Introduction
Hepatocellular carcinoma (HCC) ranks sixth in cancer
morbidity and fourth in cancer-related mortality globally. It is a
global disease burden, especially in Asia, where it accounts for
~72% of HCC cases (1,2). Owing to the late presentation of
symptoms, >50% of patients with HCC are diagnosed at an advanced
stage (3). Aside from the
traditional molecular classification, HCC has recently begun to be
classified according to the immunological environment, including
active immune phenotypes (with enriched T cell response effectors),
exhausted immune phenotypes [featured by T cell exhaustion,
immunosuppressive macrophages and transforming growth factor β
(TGFβ) signaling], and excluded immune phenotypes
(immunosuppressive signatures in the surrounding tissues of the
tumor but with little immune gene expression in the tumor core)
(4). Moreover, the aforementioned
immunological classification of advanced HCC is associated with
different survival rates, which attracts the attention of
clinicians to HCC immunity (5).
Mucosa-associated lymphoid tissue 1 (MALT1) is an
intracellular signaling gene with both protease activity and
scaffold function. It facilitates tumorigenesis by modulating
cancer cell proliferation, migration and stemness in several solid
cancers (6–10). A previous study reported that MALT1
serves as an oncogene by enhancing tumor cell proliferation and
invasion in prostate carcinoma (8),
and another study demonstrated that the MALT1 gene potentiates the
crosstalk between TGFβ and nuclear factor κB (NF-κB) to participate
in tumor progression (10).
Notably, one study reported that MALT1 paracaspase was upregulated
and facilitated cancer growth in an HCC cell line (9).
In addition to the direct oncogenic role, MALT1 also
activates NF-κB signaling to regulate cytotoxic T lymphocytes and
immune escape (9,11,12).
For instance, a previous study reported that MALT1 restrained
antitumor immunity by facilitating cluster of differentiation
(CD)8+ T cell exhaustion (9). Another study reported that MALT1
decreased the activity of tumor-infiltrating CD8+ T
cells and elevated the immunosuppressive effects of regulatory T
cells (Tregs) in malignant melanoma (11). Notably, a previous study reported
that MALT1 induced adaptive immune resistance and thereby weakened
the response of tumor cells to immune-checkpoint inhibitor (ICI)
treatment (12). Furthermore, the
ICI-involved systemic treatment modality emerges with the evolving
therapeutic landscape of advanced HCC and brings certain survival
benefits (13,14). For instance, a phase III clinical
trial (KEYNOTE-240) found that pembrolizumab following sorafenib
plus best supportive care prolonged the survival of patients with
advanced HCC compared to those with placebo plus best supportive
care [hazard ratio (HR)=0.781, P=0.0238] (15). Another study showed that
atezolizumab plus bevacizumab resulted in a better progression-free
survival (PFS) compared to sorafenib in patients with unresectable
HCC (median PFS, 6.8 vs. 4.3 months) (16). However, the ICI efficacy is varied
among each patient with advanced HCC and, the treatment response of
ICI is still unmet in certain patients (17).
Therefore, the present study aimed to assess the
clinical significance of MALT1 for estimating ICI treatment
outcomes in patients with advanced HCC, which, to the best of our
knowledge, has not been reported yet.
Materials and methods
Subjects
A total of 51 patients with advanced HCC who were
treated with an ICI or ICI-based therapy in Handan Central Hospital
(Handan, China) between February 2020 and November 2022 were
consecutively enrolled in the present study. The inclusion criteria
were as follows: i) Diagnosis with primary HCC using a pathological
method; ii) Barcelona Clinic Liver Cancer stage C (18) [also recognized as China liver cancer
staging (CNLC) stage III (19)];
iii) age ≥18 years old; iv) Eastern Cooperative Oncology Group
Performance Status (ECOG PS) score ≤2 (20); v) Child-Pugh stage A or B (21); and vi) scheduled to receive ICI or
ICI-based treatment. The exclusion criteria were as follows: i)
Additional malignant diseases; ii) absence of measurable lesion to
be assessed using Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1 (22); iii)
refusal to provide peripheral blood (PB) sample for use in the
present study; and iv) pregnancy or lactation. Furthermore, 50
healthy participants were enrolled as healthy controls (HCs), whose
eligibility criteria were as follows: i) No signs of abnormalities
in recent physical examinations; ii) age and sex-matched with
patients with advanced HCC; and iii) willingness to cooperate with
this study. The Ethics Committee of Handan Central Hospital
approved the present study and all subjects gave their written
informed consent to participate.
Data and samples
Clinical characteristics were collected from
patients with advanced HCC, including demographics and
disease-related characteristics. PB samples were obtained from
patients with advanced HCC before treatment initiation, whilst
samples from HCs were obtained at enrollment.
After PB sample collection, PB mononuclear cells
(PBMCs) were isolated using the Ficoll-Paque®
centrifugation machine (GE Healthcare). Subsequently, the levels of
MALT1 in PBMCs were detected using reverse transcription
(RT)-quantitative (q)PCR. The RNeasy® Protect Mini Kit
(Qiagen GmbH) was used for total RNA extraction, and then the
PrimeScript™ RT Reagent Kit (Takara Biotechnology Co., Ltd.) was
used for RT (37°C for 15 min, 85°C for 5 sec). Subsequently, qPCR
(1 cycle of 95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C
for 10 sec) was performed using the TB Green® Fast qPCR
Mix (Takara Biotechnology Co., Ltd.). GAPDH was set as an internal
reference. The quantitation of MALT1 was calculated using the
2−ΔΔCq method (23). The
sequences of primer for MALT1 and GAPDH were the same as in a
previous study (24).
Treatment regimen
The present study was an observational study and the
authors did not intervene in the treatment of the enrolled
patients. ICI monotherapy or ICI-based treatments were administered
according to the disease status of the patients, physician
consultations and willingness of the patients to undergo the
treatments. The regimens included: i) Camrelizumab + apatinib
(25); ii) pembrolizumab +
lenvatinib (26); iii) sintilimab +
lenvatinib (27); iv) atezolizumab
+ bevacizumab (28); v) sintilimab
monotherapy (29); vi) camrelizumab
monotherapy (30); vii)
atezolizumab monotherapy (31); and
viii) nivolumab monotherapy (32).
In detail, the dosage was as follows: 200 mg camrelizumab was
administered intravenously every 2 weeks; 250 mg apatinib was given
orally on day 1 of a 21-day cycle; 200 mg pembrolizumab was
administered intravenously on day 1 of a 21-day cycle; 8 mg
lenvatinib for bodyweight <60 kg and 12 mg for bodyweight ≥60 kg
was administered orally once daily; 200 mg sintilimab was given
intravenously on day 1 of a 21-day cycle; 1,200 mg atezolizumab was
administered intravenously on day 1 of a 21-day cycle; 15 mg/kg
bevacizumab was given intravenously on day 1 over a 21-day cycle;
and 3 mg/kg nivolumab was administered intravenously every 2 weeks.
The drug treatment was continued until disease progression,
intolerable toxicity or voluntarily withdrawal from the
treatment.
Follow-up and evaluation
Patients with advanced HCC underwent routine
follow-ups, with a median follow-up of 13.3 months (range, 1.4–29.4
months). The last follow-up was performed in March 2023. During the
follow-up, patients received imaging examinations every 2 cycles
(~42 days). Based on treatment response data after 4 cycles (~3
months), the objective response rate (ORR) and disease control rate
(DCR) were calculated, which was assessed according to RECIST
version 1.1 (33). The ORR was
defined as the sum of complete response (CR) and partial response
(PR) rates, whereas the DCR was defined as the sum of CR, PR and
stable disease (SD) rates. In addition, the PFS and overall
survival (OS) were calculated according to the disease status or
death of a patient.
Statistical analysis
SPSS 26.0 (IBM Corp.) and GraphPad Prism 7.01
(Dotmatics) were used for analyzing data and plotting figures,
respectively. The Wilcoxon rank-sum test was used for comparison
analysis and the Spearman's rank correlation coefficient test was
used for correlation analysis. The receiver operating
characteristics curve demonstrated the ability of MALT1 to
differentiate patients with advanced HCC from HCs. To estimate the
effect of MALT1 on prognosis in patients with advanced HCC, MALT1
was divided into high and low levels by its median value. The
Kaplan-Meier curve was used to assess the PFS and OS, in which the
log-rank test was used for comparing PFS and OS between patients
with high and low MALT1. Univariate and multivariate Cox regression
models were used to identify factors associated with PFS and OS, in
which the forward stepwise method was performed in the multivariate
model. All factors included in the univariate model were put into
the forward stepwise-multivariate model. P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of patients with
advanced HCC
Among the 51 patients with advanced HCC, there were
7 (13.7%) females and 44 (86.3%) males, whose mean age was 59.0±8.3
years. A total of 13 (25.5%), 36 (70.6%) and 2 (3.9%) patients had
ECOG PS scores of 0, 1, and 2, respectively. Moreover, 44 (86.3%),
21 (41.2%) and 32 (62.7%) patients had portal vein invasion,
hepatic vein invasion and extrahepatic disease, respectively. A
total of 19 (37.3%) and 32 (62.7%) patients were diagnosed as CNLC
stage IIIa and IIIb, respectively. Detailed information regarding
the patients is presented in Table
I.
 | Table I.Characteristics of patients with
advanced hepatocellular carcinoma (n=51). |
Table I.
Characteristics of patients with
advanced hepatocellular carcinoma (n=51).
| Characteristic | Value |
|---|
| Age, years | 59.0±8.3 |
| Sex |
|
|
Female | 7 (13.7) |
|
Male | 44 (86.3) |
| History of
drinking |
|
|
Yes | 30 (58.8) |
| No | 21 (41.2) |
| HBV |
|
|
Yes | 40 (78.4) |
| No | 11 (21.6) |
| Liver
cirrhosis |
|
|
Yes | 28 (54.9) |
| No | 23 (45.1) |
| ECOG PS score |
|
| 0 | 13 (25.5) |
| 1 | 36 (70.6) |
| 2 | 2 (3.9) |
| Child-Pugh
stage |
|
| A | 33 (64.7) |
| B | 18 (35.3) |
| Largest tumor size,
cm | 8.8 (6.7–11.2) |
| Portal vein
invasion |
|
|
Yes | 44 (86.3) |
| No | 7 (13.7) |
| Hepatic vein
invasion |
|
|
Yes | 21 (41.2) |
| No | 30 (58.8) |
| Extrahepatic
disease |
|
|
Yes | 32 (62.7) |
| No | 19 (37.3) |
| BCLC stage C | 51 (100.0) |
| CNLC stage |
|
|
IIIa | 19 (37.3) |
|
IIIb | 32 (62.7) |
| AFPa, ng/ml | 226.3
(26.8–2219.6) |
| PD-L1 CPS |
|
| ≥1 | 37 (72.5) |
|
<1 | 14 (27.5) |
Treatment information of patients with
advanced HCC
A total of 19 (37.3%) patients received ICI therapy
as a first-line treatment, whilst 32 (62.7%) patients were treated
with ICI therapy as a second-line treatment. Furthermore, 11
(21.6%), 5 (9.8%), 5 (9.8%), 4 (7.8%), 9 (17.6%), 8 (15.7%), 6
(11.8%) and 3 (5.9%) patients received camrelizumab + apatinib,
pembrolizumab + lenvatinib, sintilimab + lenvatinib, atezolizumab +
bevacizumab, sintilimab monotherapy, camrelizumab monotherapy,
atezolizumab monotherapy and nivolumab monotherapy, respectively
(Table II).
 | Table II.Treatment information of patients
with advanced hepatocellular carcinoma (n=51). |
Table II.
Treatment information of patients
with advanced hepatocellular carcinoma (n=51).
| Item | n (%) |
|---|
| Treatment line |
|
| 1 | 19 (37.3) |
| 2 | 32 (62.7) |
| Regimen |
|
|
Camrelizumab + apatinib | 11 (21.6) |
|
Pembrolizumab +
lenvatinib | 5 (9.8) |
|
Sintilimab + lenvatinib | 5 (9.8) |
|
Atezolizumab +
bevacizumab | 4 (7.8) |
|
Sintilimab monotherapy | 9 (17.6) |
|
Camrelizumab monotherapy | 8 (15.7) |
|
Atezolizumab monotherapy | 6 (11.8) |
|
Nivolumab monotherapy | 3 (5.9) |
Blood MALT1 levels in patients with
advanced HCC and HCs
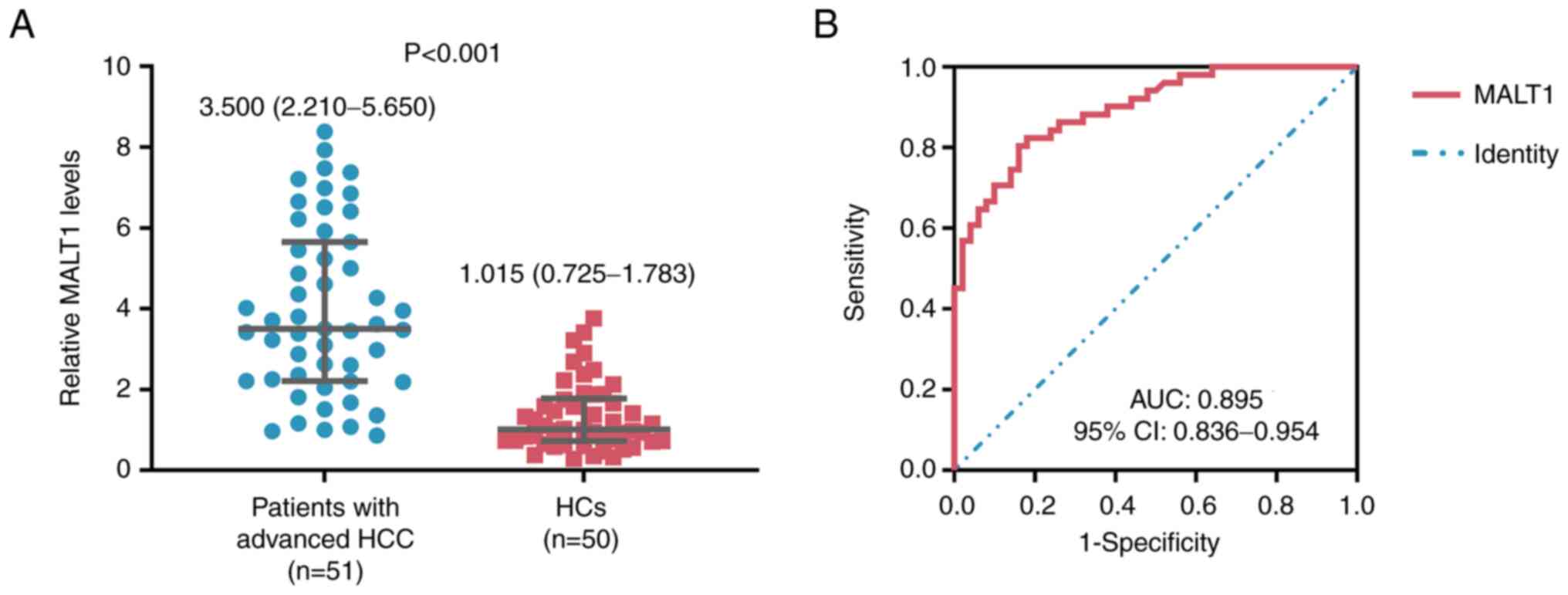
Blood MALT1 levels were significantly increased in
patients with advanced HCC compared with HCs (P<0.001; Fig. 1A) and it possessed a good ability to
distinguish patients with advanced HCC from HCs (area under the
curve, 0.895; 95% confidence interval, 0.836–0.954; Fig. 1B).
Relationship between blood MALT1
levels and tumor features in patients with advanced HCC
Blood MALT1 levels were significantly increased in
patients with portal vein invasion (vs. without portal vein
invasion; P=0.010), extrahepatic disease (vs. without extrahepatic
disease; P=0.026) and α-fetoprotein (AFP) ≥200 ng/ml (vs. AFP
<200 ng/ml; P=0.040). However, blood MALT1 levels were not
significantly correlated with ECOG PS score (r=0.193, P=0.175) or
significantly varied in patients with Child-Pugh stage A (vs. stage
B; P=0.145), largest tumor size >10 cm (vs. ≤10 cm; P=0.053),
hepatic vein invasion (vs. without; P=0.157) or programmed cell
death 1 ligand 1 combined positive score (PD-L1 CPS) ≥1 (vs. PD-L1
CPS<1; P=0.095) (Table
III).
 | Table III.Relationship between
mucosa-associated lymphoid tissue 1 in patients with advanced
hepatocellular carcinoma and different tumor features. |
Table III.
Relationship between
mucosa-associated lymphoid tissue 1 in patients with advanced
hepatocellular carcinoma and different tumor features.
| Feature | MALT1, median
(IQR) | P-value |
|---|
| ECOG PS score |
| 0.175a |
| 0 | 2.250
(1.595–5.055) |
|
| 1 | 3.665
(2.905–5.853) |
|
| 2 | 4.520
(2.050-NA) |
|
| Child-Pugh
stage |
| 0.145b |
| A | 3.420
(2.125–5.120) |
|
| B | 4.315
(3.073–6.555) |
|
| Largest tumor size
>10 cm |
| 0.053b |
| No | 3.100
(1.745–5.120) |
|
|
Yes | 3.985
(3.350–6.413) |
|
| Portal vein
invasion |
| 0.010b |
| No | 1.810
(1.160–2.880) |
|
|
Yes | 3.875
(2.420–6.145) |
|
| Hepatic vein
invasion |
| 0.157b |
| No | 3.405
(2.198–4.903) |
|
|
Yes | 3.800
(2.285–6.820) |
|
| Extrahepatic
disease |
| 0.026b |
| No | 3.230
(1.810–3.800) |
|
|
Yes | 4.740
(2.308–6.615) |
|
| AFP ≥200 ng/ml |
| 0.040b |
| No | 2.980
(2.195–4.315) |
|
|
Yes | 4.315
(3.133–6.278) |
|
| PD-L1 CPS ≥1 |
| 0.095b |
| No | 4.580
(3.193–7.045) |
|
|
Yes | 3.420
(2.195–4.935) |
|
Association between blood MALT1 levels
and clinical response in patients with advanced HCC who received
ICI therapy
After 4 cycles of ICI therapy, 0 (0.0%), 15 (29.4%),
20 (39.2%) and 16 (31.4%) patients with advanced HCC had CR, PR, SD
and progressive disease, respectively; thus, the ORR and DCR were
29.4 and 68.6%, respectively (Fig.
2A). Notably, blood MALT1 levels were significantly decreased
in patients with ORR (vs. without ORR; P=0.043; Fig. 2B) and DCR (vs. without DCR; P=0.004)
(Fig. 2C).
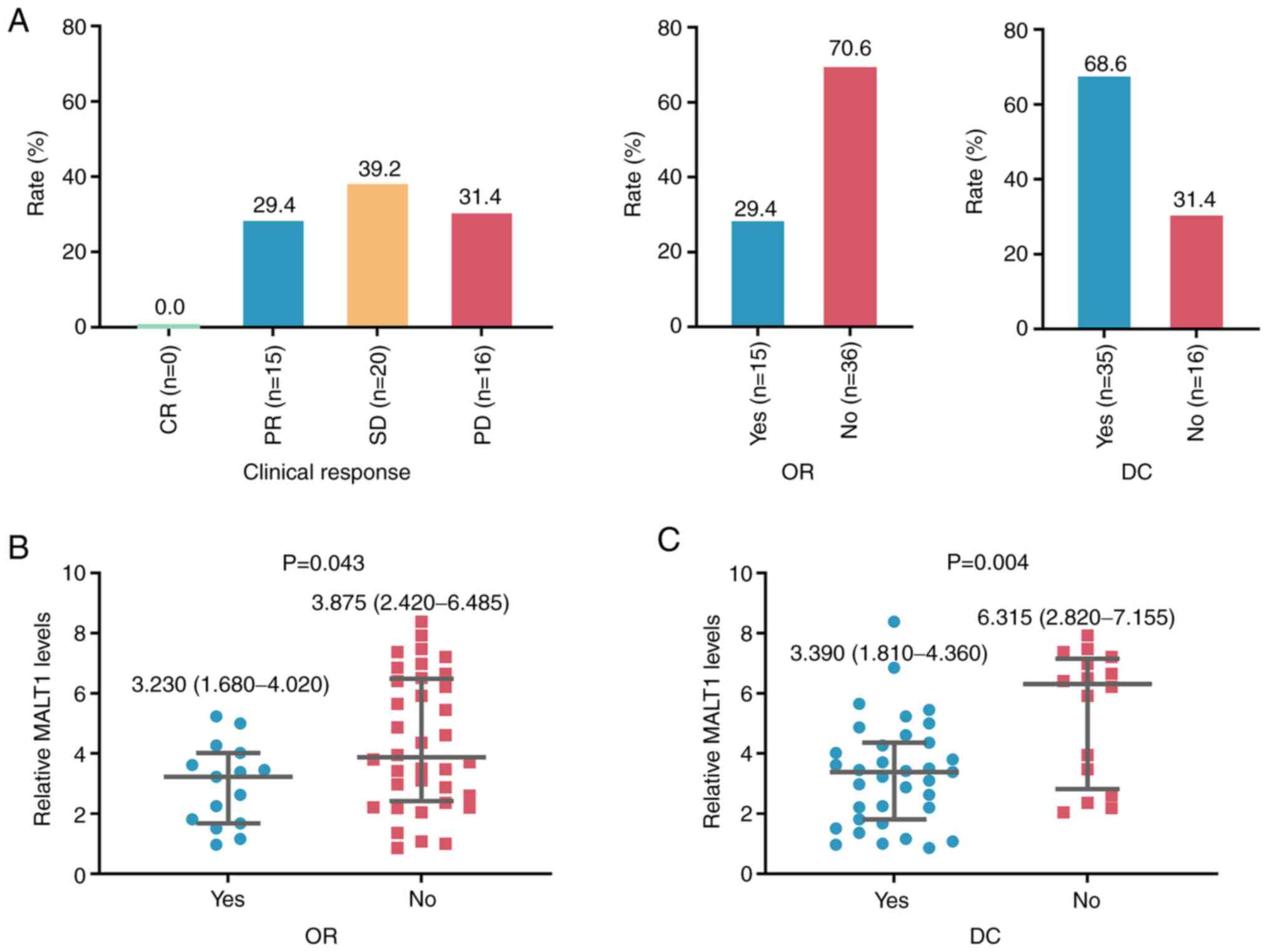 | Figure 2.Blood MALT1 levels are negatively
associated with ICI treatment response. (A) Proportions of patients
with advanced HCC with different treatment responses after ICI
therapy. Association between blood MALT1 levels and (B) ORR and (C)
DCR in patients with advanced HCC who received ICI therapy. The
median (interquartile range) level of MALT1 in patients with and
without OR was 3.230 (1.680–4.020) and 3.875 (2.420–6.485),
respectively; and it was 3.390 (1.810–4.360) and 6.315
(2.820–7.155) in patients with and without DC, accordingly. MALT1,
mucosa-associated lymphoid tissue 1; HCC, hepatocellular carcinoma;
OR, objective response; DC, disease control; ICI, immune-checkpoint
inhibitor; CR, complete response; PR, partial response; SD, stable
disease; PD, progressive disease. |
Association between blood MALT1 levels
and PFS and OS in patients with advanced HCC who received ICI
therapy
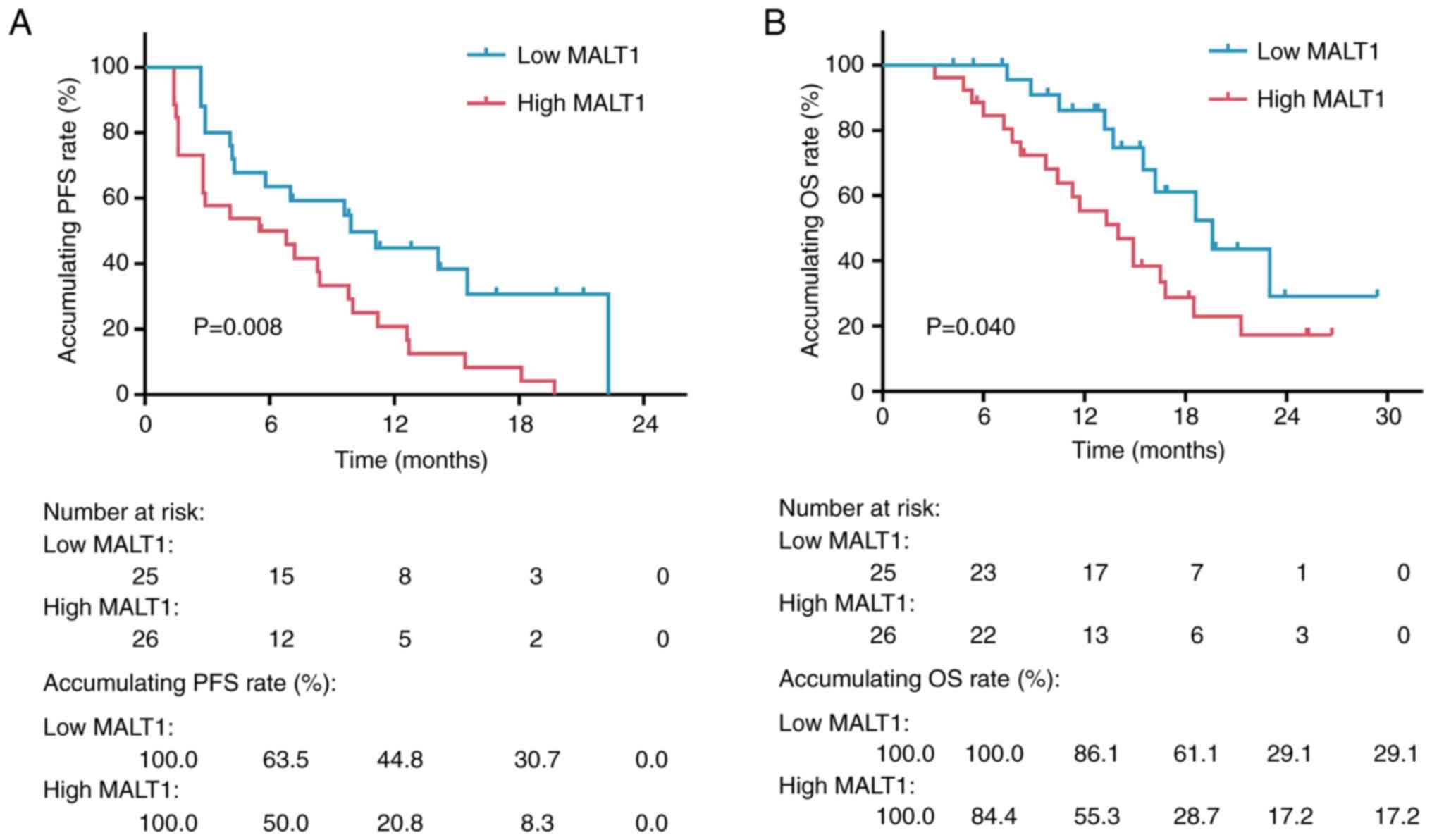
Accumulating PFS was shortened in patients with high
blood MALT1 levels compared to those with low blood MALT1 levels
(P=0.008). Specifically, the 6-, 12-18- and 24-month accumulating
PFS rates in patients with high blood MALT1 levels were 50.0, 20.8,
8.3 and 0.0%, respectively, whereas they were 63.5, 44.8, 30.7 and
0.0% in patients with low blood MALT1 levels (Fig. 3A).
Moreover, accumulating OS was shortened in patients
with high blood MALT1 levels in comparison with those with low
blood MALT1 levels (P=0.040). Specifically, the 6-, 12-, 18-, 24-
and 30-month cumulative OS rates were 84.4, 55.3, 28.7, 17.2 and
17.2% in patients with high blood MALT1 levels, respectively,
whereas the rates at the aforementioned time points were 100.0,
86.1, 61.1, 29.1 and 29.1% in patients with low blood MALT1 levels,
respectively (Fig. 3B).
Risk factors associated with a shorter
PFS in patients with advanced HCC who received ICI therapy
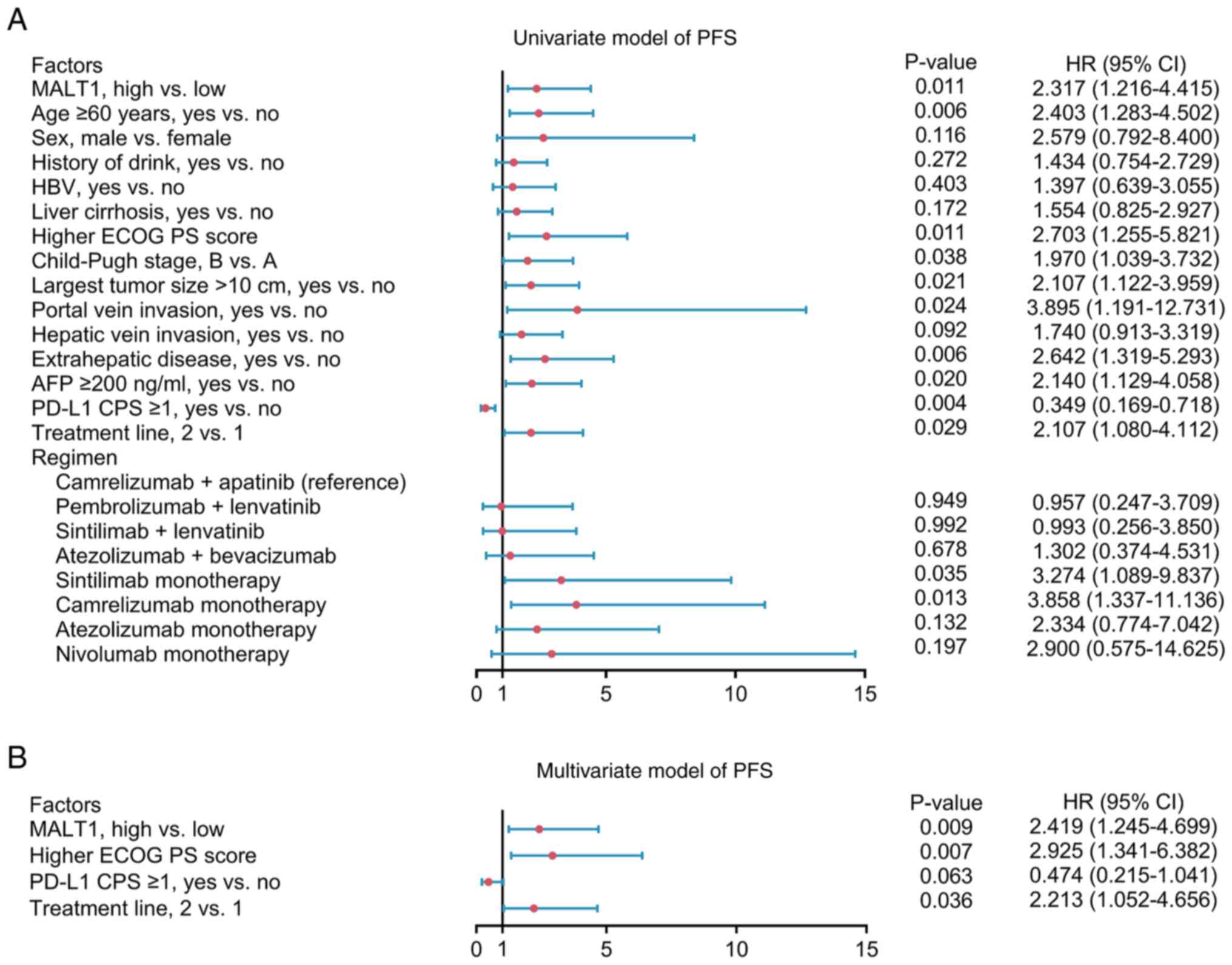
High blood MALT1 levels (P=0.011), age ≥60 years
(P=0.006), higher ECOG PS score (P=0.011), Child-Pugh stage B (vs.
A) (P=0.038), largest tumor size >10 cm (P=0.021), portal vein
invasion (P=0.024), extrahepatic disease (P=0.006), AFP ≥200 ng/ml
(P=0.020), treatment line of 2 (vs. 1; P=0.029), sintilimab
monotherapy (vs. camrelizumab + apatinib; P=0.035), and
camrelizumab monotherapy (vs. camrelizumab + apatinib; P=0.013)
were significantly associated with a shorter PFS; however, PD-L1
CPS ≥1 (P=0.004) was significantly associated with a longer PFS in
patients with advanced HCC who received ICI therapy (Fig. 4A). After adjustment, high blood
MALT1 levels [HR=2.419; P=0.009], higher ECOG PS score (HR=2.925;
P=0.007) and treatment line of 2 (vs. 1; HR=2.213; P=0.036) were
independent factors significantly associated with a shorter PFS in
patients with advanced HCC who received ICI therapy (Fig. 4B).
Risk factors associated with a shorter
OS in patients with advanced HCC who received ICI therapy
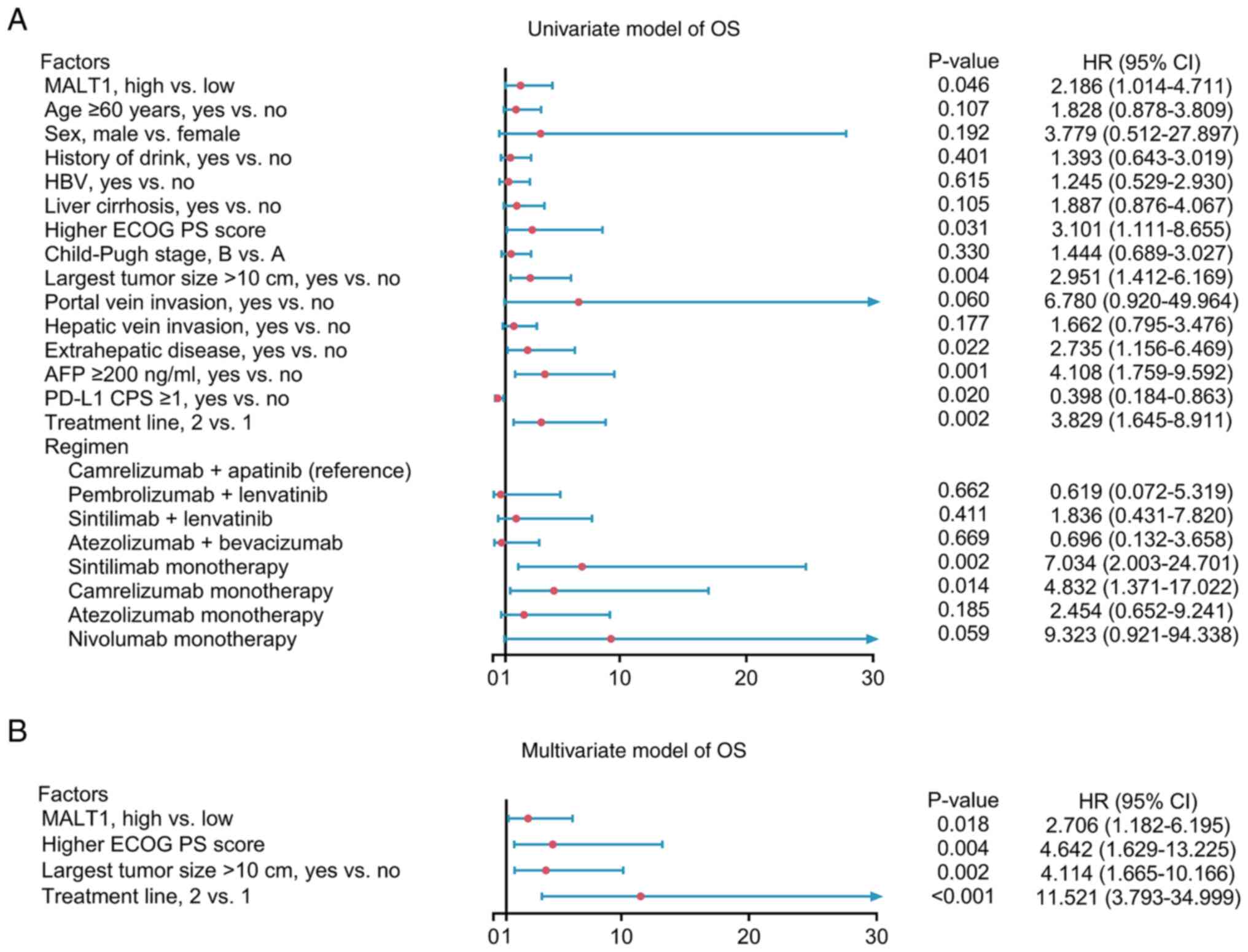
High blood MALT1 levels (P=0.046), higher ECOG PS
score (P=0.031), largest tumor size >10 cm (P=0.004),
extrahepatic disease (P=0.022), AFP ≥200 ng/ml (P=0.001), treatment
line of 2 (vs. 1; P=0.002), sintilimab monotherapy (vs.
camrelizumab + apatinib; P=0.002) and camrelizumab monotherapy (vs.
camrelizumab + apatinib; P=0.014) were significantly associated
with a shortened OS; however, PD-L1 CPS ≥1 (P=0.020) was
significantly associated with a longer OS in patients with advanced
HCC who received ICI therapy (Fig.
5A). Furthermore, high blood MALT1 levels (HR=2.706; P=0.018),
higher ECOG PS score (HR=4.642; P=0.004), largest tumor size >10
cm (HR=4.114; P=0.002) and treatment line of 2 (vs. 1; HR=11.521;
P<0.001) were independently significantly associated with a
shorter OS in patients with advanced HCC who received ICI therapy
(Fig. 5B).
Discussion
Although the oncogenic role of MALT1 is
well-elucidated, only two previous studies have investigated MALT1
in HCC, to the best of our knowledge (34,35).
For instance, one of the aforementioned studies reported that MALT1
inhibited HCC cell apoptosis and facilitated HCC progression
through competitively binding to tumor necrosis factor
receptor-associated factor (TRAF)6 with TRAF-interacting protein
with Forkhead-associated domain (34). The other study reported that MALT1
was elevated and promoted migration, invasion and tumor-forming
ability in human HCC cell lines (35). The aforementioned studies provide
evidence of molecular implications of MALT1 in HCC, whereas the
clinical role of blood MALT1 in patients with advanced HCC remains
unclear.
The present study demonstrated that blood MALT1
levels were elevated in patients with advanced HCC compared with
HCs, and increased blood MALT1 levels was associated with portal
vein invasion, extrahepatic disease and AFP ≥200 ng/ml in patients
with advanced HCC. A possible explanation could be as follows: i)
MALT1 is a well-known oncogene, whose elevation promoted tumor
development (6,7). Consequently, blood MALT1 levels were
elevated in patients with advanced HCC compared with that in HCs;
and ii) MALT1 has been reported to promote migration and invasion
in an HCC cell line (35). As a
result, blood MALT1 levels were positively associated with portal
vein invasion and extrahepatic disease in patients with advanced
HCC.
AFP, identified 60 years ago, is the most widely
used serum biomarker to detect HCC and predict the prognosis
(36). The results of the present
study demonstrated that elevated blood MALT1 levels were associated
with AFP ≥200 ng/ml in patients with advanced HCC, which may be
explained as follows: MALT1 aggravated the progression of HCC which
is typically reflected by elevated AFP (37). Consequently, elevated blood MALT1
levels was associated with AFP ≥200 ng/ml in patients with advanced
HCC. As AFP is a well-known marker of HCC, this finding of the
present study further provides evidence supporting the clinical
utilization of MALT1 in patients with advanced HCC.
Furthermore, MALT1 has recently gained additional
attention due to its role in regulating the immunological
environment (9,11,38,39).
For example, a previous study reported that MALT1 paracaspase
activity mediated the T cell receptor-induced NF-κB activation in
Tregs, which induced the conversion of resting Tregs into effector
Tregs, thus facilitating the immune escape of tumor cells.
Conversely, inhibiting MALT1 paracaspase activity could enhance
antitumor immunity (11). Another
study reported that MALT1 self-cleavage promoted interleukin-2
expression in conventional CD4+ T cells to regulate Treg
homeostasis. Moreover, inhibition of MALT1 self-cleavage can cause
Treg deficit, which enhances the antitumor immune reactivity
(40). Based on the aforementioned
results, a bioinformatic analysis identified that MALT1 could
eliminate the antitumor effect of ICI by impairing the activation
of CD8+ T cells (39).
Notably, the density of liver-infiltrated Treg cells is increased
in HCC and associated with the suppression of antitumor immunity,
meanwhile, exhausted CD8+ T cells are the landmark of
the HCC tumor microenvironment (41–43).
Therefore, the regulatory role of MALT1 on Treg cells and
CD8+ T cells suggests its involvement in antitumor
immunotherapy of HCC. In the current study, it was demonstrated
that blood MALT1 levels were negatively associated with ORR and DCR
in patients with advanced HCC who received ICI therapy. The
possible reasons are as follows: i) MALT1 attenuated the immune
surveillance function of CD8+ T cells and promoted Treg
cell-mediated immune escape, which further restrained the treatment
response of ICI therapy (12,44,45);
and ii) MALT1 activated dendritic cells to regulate
immunosuppressive factors, thus the immunotherapy resistance of HCC
cells was facilitated (46). Blood
MALT1 levels were therefore negatively associated with a reduced
ORR and DCR after ICI therapy in patients with advanced HCC.
Apart from treatment response, the present study
also demonstrated that high blood MALT1 levels were an independent
risk factor for a shortened PFS and OS in patients with advanced
HCC who received ICI therapy. The possible explanations are as
follows: i) MALT1, together with B-cell lymphoma/leukemia 10
(BCL10) and caspase recruitment domain family member (CARD) to form
the CARD-BCL10-MALT1 (CBM) complex, promoted tumor progression and
resulted in a worse survival in patients with advanced HCC
(47); and ii) MALT1 restrained the
treatment response towards ICI; thus, the survival benefits of ICI
therapy were impaired in patients with advanced HCC. Therefore,
high blood MALT1 levels were independently associated with a
shortened PFS and OS in patients with advanced HCC who received ICI
therapy.
However, the present study had the following
limitations: i) Considering that ICI treatment was only recently
used in advanced HCC, the present study could only enroll 51
eligible patients, and the small sample size weakened the
statistical power; ii) the mean age of the enrolled patients was
59.0±8.3 years, whilst the prognostic value of blood MALT1 levels
in elderly patients with HCC (generally defined as age ≥65 years)
remained unknown; and iii) MALT1 may have formed a CBM complex to
exert a biological regulatory effect; however, the other two
components of the CBM complex (BCL10 and CARD) were not detected in
the present study, which warrants further investigations; and iv)
the change of blood MALT1 during treatment was not evaluated and
its association with treatment response and survival should be
explored in the future.
In summary, high blood MALT1 levels reflect a worse
ICI-treatment response and survival in patients with advanced HCC,
and therefore, this may be a potential target to improve ICI
treatment outcomes in patients with advanced HCC that warrants
further exploration.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology Research
and Development Project of Handan (grant no. 23422083200).
Availability of data and materials
The datasets generated in the present study may be
requested from the corresponding author.
Authors' contributions
WM and LT designed the study and analyzed the data.
YY and BD collected the data and reviewed the relevant literature.
WM, YY, BD and LT wrote the original draft. LW reviewed the
relevant literature, analysed the data, prepared the tables and
figures, and revised the manuscript. WM and LT confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Handan Central Hospital
(Handan, China) approved the present study, and all subjects
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singal AG, Lampertico P and Nahon P:
Epidemiology and surveillance for hepatocellular carcinoma: New
trends. J Hepatol. 72:250–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JW, Chen M, Colombo M, Roberts LR,
Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS and
Sherman M: Global patterns of hepatocellular carcinoma management
from diagnosis to death: The BRIDGE Study. Liver Int. 35:2155–2166.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giraud J, Chalopin D, Blanc JF and Saleh
M: Hepatocellular carcinoma immune landscape and the potential of
immunotherapies. Front Immunol. 12:6556972021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, De Baere T, Kulik L, Haber PK,
Greten TF, Meyer T and Lencioni R: Locoregional therapies in the
era of molecular and immune treatments for hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 18:293–313. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Neill TJ, Tofaute MJ and Krappmann D:
Function and targeting of MALT1 paracaspase in cancer. Cancer Treat
Rev. 117:1025682023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomez Solsona B, Schmitt A,
Schulze-Osthoff K and Hailfinger S: The Paracaspase MALT1 in
Cancer. Biomedicines. 10:3442022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsui KH, Chang KS, Sung HC, Hsu SY, Lin
YH, Hou CP, Yang PS, Chen CL, Feng TH and Juang HH:
Mucosa-Associated lymphoid tissue 1 is an oncogene inducing cell
proliferation, invasion, and tumor growth via the upregulation of
NF-κB activity in human prostate carcinoma cells. Biomedicines.
9:2502021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Deng N, Yang N, Zhao X and Lin X:
Malt1 protease is critical in maintaining function of regulatory T
cells and may be a therapeutic target for antitumor immunity. J
Immunol. 202:3008–3019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazi FA, Cakiroglu E, Uysal M, Kalyoncu M,
Demirci D, Sozeri PYG, Yilmaz GO, Ozhan SE and Senturk S: The
paracaspase MALT1 is a downstream target of Smad3 and potentiates
the crosstalk between TGF-β and NF-kB signaling pathways in cancer
cells. Cell Signal. 105:1106112023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenbaum M, Gewies A, Pechloff K, Heuser
C, Engleitner T, Gehring T, Hartjes L, Krebs S, Krappmann D,
Kriegsmann M, et al: Bcl10-controlled Malt1 paracaspase activity is
key for the immune suppressive function of regulatory T cells. Nat
Commun. 10:23522019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Pilato M, Kim EY, Cadilha BL, Prussmann
JN, Nasrallah MN, Seruggia D, Usmani SM, Misale S, Zappulli V,
Carrizosa E, et al: Targeting the CBM complex causes T(reg) cells
to prime tumours for immune checkpoint therapy. Nature.
570:112–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang
M, Li Y and Jiang X: Immunotherapy for Hepatocellular Carcinoma:
Current Status and Future Prospects. Front Immunol. 12:7651012021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A Randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM, Pinyol R, Kelley RK,
El-Khoueiry A, Reeves HL, Wang XW, Gores GJ and Villanueva A:
Molecular pathogenesis and systemic therapies for hepatocellular
carcinoma. Nat Cancer. 3:386–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Sun H, Wang Z, Cong W, Wang J,
Zeng M, Zhou W, Bie P, Liu L, Wen T, et al: Guidelines for the
diagnosis and treatment of hepatocellular carcinoma (2019 Edition).
Liver Cancer. 9:682–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartz LH, Litiere S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Zhang X, Lan L, Xu G, Li Y and
Huang S: MALT1 positively correlates with Th1 cells, Th17 cells,
and their secreted cytokines and also relates to disease risk,
severity, and prognosis of acute ischemic stroke. J Clin Lab Anal.
35:e239032021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia Y, Tang W, Qian X, Li X, Cheng F, Wang
K, Zhang F, Zhang C, Li D, Song J, et al: Efficacy and safety of
camrelizumab plus apatinib during the perioperative period in
resectable hepatocellular carcinoma: A single-arm, open label,
phase II clinical trial. J Immunother Cancer. 10:e0046562022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finn RS, Ikeda M, Zhu AX, Sung MW, Baron
AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, et al:
Phase Ib study of lenvatinib plus pembrolizumab in patients with
unresectable hepatocellular carcinoma. J Clin Oncol. 38:2960–2970.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning S, Li X, Ma X, Liu J and Chang X:
Efficacy of TACE combined with lenvatinib plus sintilimab for
hepatocellular carcinoma with tumor thrombus in the inferior vena
cava and/or right atrium. J Hepatocell Carcinoma. 10:1511–1525.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Q, Wang Y, Ungchusri E, Patel M, Kumari
D, Van Ha T, Pillai A, Liao CY and Ahmed O: Combination of
transarterial radioembolization with atezolizumab and bevacizumab
for intermediate and advanced staged hepatocellular carcinoma: A
preliminary report of safety and feasibility. J Interv Med.
6:187–193. 2023.PubMed/NCBI
|
|
29
|
Chen J, Hu X, Li Q, Dai W, Cheng X, Huang
W, Yu W, Chen M, Guo Y and Yuan G: Effectiveness and safety of
toripalimab, camrelizumab, and sintilimab in a real-world cohort of
hepatitis B virus associated hepatocellular carcinoma patients. Ann
Transl Med. 8:11872020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren Y, Liu Z, Makamure J, Kan X, Song S,
Liu Y, Qian K, Zheng C and Liang B: Addition of camrelizumab to
transarterial chemoembolization in hepatocellular carcinoma with
untreatable progression. Technol Cancer Res Treat.
21:153303382211313852022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Gong JF, Pan HM, Bai YX, Liu TS,
Cheng Y, Chen YC, Huang JY, Xu TT, Ge FJ, et al: Atezolizumab
therapy in Chinese patients with locally advanced or metastatic
solid tumors: An open-label, phase I study. Beijing Da Xue Xue Bao
Yi Xue Ban. 54:971–980. 2022.(In Chinese). PubMed/NCBI
|
|
32
|
El-Khoueiry AB, Trojan J, Meyer T, Yau T,
Melero I, Kudo M, Hsu C, Kim TY, Choo SP, Kang YK, et al: Nivolumab
in sorafenib-naive and sorafenib-experienced patients with advanced
hepatocellular carcinoma: 5-year follow-up from CheckMate 040. Ann
Oncol. 35:381–391. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen W, Du R, Li J, Luo X, Zhao S, Chang
A, Zhou W, Gao R, Luo D, Wang J, et al: TIFA suppresses
hepatocellular carcinoma progression via MALT1-dependent and
-independent signaling pathways. Signal Transduct Target Ther.
1:160132016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurden-Pekmezci A, Cakiroglu E, Eris S,
Mazi FA, Coskun-Deniz OS, Dalgic E, Oz O and Senturk S: MALT1
paracaspase is overexpressed in hepatocellular carcinoma and
promotes cancer cell survival and growth. Life Sci. 323:1216902023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu X, Chen R, Wei Q and Xu X: The
landscape of alpha fetoprotein in hepatocellular carcinoma: Where
are we? Int J Biol Sci. 18:536–551. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng Y, Zhu M and Li M: Effects of
alpha-fetoprotein on the occurrence and progression of
hepatocellular carcinoma. J Cancer Res Clin Oncol. 146:2439–2446.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frizinsky S, Rechavi E, Barel O, Najeeb
RH, Greenberger S, Lee YN, Simon AJ, Lev A, Ma CA, Sun G, et al:
Novel MALT1 mutation linked to immunodeficiency, immune
dysregulation, and an abnormal T cell receptor repertoire. J Clin
Immunol. 39:401–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang N, Ji F, Cheng L, Lu J, Sun X, Lin X
and Lan X: Knockout of immunotherapy prognostic marker genes
eliminates the effect of the anti-PD-1 treatment. NPJ Precis Oncol.
5:372021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baens M, Stirparo R, Lampi Y, Verbeke D,
Vandepoel R, Cools J, Marynen P, de Bock CE and Bornschein S: Malt1
self-cleavage is critical for regulatory T cell homeostasis and
anti-tumor immunity in mice. Eur J Immunol. 48:1728–1738. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Magen A, Hamon P, Fiaschi N, Soong BY,
Park MD, Mattiuz R, Humblin E, Troncoso L, D'Souza D, Dawson T, et
al: Intratumoral dendritic cell-CD4(+) T helper cell niches enable
CD8(+) T cell differentiation following PD-1 blockade in
hepatocellular carcinoma. Nat Med. 29:1389–1399. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barsch M, Salie H, Schlaak AE, Zhang Z,
Hess M, Mayer LS, Tauber C, Otto-Mora P, Ohtani T, Nilsson T, et
al: T-cell exhaustion and residency dynamics inform clinical
outcomes in hepatocellular carcinoma. J Hepatol. 77:397–409. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Jiang Z and Zhang L: Dual effect
of T helper cell 17 (Th17) and regulatory T cell (Treg) in liver
pathological process: From occurrence to end stage of disease. Int
Immunopharmacol. 69:50–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng X, Jin W, Wang S and Ding H:
Progression on the roles and mechanisms of tumor-infiltrating T
lymphocytes in patients with hepatocellular carcinoma. Front
Immunol. 12:7297052021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lainé A, Labiad O, Hernandez-Vargas H,
This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA,
Paidassi H and Marie JC: Regulatory T cells promote cancer
immune-escape through integrin αvβ8-mediated TGF-β activation. Nat
Commun. 12:62282021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Q, He J, Li S, Tian C, Yang J, Yuan H,
Lu Y, Fagone P, Nicoletti F and Xiang M: The combination of
gemcitabine and ginsenoside Rh2 enhances the immune function of
dendritic cells against pancreatic cancer via the
CARD9-BCL10-MALT1/NF-κB pathway. Clin Immunol. 248:1092172023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hayashi H, Chiba T, Mihara-Tomiyama N,
Negishi T, Kodama Y, Sakashita H and Imai K: Domain structures of
mucosa-associated lymphoid tissue lymphoma translocation 1 protein
for nuclear localization in oral carcinoma cells and the
proliferation inhibition. Biochem Biophys Res Commun. 522:799–804.
2020. View Article : Google Scholar : PubMed/NCBI
|