Introduction
Chronic lymphocytic leukemia (CLL) is an indolent
hematological malignancy characterized by the clonal proliferation
of mature B lymphocytes (1). CLL is
more prevalent in elderly patients, with a median patient age of 72
years at diagnosis (2). Results of
a previous study demonstrated that CLL was associated with the
development of secondary tumors, such as lung and skin cancer
(3). In addition, CLL was
associated with the development of hematological malignancies, such
as myelodysplastic syndrome and acute myeloid leukemia (AML)
(4). Notably, the number of
patients diagnosed with concomitant AML and untreated CLL has
increased in the last two decades due to advances in flow cytometry
(FCM) analysis, as FCM can reduce the rate of misdiagnosis of this
disease through immunophenotyping techniques. The prognosis of
patients with concomitant AML and untreated CLL remains poor, due
to limited treatment options. For example, as they are often older
and more susceptible to infection, the majority of patients are not
eligible for intensive chemotherapy and hematopoietic stem cell
transplantation (5).
Bcl-2, an inhibitor of apoptosis, effectively
suppresses apoptosis in diverse cellular systems (6). Results of numerous studies reported
that members of the Bcl-2 family were abnormally expressed in
various tumors, such as leukemia, lymphoma and breast cancer, and
were associated with pathogenesis and chemotherapeutic resistance
(7,8). Venetoclax, which selectively binds to
Bcl-2 to inhibit expression, thus inducing healthy apoptotic
responses and protecting against cancer cell development, was the
first Bcl-2 inhibitor to be discovered (5). Venetoclax was approved by the United
States Food and Drug Administration for the treatment of CLL in
2016, and was approved for the treatment of AML that is ineligible
for intensive chemotherapy in 2018. Venetoclax is considered an
advance in traditional medicine methods for malignant
hematology.
The pathogenesis, characteristics, treatment and
prognosis of concomitant AML and untreated CLL remain poorly
understood, due to a low incidence rate compared with CLL (4.9 per
100,000 individuals per year) and AML (4.34 per 100,000 individuals
per year (1,9). The present study reports the case of a
58-year-old male diagnosed with concurrent AML and untreated CLL.
In addition, the data of 21 patients diagnosed with AML and
untreated CLL reported between January 2000 and December 2023 were
retrospectively analyzed, and the clinical characteristics of these
patients were summarized.
Case report
A 58-year-old man was referred to Tianjin Union
Medical Center of Nankai University (Tianjin, China) with
asymptomatic leukocytosis, found via a routine blood test, in
October 2022. The routine blood examination revealed a white blood
cell (WBC) count of 11.71×109/l (normal range,
3.5–9.5×109), with 48.51% lymphocytes (normal range,
20–50%), 6.32% monocytes (normal range, 3–10%) and 44.97%
neutrophils (normal range, 40–75%). In addition, the routine blood
examination revealed levels of hemoglobin at 104 g/l (normal range,
115–150 g/l) and a platelet count of 98×109/l (normal
range, 125–350×109). At 1 month prior to referral, the
patient was diagnosed with acute cerebral infarction due to blurred
vision in both eyes. The patient was previously healthy with no
malignancies and no history of associated treatments. The patient
did not present with a fever, night sweats or weight loss, and had
no history of exposure to toxins or radiation. A physical
examination revealed neither lymphadenopathy nor
hepatosplenomegaly. The patient refused a bone marrow aspiration
for personal reasons. A peripheral blood smear revealed small
lymphocytes, and no blasts or abnormal monocytes. Results of the
FCM analysis using a Navios 10 color flow cytometer with Kaluza
analysis software (Beckman Coulter, Inc.) revealed a small B-cell
population (41.43% of total cellularity), with positive CD5, CD19,
CD20 and CD23 expression, negative CD10 expression and λ-light
chain restriction in the peripheral blood. Thus, a diagnosis of CLL
(Binet C and Rai IV) was made (1).
As the patient was recovering from acute cerebral infarction,
rituximab (375 mg/m2 on day 0) plus cyclophosphamide
(200 mg on day 1) and prednisone (60 mg/m2 on days 1–3)
chemotherapy was administered. In November 2022, the WBC count of
the patient increased to 65.66×109/l, with 13.27%
lymphocytes, 73.00% monocytes and 13.23% neutrophils. Results of
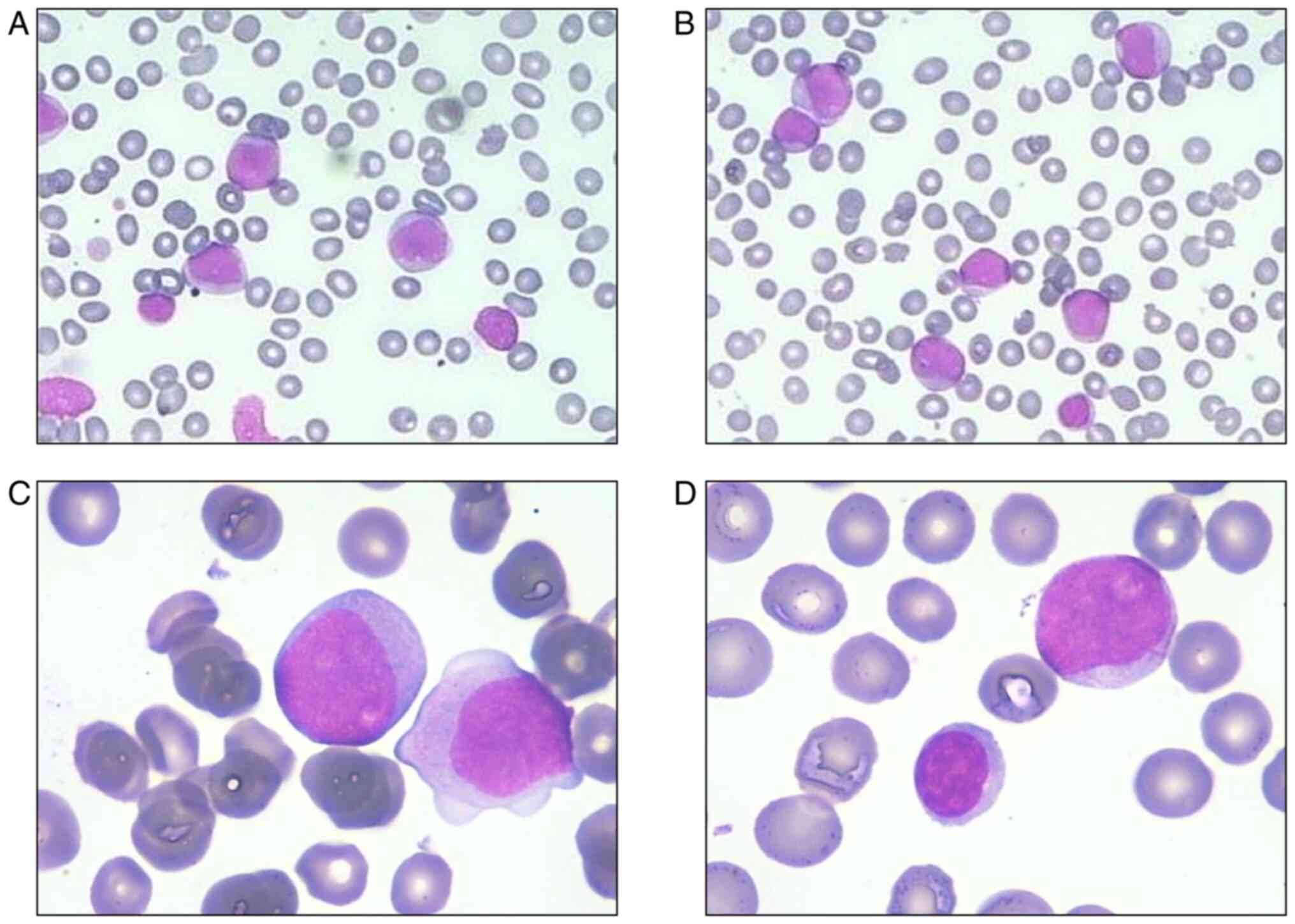
the peripheral blood smear revealed protocytosis, monocytosis and
small lymphocytes (Fig. 1). The
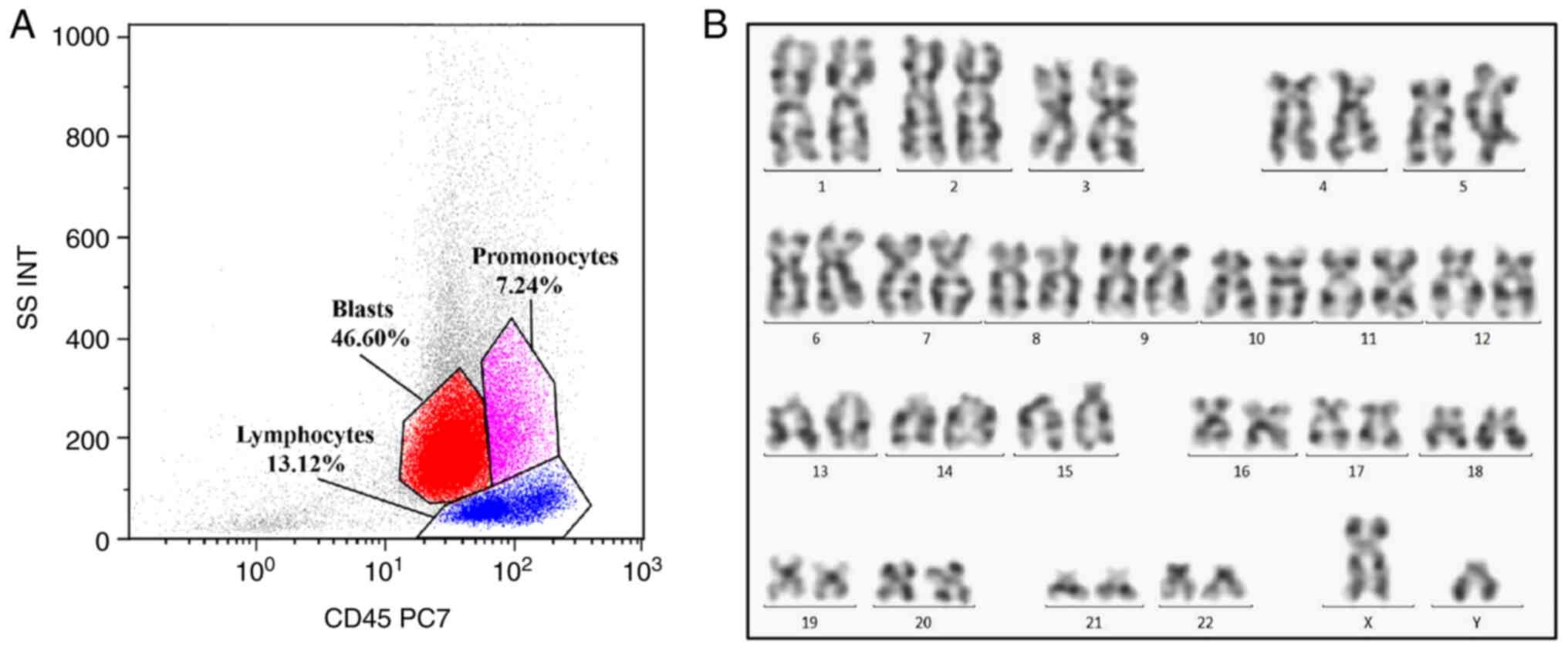
patient subsequently consented to a bone marrow aspiration. FCM
analysis revealed a primitive myeloid cell population (expressing
CD117, CD34, CD33, CD13, human leukocyte antigen-DR and CD7)
accounting for 46.60% of total cellularity, an abnormal monocyte
population (expressing CD4, CD11b, CD14, CD33, CD36 and CD64)
accounting for 7.24% of total cellularity and a monoclonal B
lymphocyte population (expressing CD5, CD19, CD22, CD23, CD200 and
λ-light chain) accounting for 13.12% of total cellularity (Fig. 2). Cytogenetic analysis revealed a
normal karyotype, and the hot spot fusion gene of leukemia was
negative. Results of the gene rearrangement assay demonstrated
positive immunoglobulin heavy-chain variable region gene expression
in monoclonal B lymphocytes (IGH Somatic Hypermutation Detection
Kit; Invivoscribe, Inc.). Based on these findings, disease
progression was observed in the patient, and a diagnosis of AML-M2
with co-existing untreated CLL was determined. The corresponding
treatment regimen consisted of 40 mg/m2 daunorubicin on
days 1 to 3 and 80 mg/m2 cytarabine on days 1 to 7 (DA
regimen), combined with a standard dose of 375 mg/m2
rituximab on day 0 and 400 mg venetoclax on days 1 to 7 (VR
regimen) every 28 days. This regimen was used as induction
chemotherapy at 1 month after initial admission to the hospital.
The patient achieved a morphological complete remission (CR) in
both AML and CLL after the first course of chemotherapy. Notably,
the patient refused further chemotherapy and died due to hemoptysis
1 month after the AML diagnosis.
Discussion
On admission to the hospital, the patient presented
with leukocytosis as the first symptom. As the patient refused a
bone marrow aspiration at the first presentation, and results of
the peripheral blood smear and FCM analysis revealed no blasts, it
was not possible to accurately determine the pre-treatment disease
status. However, a previous study revealed that secondary tumors
associated with treated CLL often occurred 1–3 years after
chemotherapy, and the development of AML associated with therapy
often occurred after 5–7 years, with an incidence of ~1% (10). In the present case, the duration
between the appearance of blasts and initial treatment was 1 month;
thus, the development of secondary AML was considered unlikely.
However, the distribution of myeloblasts in the bone marrow and
peripheral blood were typical of AML (11); thus, the patient was diagnosed with
concurrent untreated CLL and AML-M2.
The PubMed database (https://pubmed.ncbi.nlm.nih.gov/) was screened for
associated literature between January 2000 and December 2023, and
the terms ‘acute myeloid leukemia’ and ‘chronic lymphocytic
leukemia’ were used during the retrieval process. Patients with a
history of CLL treatment were excluded. The data of 21 patients
with concurrent untreated CLL and AML were extracted on July 2023.
AML and CLL were defined based on the latest World Health
Organization classification (12,13),
and patients who had any history of exposure to chemotherapy or
radiation were excluded. The clinical information of all patients
is displayed in Table I (14–33).
Results from the studies demonstrated a higher incidence of
concurrent untreated CLL and AML in older individuals, with a
median patient age at diagnosis of AML of 69 years (range, 52–86
years). Moreover, the male:female ratio was 6.33/1 and AML-M2 was
the most frequent subtype. Complex karyotype was observed in 4
cases, and this was associated with the poorest prognosis. In
addition to the patient described in the present study, 2 patients
were treated with venetoclax, and they exhibited an improved
prognosis. The CR rate of patients with AML was 27.27%, and the
median overall survival time was only 3 months after the AML
diagnosis. Moreover, patients who achieved CR tend to survive
longer than those who did not.
 | Table I.Characteristics of previously reported
patients with concomitant acute myeloid leukemia and untreated
chronic lymphocytic leukemia. |
Table I.
Characteristics of previously reported
patients with concomitant acute myeloid leukemia and untreated
chronic lymphocytic leukemia.
|
|
|
|
| BM
immunophenotype |
|
|
|
|
|
|---|
| First author,
year | Age, years | Sex | FAB subtype | Blasts, % | Lymphocytes, % | Cytogenetics | Treatment | CR | Survival time,
months | (Refs.) |
|---|
| Xie et al,
2000 | 64 | M | M5 | 78.00 | 0.75 | −7 | Cytarabine |
|
| (14) |
| Ornellas De Souza
et al, 2001 | 70 | F | M2 | 27.00 | 49.00 | 47,XX,+12[10]/
46,XX,del(5)(q31), t(8;13)(q22; q21)[4]/46,XX[6] | Hydroxyurea | No | <1 | (17) |
| Miller et
al, 2001 | 55 | M | M4 |
|
|
|
|
|
| (15) |
| Muta et al,
2002 | 84 | M | M2 | 21.30 | 56.60 | 56,XY,+1,+6,+
8,add(10)(q26), +11,+11,+13,+14,+15,+21,+21
[19/20]/46,XY[1/20] | No
chemotherapy | No | 3 | (16) |
| Lu et al,
2006 | 59 | M | M4 |
|
| inv(16)/+22 | DA | Yes | >12 | (18) |
| Gottardi et
al, 2006 | 69 | M | M2 |
|
| 46,XY | Hydroxyurea | No | 9 | (19) |
| Katz et al,
2010 | 76 | F | M5 |
|
|
| Hydroxyurea | No | <1 | (22) |
| Zhang et al,
2011 | 80 | M | M0 |
|
| 46,XY,t(2;5;11)
(q31,3;q21,2;p15), t(5,11)(q21.1;q14.2),t(5,11) (q35.2;p15) |
|
|
| (23) |
| DeFilipp et
al, 2012 | 55 | M | M2 | 46.50 | 26.60 | −7q | 7+3+3, HiDAC,
allo-HSCT | Yes | >12 | (24) |
| Su et al,
2017 | 52 | M | M3 |
|
|
t(15;17)(q22;q12) | ATRA
hydroxyurea | No | <1 | (33) |
| Kajtar et
al, 2015 | 74 | M | M1 | 28.00 | 40.00 | 46,XY | No
chemotherapy | No | 9 | (25) |
| Milosevic,
2016 | 76 | M |
| 30.00 | 65.00 | 46,XY |
| No | 2 | (26) |
| Al Mussaed et
al, 2016 | 77 | M | M5 | 60.00 | 30.00 | 46,XY | Hydroxyurea | No | <1 | (28) |
| Ito et al,
2017 | 65 | M |
| 30.00 | 17.00 | 46,XY | DA | No | 6 | (29) |
| Lee et al,
2017 | 76 | M |
| 21.60 | 16.60 |
46,XY,del(13)(q14),add(14)(q32)
[3]/46,XY[17] | Decitabine | No |
| (30) |
| Boddu et al,
2019 | 71 | M | M3 | 18.00 | 0.07 |
46,XY,t(15;17)(q24;q21)[5]/46,XY[15] | ATRA + ATO | Yes | >12 | (32) |
| Shoyele and Gupta,
2018 | 65 | M | M4 | 34.00 | 3.20 | inv(16)(p13.1q
22) | DA | No | <1 | (21) |
| Licci, 2020 | 86 | M |
|
|
|
|
| No | <1 | (27) |
| Chen et al,
2021 | 66 | M | M2a | 46.57 | 14.70 |
45,XY,-7[10]/46,XY[5] | Cytarabine +
azacitidine, venclexta + azacitidine | Yes | >12 | (20) |
|
| 62 | F | M1 | 29.78 | 50.42 |
| HA, cytarabine +
VP16, HA + VP16 | Yes | >12 |
|
| Kiso et al,
2021 | 69 | M |
| 21.40 | 34.80 |
46,XY,add(1)(p36.1),del1(1)(p?),
−5,-7,-8,-10,-12,-13,-14,-16,add (19)(p13),-21,+8mar [4]/46,
XY[16] | BR, venetoclax | No | 3 | (31) |
| Present study | 58 | M | M2 | 46.6 | 13.12 | 46,XY | DA, venetoclax,
rituximab | Yes | 1 |
|
The results of the present study demonstrate that
the prognosis of patients with concurrent untreated CLL and AML
remain poor due to a limited response to chemotherapy. However, a
combination regimen of venetoclax and rituximab may improve
outcomes in this subset of patients. Results of previous studies
demonstrated that the limited response to chemotherapy in these
patients may be due to poor humoral and cell-mediated immunity,
including hypogammaglobulinemia and abnormal T-cell subsets,
resulting in both impaired antitumor responses and increased
susceptibility to infection (29,34).
In addition, aberrant TP53 expression also played a key role in the
poor prognosis of patients with untreated CLL and AML (35).
The mechanisms underlying concurrent untreated CLL
and AML remain to be fully elucidated. The majority of studies
supported the interpretation that the emergence of myeloblasts was
not associated with that of monoclonal B lymphocytes. In some
cases, the presence of different cytogenetic and biomolecular
alterations has been reported (17). Lu et al (18) reported a case of concurrent AML with
inversion (16) and CLL, and
revealed that the CLL cells did not possess the same chromosome
aberrations as myeloblasts, indicating that the myeloblasts and
monoclonal B lymphocytes originated from disparate clones. Shoyele
and Gupta (21) revealed that the
myeloblast nuclei exhibited MYH11/CBFB fusion without trisomy 12
(in ~33% of patients with CLL); thus, supporting the hypothesis
that AML and CLL were clonally independent. Results of an
immunoglobulin H gene rearrangement assay demonstrated that AML and
CLL had different origins (19).
Moreover, Graf (36) confirmed that
progenitor cells with plasticity undergo neoplastic transformation
in the process of hematopoietic differentiation, and may develop
into two independent lineages; namely, myeloid and lymphoid. Thus,
patients may exhibit characteristics associated with leukemia, as
well as certain tumor susceptibility factors affecting two or more
hematopoietic stem cells, highlighting that CLL and AML may
originate from different cell lines. In the present case, the
patient refused a bone marrow aspiration at the onset of disease,
and next-generation sequencing and single-cell sequencing were not
performed. Thus, the origin of the disease was not determined. The
coexistence of CLL and AML is inferred from previous domestic and
international reports, and the patient's disease progression. We
consider that these are two different groups of cells with
different origins and different driving genes. Further
investigations into the pathogenesis of concurrent untreated CLL
and AML are required.
Due to the nature of the disease, timely treatment
is required. In previous cases, the treatment of concurrent
untreated CLL and AML was based on the treatment of AML; however,
the response was not optimal. As patients with concurrent untreated
CLL and AML are often older and more susceptible to infection, the
development of an effective combination therapy regimen is
required. Following advances in medicine, the treatment of
hematological diseases tends to include targeted treatment regimens
using small molecule inhibitors and antibodies. Since its approval,
venetoclax has demonstrated efficacy in the treatment of both AML
and CLL (37–39). Results of a previous study
demonstrated that the progression-free survival time of patients
treated with a combination of venetoclax and rituximab was
significantly longer than that of patients treated with a
combination of bendamustine and rituximab. In addition, patients
treated with a combination of venetoclax and rituximab demonstrated
a higher minimal residual disease negativity rate, compared with
those treated with a combination of bendamustine and rituximab
(40). At present, numerous
combination therapies that include venetoclax are considered for
the treatment of CLL. Thus, the patient described in the present
study was treated with a standard DA + VR regimen and achieved
morphological CR after one course of chemotherapy. This response
highlights that this combination therapy may be effective in
patients diagnosed with concurrent AML and untreated CLL. However,
future clinical trials are required to validate the findings of the
present study.
In conclusion, together with the present study, the
results of previous studies demonstrated that venetoclax is
effective in the treatment of numerous types of leukemia. Thus, the
comprehensive use of targeted drugs with different mechanisms may
improve the prognosis of patients with relapse and refractory
hematological malignancies, and improve the life quality of
patients.
Acknowledgements
Not applicable.
Funding
Funding was provided by the Tianjin Key Medical Discipline
(Specialty) Construction Project (grant no. TJYXZDXK-053B), the
Tianjin Science and Technology Plan Project (grant no.
21JCYBJC00290) and the Hospital-level Project of Tianjin Union
Medical Center of Nankai University (grant no. 2021YJ014).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XLZ, XFW and YFC made substantial contributions to
the conception and design of the manuscript. XLZ provided
suggestions for patient treatment and gave final approval of the
version to be published. YFC and LYY drafted the manuscript and
revised it critically for important content. YFC, LYY, XXL, XW and
QQZ retrieved data from the literature, and analyzed the data. QQZ
managed the patient, provided suggestions for patient treatment,
and collected and analyzed patient data. XLZ, XFW and YFC confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of the
article was obtained from the patient on diagnosis.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hallek M and Al-Sawaf O: Chronic
lymphocytic leukemia: 2022 update on diagnostic and therapeutic
procedures. Am J Hematol. 96:1679–1705. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tadmor T and Polliack A: Optimal
management of older patients with chronic lymphocytic leukemia:
Some facts and principles guiding therapeutic choices. Blood Rev.
26:15–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schöllkopf C, Rosendahl D, Rostgaard K,
Pipper C and Hjalgrim H: Risk of second cancer after chronic
lymphocytic leukemia. Int J Cancer. 121:151–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tambaro FP, Garcia-Manero G, O'Brien SM,
Faderl SH, Ferrajoli A, Burger JA, Pierce S, Wang X, Do KA,
Kantarjian HM, et al: Outcomes for patients with chronic
lymphocytic leukemia and acute leukemia or myelodysplastic
syndrome. Leukemia. 30:325–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niscola P, Noguera NI, Catalano G, Cupelli
L, Fratoni S, Giovannini M, Mazzone C, Neri B, Scaramucci L,
Trawinska MM, et al: Double remission of simultaneously occurring
secondary AML and CLL by venetoclax monotherapy. Acta Oncol.
58:888–8890. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Y and Ye H: Progress in understanding
the mechanisms of resistance to BCL-2 inhibitors. Exp Hematol
Oncol. 11:312022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Del Poeta G, Venditti A, Del Principe MI,
Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A,
Mazzone C, et al: Amount of spontaneous apoptosis detected by
Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML).
Blood. 101:2125–2131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stubbins RJ, Francis A, Kuchenbauer F and
Sanford D: Management of acute myeloid leukemia: A review for
general practitioners in oncology. Curr Oncol. 29:6245–6259. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerra VA, DiNardo C and Konopleva M:
Venetoclax-based therapies for acute myeloid leukemia. Best Pract
Res Clin Haematol. 32:145–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen WL, Hsu YJ, Tsai WC and Tsao YT: An
unusual case of febrile neutropenia: Acute myeloid leukemia
presenting as myeloid sarcoma of the spleen. J Natl Med Assoc.
100:957–959. 2008.PubMed/NCBI
|
|
12
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World Health
Organization Classification of Haematolymphoid Tumours: Lymphoid
Neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie XY, Filie AC, Jasper GA, Fukushima PI
and Stetler-Stevenson M: Diagnosis of unexpected acute myeloid
leukemia and chronic lymphocytic leukemia: A case report
demonstrating the perils of restricted panels in flow cytometric
immunophenotyping. Cytometry. 42:114–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller MK, Strauchen JA, Nichols KT and
Phelps RG: Concurrent chronic lymphocytic leukemia cutis and acute
myelogenous leukemia cutis in a patient with untreated CLL. Am J
Dermatopathol. 23:334–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muta T, Okamura T and Niho Y: Acute
myelogenous leukemia concurrent with untreated chronic lymphocytic
leukemia. Int J Hematol. 75:187–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ornellas De Souza MH, de Souza Fernandez
T, Diamond HR, Maioli MC, Pitanga Bacha PC and De Lucena SB:
Cytogenetic and immunophenotypic evidence of independent clonal
origins of concomitant chronic lymphocytic leukaemia and acute
myeloid leukaemia. Eur J Haematol. 66:281–283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu CM, Murata-Collins JL, Wang E, Siddiqi
I and Lawrence H: Concurrent acute myeloid leukemia with
inv(16)(p13.1q22) and chronic lymphocytic leukemia: Molecular
evidence of two separate diseases. Am J Hematol. 81:963–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottardi M, Gattei V, Degan M, Bomben R,
Zucchetto A, Tecchio C, Laurino L, Zanatta L, Dei Tos AP,
Mordacchini M, et al: Concomitant chronic lymphocytic leukemia and
acute myeloid leukemia: Evidence of simultaneous expansion of two
independent clones. Leuk Lymphoma. 47:885–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen RR, Zhu LX, Wang LL, Li XY, Sun JN,
Xie MX, Zhu JJ, Zhou D, Li JH, Huang X, et al: Synchronous
diagnosis and treatment of acute myeloid leukemia and chronic
lymphocytic leukemia: Two case reports. World J Clin Cases.
9:9144–9150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shoyele O and Gupta G: Synchronous
Diagnosis of de novo acute myeloid leukemia with inv(16)(p13q22)
and chronic lymphocytic leukemia: A case report and review of the
literature. Ann Clin Lab Sci. 48:790–796. 2018.PubMed/NCBI
|
|
22
|
Katz JB, Curran AL, Zemba-Palko V, Dabrow
MB and Denshaw-Burke MT: Synchronous diagnosis of chronic
lymphocytic leukemia and acute myeloid leukemia. J Clin Oncol.
28:e726–e728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Kim YM, Lu X, Wang X, Pang H, Li
Y, Li S and Lee JY: Characterization of a novel t(2;5;11) in a
patient with concurrent AML and CLL: A case report and literature
review. Cancer Genet. 204:328–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeFilipp Z, Huynh DV, Fazal S and Sahovic
E: Allogeneic stem cell transplantation for acute myeloid leukemia
with del(7q) following untreated chronic lymphocytic leukemia.
Hematol Oncol Stem Cell Ther. 5:165–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kajtár B, Rajnics P, Egyed M and Alizadeh
H: Case report: Concomitant chronic lymphocytic leukaemia and
cytogenetically normal de novo acute leukaemia in a patient. Ann
Clin Lab Sci. 45:602–606. 2015.PubMed/NCBI
|
|
26
|
Milosevic I: Coexistence of chronic
lymphocytic leukemia and acute myeloid leukemia. Turk J Haematol.
33:353–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Licci S: Concurrence of chronic
lymphocytic leukaemia/small lymphocytic lymphoma and acute myeloid
leukaemia in a bone marrow biopsy. Pol J Pathol. 71:285–287. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al Mussaed E, Osman H and Elyamany G:
Simultaneous existence of acute myeloid leukemia and chronic
lymphocytic leukemia: A case report. BMC Cancer. 16:7392016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito S, Fujiwara SI, Mashima K, Umino K,
Minakata D, Nakano H, Yamasaki R, Kawasaki Y, Sugimoto M, Ashizawa
M, et al: Development of acute myeloid leukemia in patients with
untreated chronic lymphocytic leukemia. Ann Hematol. 96:719–724.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HY, Park CJ, You E, Cho YU, Jang S and
Seo EJ: A case of acute myeloid leukemia concurrent with untreated
chronic lymphocytic leukemia. Ann Lab Med. 37:336–338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiso S, Sugiura H, Kuroi T, Omote R, Toji
T, Ishikawa T, Okamoto S, Nomura N, Masunari T, Sezaki N, et al:
Concurrent onset of chronic lymphocytic leukemia and atypical
phenotype acute myeloid leukemia revealed by autopsy. Case Rep
Oncol. 14:1725–1732. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boddu P, Schlette E, Thakral B, Tang G,
Pemmaraju N, Kadia T, Ferrajoli A, Ravandi F, Wierda W and Jain N:
Acute promyelocytic leukemia in a patient with chronic lymphocytic
leukemia-A case report. Hematol Oncol Stem Cell Ther. 12:161–165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su J, Veillon D, Shackelford R, Cotelingam
J, El-Osta H, Mills G, Munker R and Devarakonda S: Acute
promyelocytic leukemia and chronic lymphocytic leukemia:
Concomitant presentation of two molecularly distinct entities. J La
State Med Soc. 169:68–70. 2017.PubMed/NCBI
|
|
34
|
Forconi F and Moss P: Perturbation of the
normal immune system in patients with CLL. Blood. 126:573–581.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stengel A, Kern W, Haferlach T,
Meggendorfer M, Fasan A and Haferlach C: The impact of TP53
mutations and TP53 deletions on survival varies between AML, ALL,
MDS and CLL: an analysis of 3307 cases. Leukemia. 31:705–711. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Graf T: Differentiation plasticity of
hematopoietic cells. Blood. 99:3089–3101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mato AR, Roeker LE, Jacobs R, Hill BT,
Lamanna N, Brander D, Shadman M, Ujjani CS, Yazdy MS, Perini GF, et
al: Assessment of the efficacy of therapies following venetoclax
discontinuation in CLL Reveals BTK inhibition as an effective
strategy. Clin Cancer Res. 26:3589–3596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bewersdorf JP, Giri S, Wang R, Williams
RT, Tallman MS, Zeidan AM and Stahl M: Venetoclax as monotherapy
and in combination with hypomethylating agents or low dose
cytarabine in relapsed and treatment refractory acute myeloid
leukemia: A systematic review and meta-analysis. Haematologica.
105:2659–2663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Held L, Siu C and Shadman M: Venetoclax as
a therapeutic option for the treatment of chronic lymphocytic
leukemia: The evidence so far. Expert Opin Pharmacother.
22:655–665. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seymour JF, Kipps TJ, Eichhorst B, Hillmen
P, D'Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la
Serna J, et al: Venetoclax-Rituximab in relapsed or refractory
chronic lymphocytic leukemia. N Engl J Med. 378:1107–1120. 2018.
View Article : Google Scholar : PubMed/NCBI
|