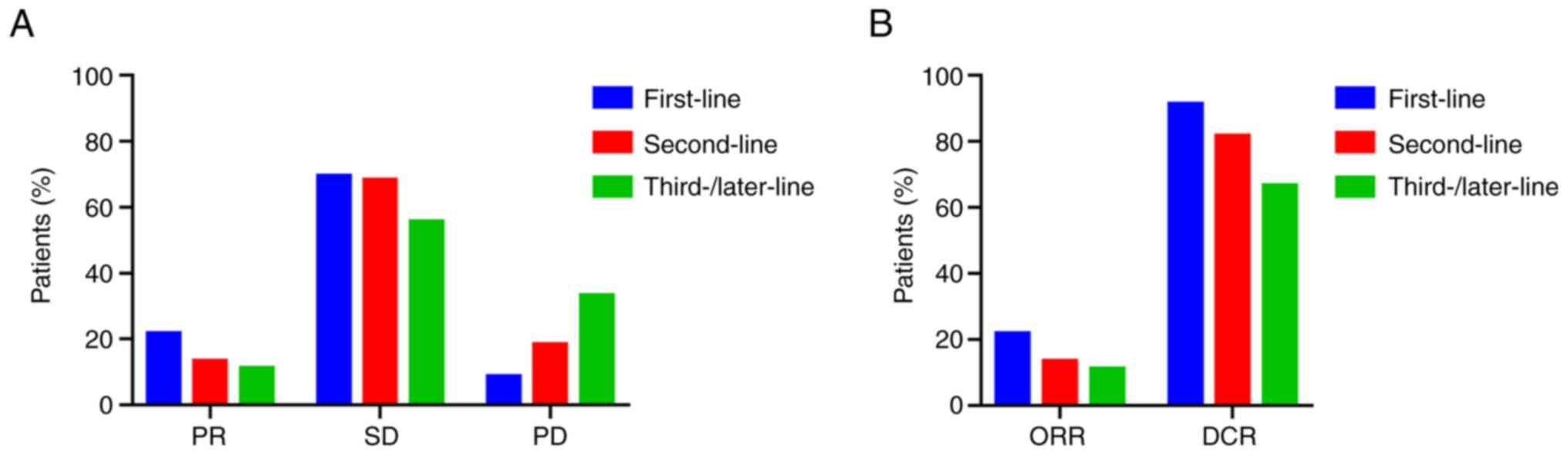
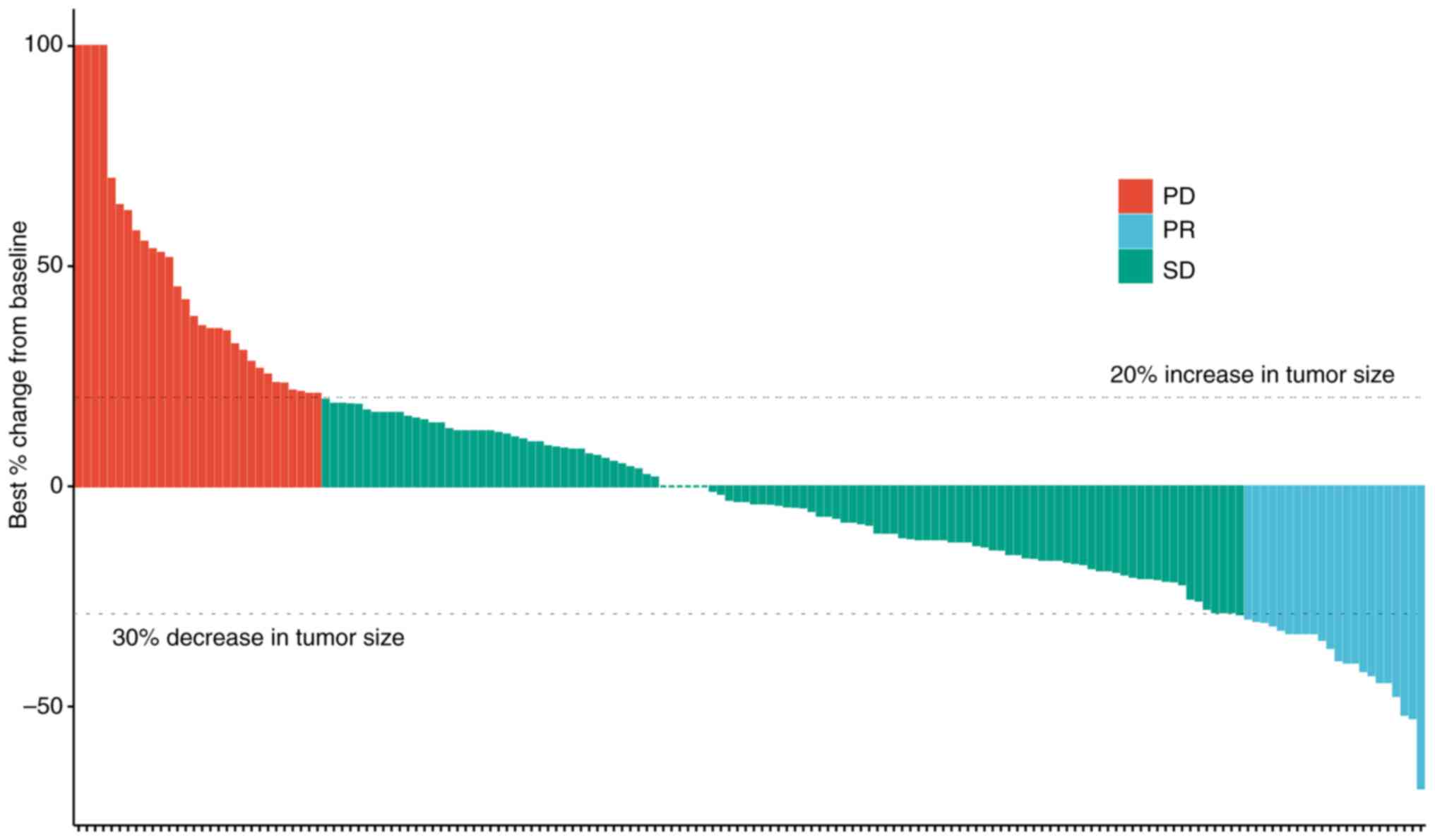
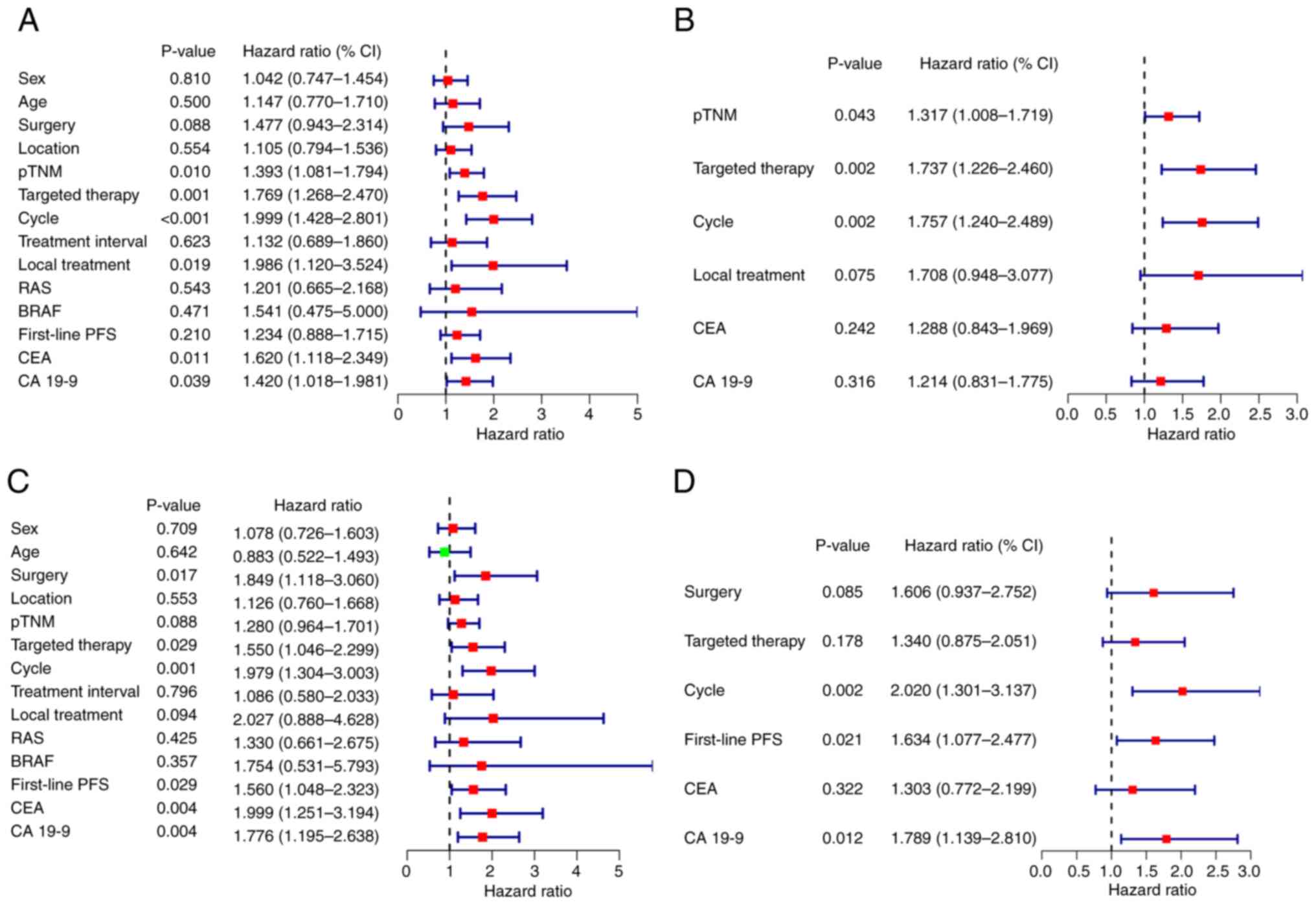
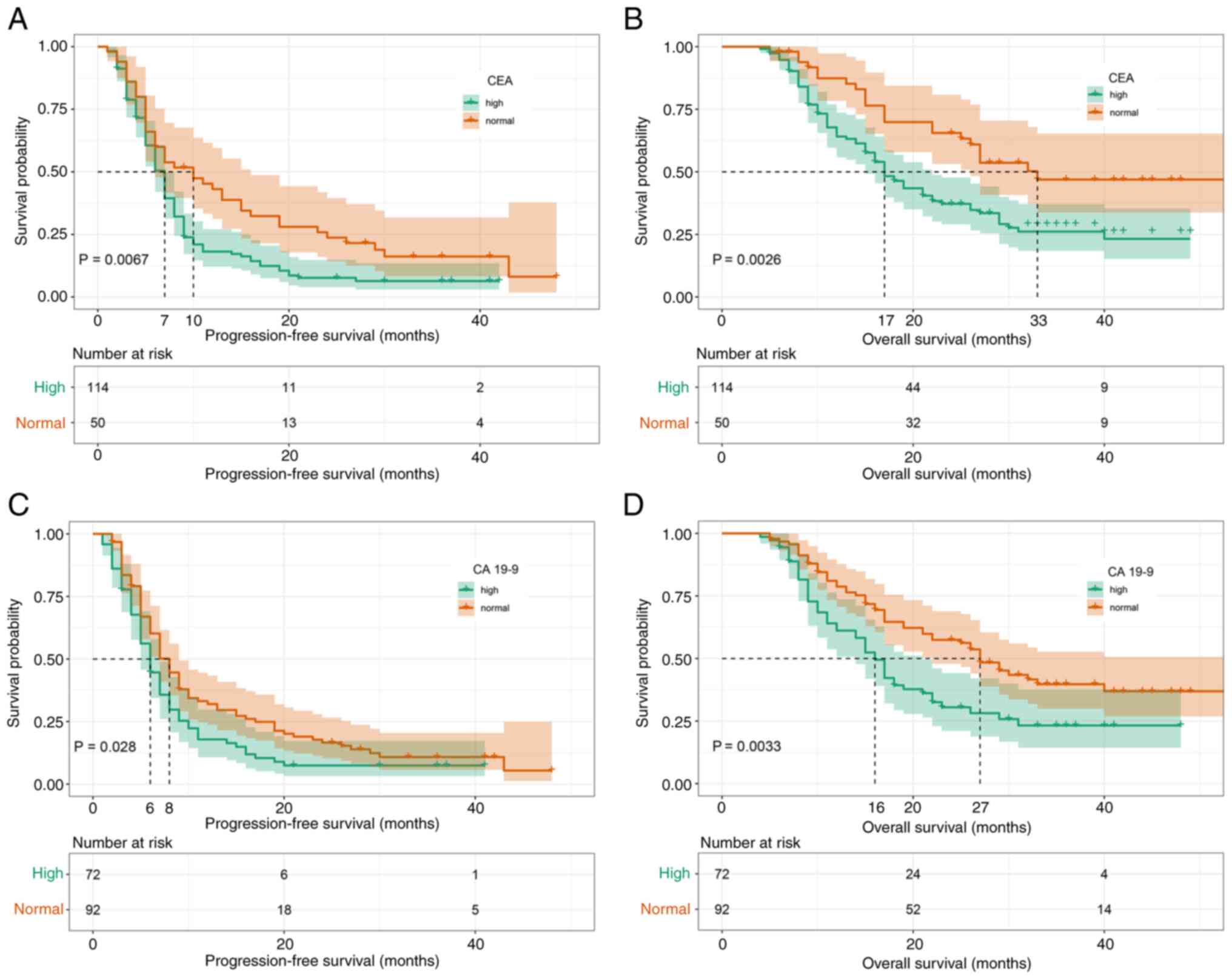
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C,
Wang J, Xiao S, Sun Q, Qian M and Zhao G: The impact of screening
on the survival of colorectal cancer in Shanghai, China: A
population based study. BMC Public Health. 19:10162019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ransom D, Wilson K, Fournier M, Simes RJ,
Gebski V, Yip D, Tebbutt N, Karapetis CS, Ferry D, Gordon S and
Price TJ: Final results of Australasian gastrointestinal trials
group ARCTIC study: An audit of raltitrexed for patients with
cardiac toxicity induced by fluoropyrimidines. Ann Oncol.
25:117–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barni S, Ghidini A, Coinu A, Borgonovo K
and Petrelli F: A systematic review of raltitrexed-based first-line
chemotherapy in advanced colorectal cancer. Anticancer Drugs.
25:1122–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batra A, Rigo R, Hannouf MB and Cheung WY:
Real-world safety and efficacy of raltitrexed in patients with
metastatic colorectal cancer. Clin Colorectal Cancer. 20:e75–e81.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aparicio J, Vicent JM, Maestu I, Garcerá
S, Busquier I, Bosch C, Llorca C, Díaz R, Fernández-Martos C and
Galán A: Multicenter phase II trial evaluating a three-weekly
schedule of irinotecan plus raltitrexed in patients with
5-fluorouracil-refractory advanced colorectal cancer. Ann Oncol.
14:1121–1125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiara S, Nobile MT, Tomasello L, Acquati
M, Taveggia P, Murolo C, Percivale P and Rosso R: Phase II trial of
irinotecan and raltitrexed in chemotherapy-naive advanced
colorectal cancer. Anticancer Res. 25:1391–1396. 2005.PubMed/NCBI
|
|
8
|
Aparicio J, de las Peñas R, Vicent JM,
Garcerá S, Llorca C, Maestu I, Yuste AL and Farrés J: Multicenter
phase I study of irinotecan plus raltitrexed in patients with
5-fluorouracil-refractory advanced colorectal cancer. Oncology.
63:42–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-standardisation and disease-specific adaptations:
Perspectives from the RECIST working group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basch E, Dueck AC, Rogak LJ, Mitchell SA,
Minasian LM, Denicoff AM, Wind JK, Shaw MC, Heon N, Shi Q, et al:
Feasibility of implementing the patient-reported outcomes version
of the common terminology criteria for adverse events in a
multicenter trial: NCCTG N1048. J Clin Oncol. 36:JCO20187886202018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Han D, Hou Y, Chen H and Chen Z:
Statistical inference methods for two crossing survival curves: A
comparison of methods. PLoS One. 10:e01167742015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong C, Ding Y, Weng S, Li G, Huang Y, Hu
H, Zhang Z, Zhang S and Yuan Y: Update in version 2021 of CSCO
guidelines for colorectal cancer from version 2020. Chin J Cancer
Res. 33:302–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fohlen A, Bordji K, Assenat E, Gongora C,
Bazille C, Boulonnais J, Naveau M, Breuil C, Pérès EA, Bernaudin M
and Guiu B: Anticancer drugs for intra-arterial treatment of
colorectal cancer liver metastases: In-vitro screening after short
exposure time. Pharmaceuticals (Basel). 14:6392021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gallois C, Hafliger E, Auclin E, Perret A,
Coutzac C, Turpin A, Pellat A, Randrian V, Basile D, Faroux R, et
al: First-line chemotherapy with raltitrexed in metastatic
colorectal cancer: An association des gastro-entérologues
oncologues (AGEO) multicentre study. Dig Liver Dis. 54:684–691.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarke SJ, Yip S, Brown C, van Hazel GA,
Ransom DT, Goldstein D, Jeffrey GM, Tebbutt NC, Buck M, Lowenthal
RM, et al: Single-agent irinotecan or FOLFIRI as second-line
chemotherapy for advanced colorectal cancer; results of a
randomised phase II study (DaVINCI) and meta-analysis [corrected].
Eur J Cancer. 47:1826–1836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bendell JC, Tournigand C, Swieboda-Sadlej
A, Barone C, Wainberg ZA, Kim JG, Pericay C, Pastorelli D, Tarazi
J, Rosbrook B, et al: Axitinib or bevacizumab plus FOLFIRI or
modified FOLFOX-6 after failure of first-line therapy for
metastatic colorectal cancer: A randomized phase II study. Clin
Colorectal Cancer. 12:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng K, Zhou YW, Chen Y, Li ZP, Qiu M and
Liu JY: Biweekly raltitrexed combined with irinotecan as
second-line therapy for patients with metastatic colorectal cancer:
A phase II trial. Cancer Control. 29:107327482210803322022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arhin ND, Shen C, Bailey CE, Matsuoka LK,
Hawkins AT, Holowatyj AN, Ciombor KK, Hopkins MB, Geiger TM, Kam
AE, et al: Surgical resection and survival outcomes in metastatic
young adult colorectal cancer patients. Cancer Med. 10:4269–4281.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong EYT, Tan GHC, Ng DWJ, Koh TPT, Kumar
M and Teo MCC: Surgical management of metastatic colorectal cancer:
A single-centre experience on oncological outcomes of pulmonary
resection vs cytoreductive surgery and HIPEC. J Gastrointest
Cancer. 48:353–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino T, Obermannová R, Bodoky G,
Garcia-Carbonero R, Ciuleanu T, Portnoy DC, Kim TW, Hsu Y, Ferry D,
Nasroulah F and Tabernero J: Baseline carcinoembryonic antigen as a
predictive factor of ramucirumab efficacy in RAISE, a second-line
metastatic colorectal carcinoma phase III trial. Eur J Cancer.
78:61–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palmieri LJ, Fihri A, Doat S, Dubreuil O,
Manceau G, Karoui M, Wagner M, Lucidarme O and Bachet JB:
Tumor-size responses to first-line is a predictor of overall
survival in metastatic colorectal cancer. Eur Radiol. 29:3871–3880.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavacchi D, Roviello G, Giommoni E, Dreoni
L, Derio S, Brugia M, Amedei A, Pillozzi S and Antonuzzo L:
Aflibercept plus FOLFIRI as second-line treatment for metastatic
colorectal cancer: A single-institution real-life experience.
Cancers (Basel). 13:38632021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennouna J, Hiret S, Bertaut A, Bouché O,
Deplanque G, Borel C, François E, Conroy T, Ghiringhelli F, des
Guetz G, et al: Continuation of bevacizumab vs cetuximab plus
chemotherapy after first progression in KRAS wild-type metastatic
colorectal cancer: The UNICANCER PRODIGE18 randomized clinical
trial. JAMA Oncol. 5:83–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda C, Honda M, Tanaka C, Fukunaga M,
Ishibashi K, Munemoto Y, Hata T, Bando H, Oshiro M, Kobayashi M, et
al: Multicenter randomized phase II clinical trial of oxaliplatin
reintroduction as a third- or later-line therapy for metastatic
colorectal cancer-biweekly versus standard triweekly XELOX (the
ORION study). Int J Clin Oncol. 21:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurwitz H, Mitchell E, Cartwright T, Kwok
A, Hu S, McKenna E and Patt YZ: A randomized, phase II trial of
standard triweekly compared with dose-dense biweekly capecitabine
plus oxaliplatin plus bevacizumab as first-line treatment for
metastatic colorectal cancer: XELOX-A-DVS (dense versus standard).
Oncologist. 17:937–946. 2012. View Article : Google Scholar : PubMed/NCBI
|