapy. J BUON. 26:313–319.
2021.PubMed/NCBI
|
|
24
|
Trukhin D, Poddubskaya E, Andric Z,
Makharadze T, Bellala RS, Charoentum C, Yanez Ruiz EP, Fulop A,
Hyder Ali IA, Syrigos K, et al: Efficacy, safety and immunogenicity
of MB02 (bevacizumab biosimilar) versus reference bevacizumab in
advanced non-small cell lung cancer: A randomized, double-blind,
phase III study (STELLA). BioDrugs. 35:429–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quintanilha JCF, Wang J, Sibley AB, Jiang
C, Etheridge AS, Shen F, Jiang G, Mulkey F, Patel JN, Hertz DL, et
al: Bevacizumab-induced hypertension and proteinuria: A genome-wide
study of more than 1000 patients. Br J Cancer. 126:265–274. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Li HM and Wang R: Effectiveness and
safety of adding bevacizumab to platinum-based chemotherapy as
first-line treatment for advanced non-small-cell lung cancer: A
meta-analysis. Front Med (Lausanne). 8:6163802021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou CH, Yang F, Jiang WJ, Zhang YC, Yang
HY, Zeng L, Liu L, Xiong Y, Zeng FX, Wang Z and Yang N: Efficacy
and safety of different doses of bevacizumab combined with
pemetrexed and platinum in first-line treatment of advanced NSCLC:
A retrospective-real world study. Front Pharmacol. 12:7271022021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|