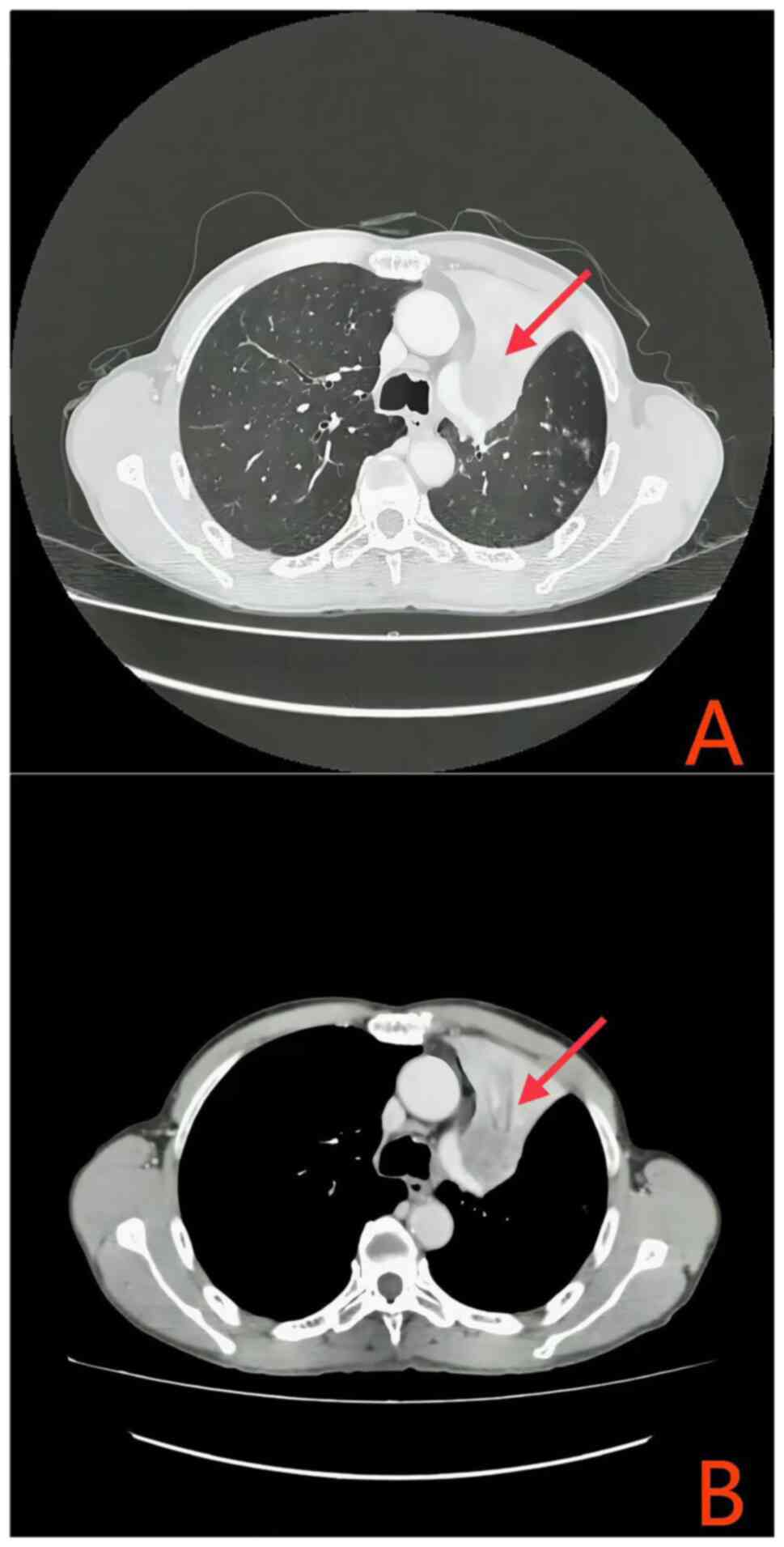
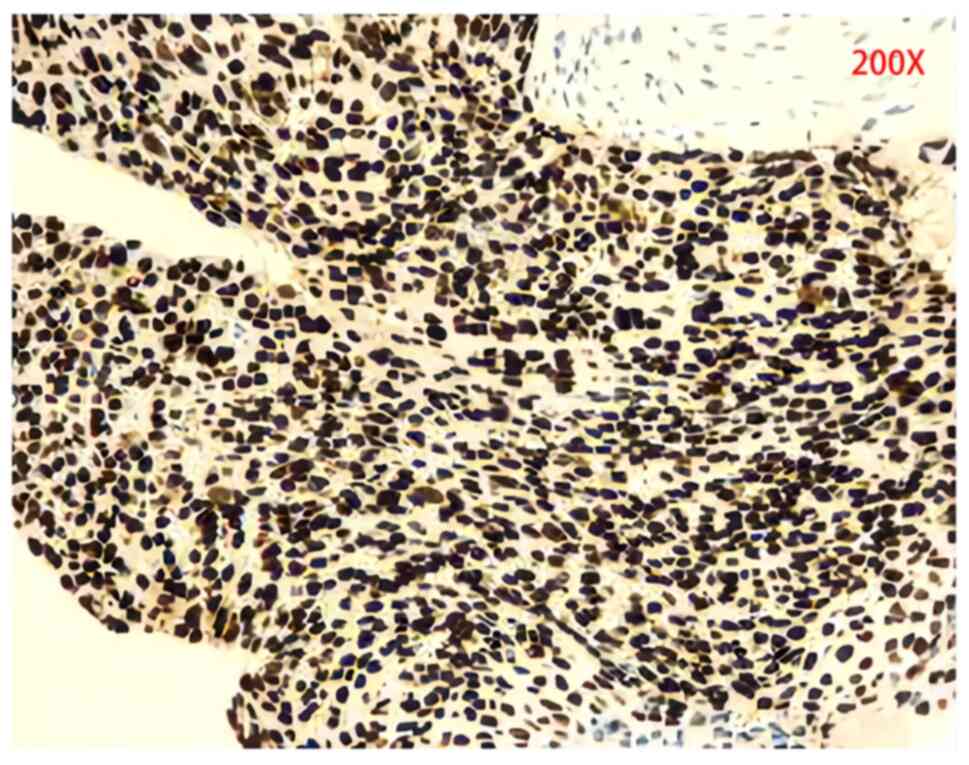
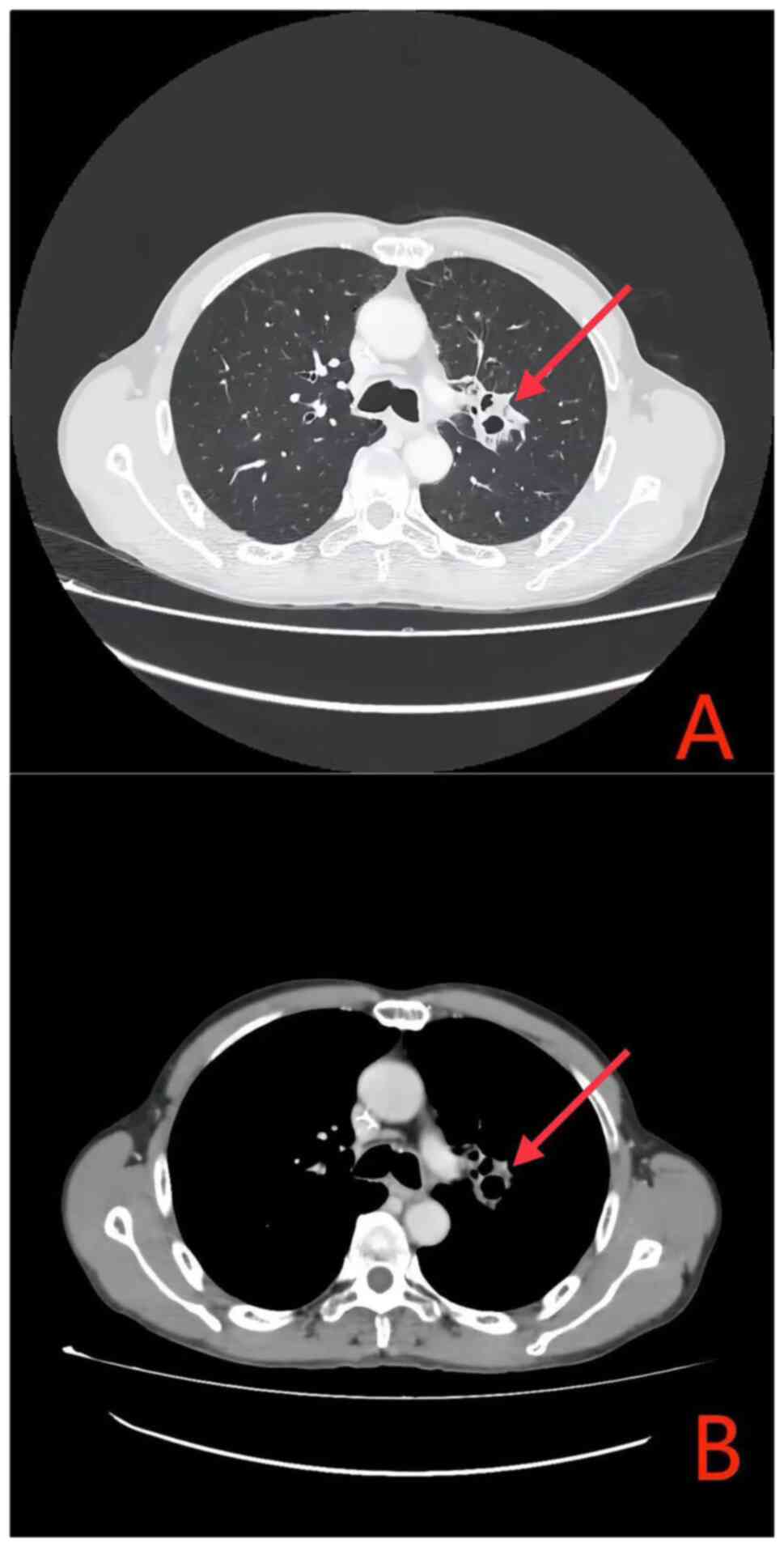
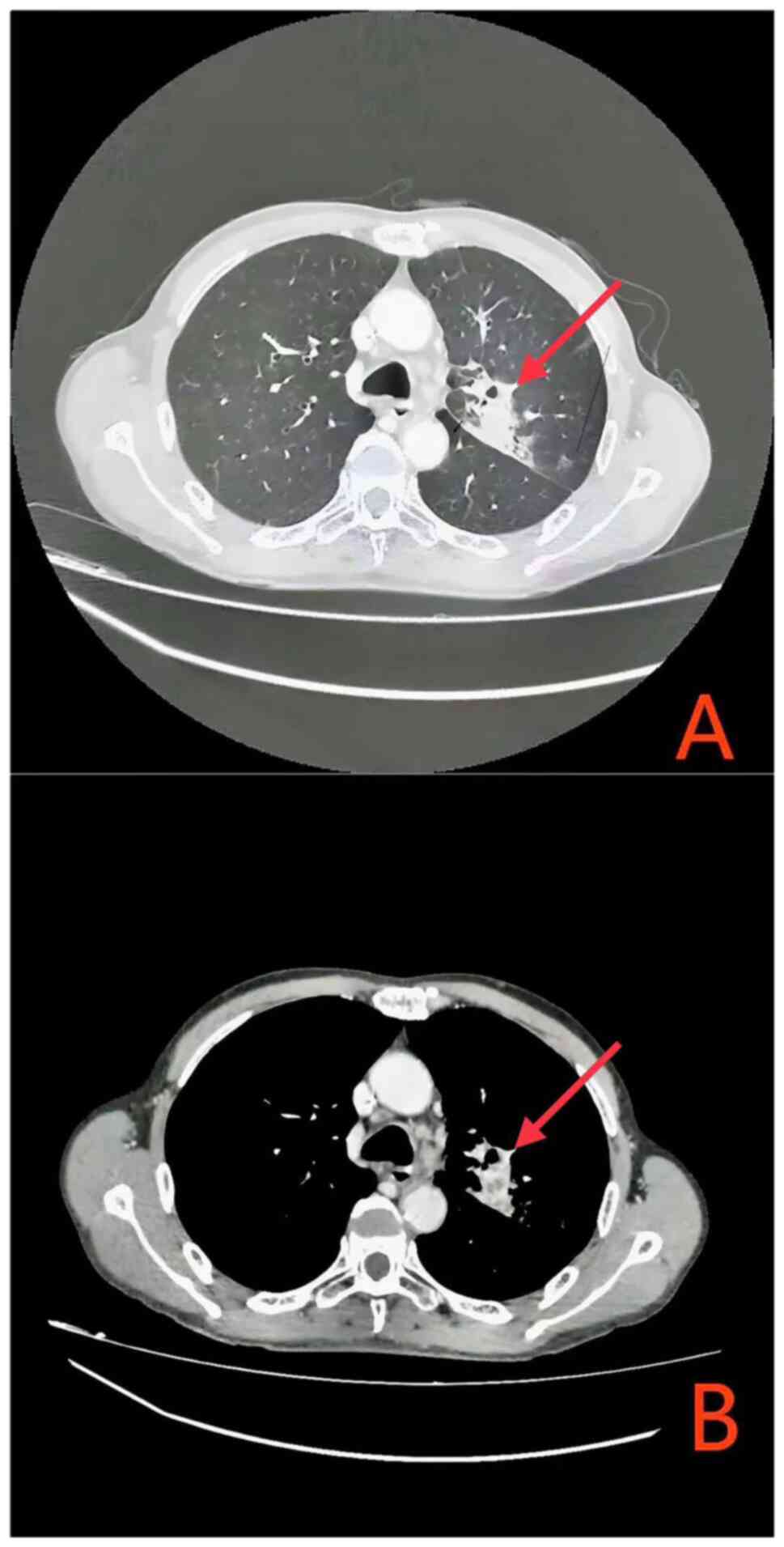
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgensztern D, Waqar S, Subramanian J,
Gao F and Govindan R: Improving survival for stage IV non-small
cell lung cancer: A surveillance, epidemiology, and end results
survey from 1990 to 2005. J Thorac Oncol. 4:1524–1529. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated Analysis From KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novello S, Kowalski DM, Luft A, Gümüş M,
Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee
KH, et al: Pembrolizumab Plus Chemotherapy in Squamous
Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III
KEYNOTE-407 Study. J Clin Oncol. 41:1999–2006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howard SC, Jones DP and Pui CH: The tumor
lysis syndrome. N Engl J Med. 364:1844–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klemencic S and Perkins J: Diagnosis and
management of oncologic emergencies. West J Emerg Med. 20:316–322.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papapanou M, Athanasopoulos AE, Georgiadi
E, Maragkos SA, Liontos M, Ziogas DC, Damaskos D and Schizas D:
Spontaneous tumor lysis syndrome in patients with solid tumors: A
scoping review of the literature. Med Oncol. 40:2332023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asamura H, Nishimura KK, Giroux DJ,
Chansky K, Hoering A, Rusch V and Rami-Porta R; Members of the
IASLC Staging and Prognostic Factors Committee and of the Advisory
Boards, and Participating Institutions, : IASLC lung cancer staging
project: The New Database to Inform Revisions in the Ninth Edition
of the TNM classification of lung cancer. J Thorac Oncol.
18:564–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perissinotti AJ, Bishop MR, Bubalo J,
Geyer MB, Goodrich A, Howard SC, Kula J, Mandayam S, Cairo MS and
Pui CH: Expert consensus guidelines for the prophylaxis and
management of tumor lysis syndrome in the United States: Results of
a modified Delphi panel. Cancer Treat Rev. 120:1026032023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN Guidelines® Insights: Non-Small Cell Lung
Cancer, Version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong H, Sun S, Chen J, Wang Z, Zhao Y,
Zhang G, Chen G, Zhou M, Zhou J, Du Y, et al: First-line penpulimab
combined with paclitaxel and carboplatin for metastatic squamous
non-small-cell lung cancer in China (AK105-302): A multicentre,
randomised, double-blind, placebo-controlled phase 3 clinical
trial. Lancet Respir Med. 12:355–365. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Lee HT, Lim H, Kim Y, Park UB and
Heo YS: Crystal structure of PD-1 in complex with an antibody-drug
tislelizumab used in tumor immune checkpoint therapy. Biochem
Biophys Res Commun. 527:226–2312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated Analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
With PD-L1 tumor proportion score of 50% or Greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Man J, Millican J, Mulvey A, Gebski V and
Hui R: Response rate and survival at key timepoints with PD-1
blockade vs chemotherapy in PD-L1 Subgroups: Meta-Analysis of
Metastatic NSCLC Trials. JNCI Cancer Spectr. 5:pkab0122021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gridelli C, Ardizzoni A, Barberis M,
Cappuzzo F, Casaluce F, Danesi R, Troncone G and De Marinis F:
Predictive biomarkers of immunotherapy for non-small cell lung
cancer: Results from an Experts Panel Meeting of the Italian
Association of Thoracic Oncology. Transl Lung Cancer Res.
6:373–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gascón M, Isla D, Cruellas M, Gálvez EM,
Lastra R, Ocáriz M, Paño JR, Ramírez A, Sesma A, Torres-Ramón I, et
al: Intratumoral versus circulating lymphoid cells as predictive
biomarkers in lung cancer patients treated with immune checkpoint
inhibitors: Is the Easiest Path the Best One? Cells. 9:15252020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KH, Kim CG and Shin EC: Peripheral
blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy.
Immune Netw. 20:e82020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng R, Tang H, You T, Xu X, Li S, Li Z,
Liu Y, Qiu W, Zhou N, Li N, et al: Peripheral CD8+CD28+ T
lymphocytes predict the efficacy and safety of PD-1/PD-L1
inhibitors in cancer patients. Front Immunol. 14:11258762023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong JY, Cho HJ, Sa JK, Liu X, Ha SY, Lee
T, Kim H, Kang W, Sinn DH, Gwak GY, et al: Hepatocellular carcinoma
patients with high circulating cytotoxic T cells and intra-tumoral
immune signature benefit from pembrolizumab: Results from a
single-arm phase 2 trial. Genome Med. 14:12022. View Article : Google Scholar : PubMed/NCBI
|