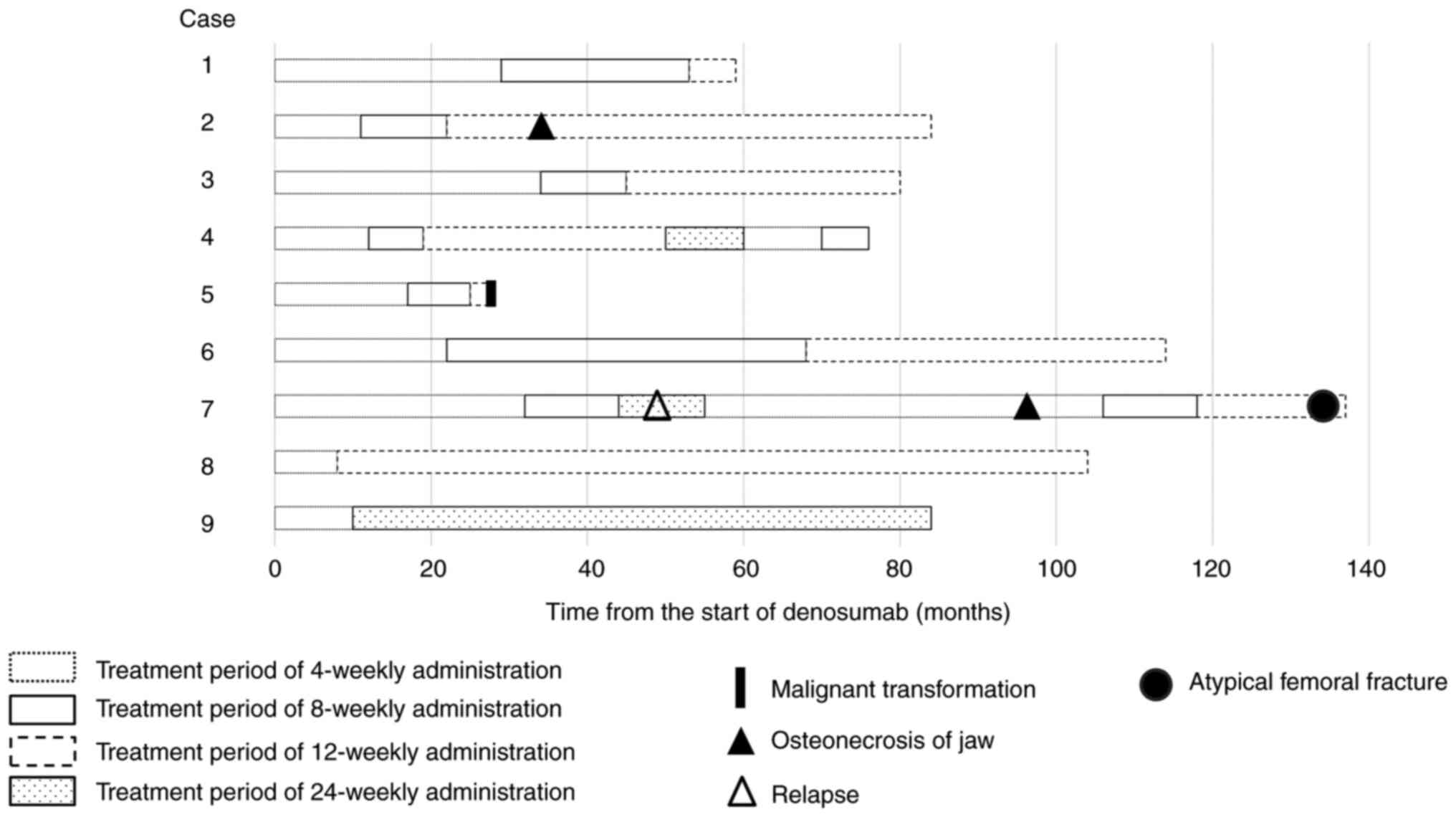
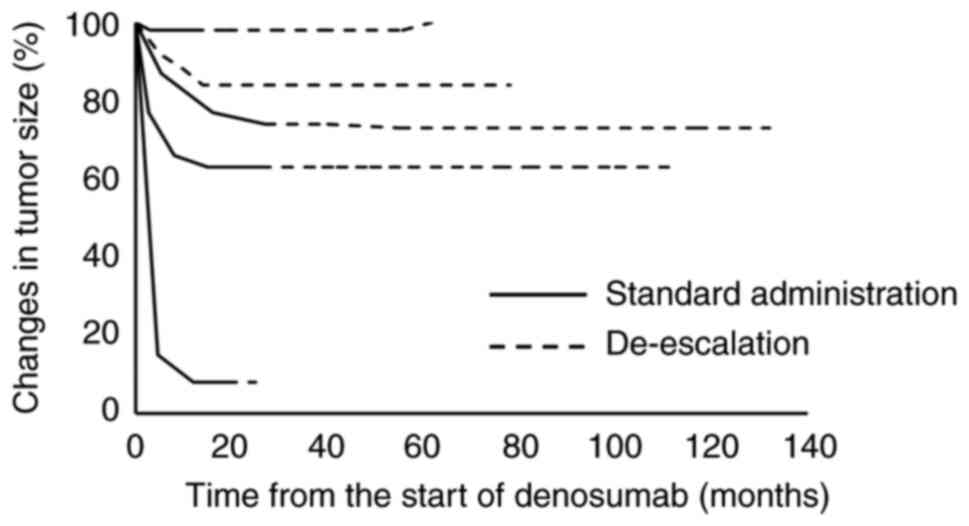
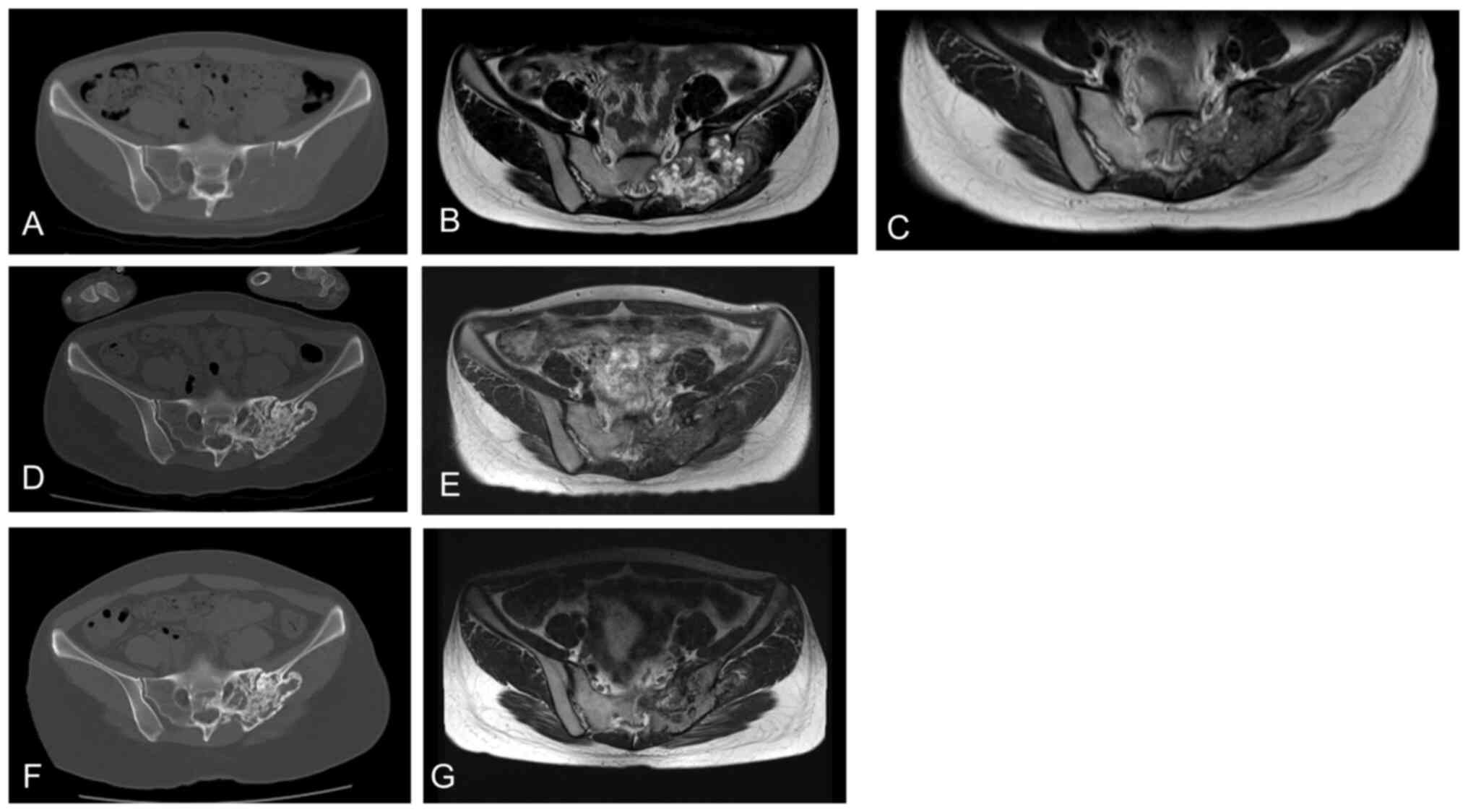
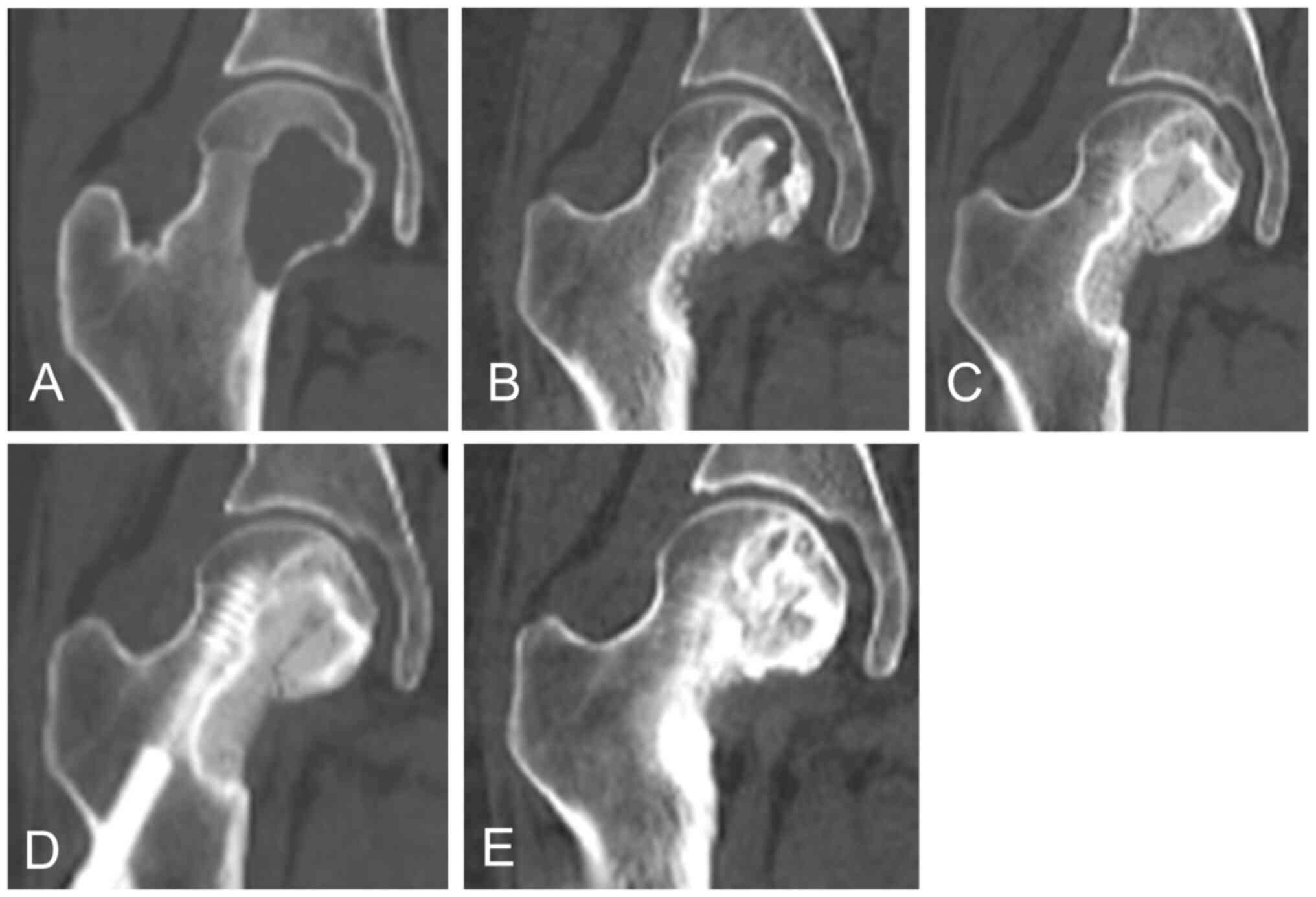
|
1
|
van der Heijden L, Dijkstra S, van de
Sande M and Gelderblom H: Current concepts in the treatment of
giant cell tumour of bone. Curr Opin Oncol. 32:332–338. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basu Mallick A and Chawla SP: Giant cell
tumor of bone: An update. Curr Oncol Rep. 22:512021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boriani S, Bandiera S, Casadei R, Boriani
L, Donthineni R, Gasbarrini A, Pignotti E, Biagini R and Schwab JH:
Giant cell tumor of the mobile spine: A review of 49 cases. Spine
(Phila Pa 1976). 37:E37–E45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jamshidi K, Bagherifard A, Mirzaei A and
Bahrabadi M: giant cell tumor of the sacrum: series of 19 patients
and review of the literature. Arch Bone Jt Surg. 5:443–450.
2017.PubMed/NCBI
|
|
5
|
Tsukamoto S, Mavrogenis AF, Kido A and
Errani C: Current concepts in the treatment of giant cell tumors of
bone. Cancers (Basel). 13:36472021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmerini E, Picci P, Reichardt P and
Downey G: Malignancy in giant cell tumor of bone: A review of the
literature. Technol Cancer Res Treat.
1:15330338198400002019.PubMed/NCBI
|
|
7
|
Hosalkar HS, Jones KJ, King JJ and Lackman
RD: Serial arterial embolization for large sacral giant-cell
tumors: Mid- to long-term results. Spine (Phila Pa 1976).
32:1107–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He SH, Xu W, Sun ZW, Liu WB, Liu YJ, Wei
HF and Xiao JR: Selective arterial embolization for the treatment
of sacral and pelvic giant cell tumor: A systematic review. Orthop
Surg. 9:139–144. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Gao J, Gao Y, Lin N, Zheng M and Ye
Z: Denosumab in giant cell tumor of bone: Current status and
pitfalls. Front Oncol. 10:5806052020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmerini E, Staals EL, Jones LB, Donati
DM, Longhi A and Randall RL: Role of (Neo) adjuvant denosumab for
giant cell tumor of bone. Curr Treat Options Oncol. 21:682020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta A, Durocher-Allen L, Popovic S,
Tozer R, Yao X and Ghert M: The role of denosumab for surgical
outcomes in patients with giant cell tumour of bone: A systematic
review. Curr Oncol. 22:1302–1313. 2021. View Article : Google Scholar
|
|
12
|
van Langevelde K and McCarthy CL:
Radiological findings of denosumab treatment for giant cell tumours
of bone. Skeletal Radiol. 49:1345–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chawla S, Blay JY, Rutkowski P, Le Cesne
A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger
L, et al: Denosumab in patients with giant-cell tumour of bone: A
multicentre, open-label, phase 2 study. Lancet Oncol. 20:1719–1729.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase II trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alaqaili SI, Abduljabbar AM, Altaho AJ,
Khan AA and Alherabi J: A: Malignant sarcomatous transformation of
benign giant cell tumor of bone after treatment with denosumab
therapy: A literature review of reported cases. Cureus.
28:e37922018.PubMed/NCBI
|
|
18
|
Palmerini E, Chawla NS, Ferrari S, Sudan
M, Picci P, Marchesi E, Leopardi MP, Syed I, Sankhala KK,
Parthasarathy P, et al: Denosumab in advanced/unresectable
giant-cell tumour of bone (GCTB): For how long? Eur J Cancer.
76:118–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matcuk GR Jr, Patel DB, Schein AJ, White
EA and Menendez LR: Giant cell tumor: Rapid recurrence after
cessation of long-term denosumab therapy. Skeletal Radiol.
44:1027–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clemons M, Liu M, Stober C, Pond G, Jemaan
Alzahrani M, Ong M, Ernst S, Booth C, Mates M, Abraham Joy A, et
al: Two-year results of a randomised trial comparing 4- versus
12-weekly bone-targeted agent use in patients with bone metastases
from breast or castration-resistant prostate cancer. J Bone Oncol.
30:1003882021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Wang L, Liu L, Zhuang J, Tang S,
Zhang T, Zhou C, Feng F, Liu R, Zhang J, et al: Efficacy and safety
of de-escalation bone-modifying agents for cancer patients with
bone metastases: A systematic review and meta-analysis. Cancer
Manag. Res. 10:3809–3823. 2018.
|
|
22
|
Tanikawa M, Yamada H, Sakata T and Mase M:
Dosing interval adjustment of denosumab for the treatment of giant
cell tumor of the sphenoid bone: A case report. Surg Neurol Int.
11:3702020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park A, Cipriano CA, Hill K, Kyriakos M
and McDonald DJ: Malignant transformation of a giant cell tumor of
bone treated with denosumab: A case report. JBJS Case Connect.
6:e782016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamaoka T, Costelloe CM, Madewell JE, Liu
P, Berry DA, Islam R, Theriault RL, Hortobagyi GN and Ueno NT:
Tumour response interpretation with new tumour response criteria vs
the World Health Organisation criteria in patients with bone-only
metastatic breast cancer. Br J Cancer. 102:651–657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: cancer response criteria and bone metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi N, Costelloe CM, Hamaoka T, Wei C,
Niikura N, Theriault RL, Hortobagyi GN, Madewell JE and Ueno NT: A
prospective study of bone tumor response assessment in metastatic
breast cancer. Clin Breast Cancer. 13:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Priebe MM and Waring WP: The interobserver
reliability of the revised American Spinal Injury Association
standards for neurological classification of spinal injury
patients. Am J Phys Med Rehabil. 70:268–270. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
European Organisation for Research and
Treatment of Cancer, . Reduced Dose-density of Denosumab for
Unresectable GCTB (REDUCE). ClinicalTrials.gov Identifier:
NCT03620149. National Library of Medicine; Bethesda, MD: 2021,
https://www.clinicaltrials.gov/ct2/show/NCT03620149January
6–2021
|
|
30
|
Nakazawa T, Inoue G, Imura T, Miyagi M,
Saito W, Namba T, Shirasawa E, Uchida K, Takahira N and Takaso M:
Remarkable regression of a giant cell tumor of the cervical spine
treated conservatively with denosumab: A case report. Int J Surg
Case Rep. 24:22–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sambri A, Medellin MR, Errani C,
Campanacci L, Fujiwara T, Donati D, Parry M and Grimer R: Denosumab
in giant cell tumour of bone in the pelvis and sacrum: Long-term
therapy or bone resection? J Orthop Sci. 25:513–519. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bukata SV, Blay JY, Rutkowski P, Skubitz
K, Henshaw R, Seeger L, Dai T, Jandial D and Chawla S: Denosumab
treatment for giant cell tumor of the spine including the sacrum.
Spine(Phila Pa 1976). 46:277–284. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broehm CJ, Garbrecht EL, Wood J and
Bocklage T: Two cases of sarcoma arising in giant cell tumor of
bone treated with denosumab. Case Rep. Med.
2015:7671982015.PubMed/NCBI
|
|
35
|
Raimondi A, Simeone N, Guzzo M, Maniezzo
M, Collini P, Morosi C, Greco FG, Frezza AM, Casali PG and
Stacchiotti S: Rechallenge of denosumab in jaw osteonecrosis of
patients with unresectable giant cell tumour of bone: A case series
analysis and literature review. ESMO Open. 5:e0006632020.
View Article : Google Scholar : PubMed/NCBI
|