Introduction
Gastrointestinal cancer is a common cause of
cancer-related mortality, of which gastric cancer exhibits the
highest mortality rate of all the gastrointestinal cancers, largely
due to the elusive nature of its early symptoms that lead to delays
in treatment (1). Risk factors for
gastric cancer include Helicobacter pylori infection,
advancing age, excessive salt consumption and dietary imbalances
(2). In early-stage gastric cancer,
tumor infiltration typically remains confined to the mucosal or
submucosal layers, irrespective of lesion size or lymph node
metastasis (3,4). Timely detection and close monitoring
of tumor progression are required to alleviate the disease burden
and mitigate the mortality rates associated with gastric cancer
(5). Presently, the increasing use
of semi-invasive endoscopic and radiological techniques is
expanding the number of treatable cases (6), and emerging studies aimed at
discerning differentially expressed molecules are gaining traction
in research (7,8). Initial attempts at employing dual
therapy with first-line platinum drugs and the chemotherapeutic
agent fluoropyrimidine yielded suboptimal outcomes in terms of
patient survival, with median survival rates still being <1 year
(2). By contrast, subsequent
targeted therapeutic modalities approved for gastric cancer
treatment, including trastuzumab, ramucizumab (an anti-angiogenic
agent) and PD-1 monoclonal antibody, have shown promise (9). However, the prognosis for the disease
remains poor, with the 5-year overall survival rate being ~25%
across all stages and reducing to <5% for the late metastatic
form of this type of cancer (10).
Consequently, there is a need for enhanced therapeutic
interventions for gastric cancer.
Lenvatinib, a multi-target tyrosine kinase
inhibitor, exerts its inhibitory effects on vascular endothelial
growth factor receptor 1-3, fibroblast growth factor receptor 1-4,
platelet-derived growth factor receptors α and β (PDGFRB), and RET
(11–13). Extensive research has indicated that
lenvatinib possesses tumor-suppressive mechanisms (14). For example, it has been discovered
to inhibit the proliferation of liver cancer cells both in
vivo and in vitro, to curb the proliferation, invasion
and migration of gallbladder cancer cells, and to promote apoptosis
via AKT signaling (15).
Furthermore, lenvatinib has demonstrated efficacy in inducing
apoptosis and autophagy in human papillary thyroid cancer cells
(16). However, investigations
(17,18) into the effects of lenvatinib on
gastric cancer remain scarce. Notably, a preclinical study has
indicated its potential to impede the growth of xenografts sourced
from patients with gastric cancer (17). In addition, a finding from a phase
II clinical trial in Japan suggested favorable outcomes when
lenvatinib was combined with pembrolizumab, an immune checkpoint
inhibitor, in the treatment of advanced gastric cancer (18). These observations indicate a
plausible role for lenvatinib in suppressing the malignant
progression of gastric cancer cells.
The present study aimed to further investigate the
effects of lenvatinib on gastric cancer cells, elucidating
underlying mechanisms through a combination of bioinformatics
analyses and experimental validation. The present study aimed to
establish a theoretical basis for the clinical application of
lenvatinib in gastric cancer treatment.
Materials and methods
Cell culture and treatment
Gastric adenocarcinoma MKN45 and gastric carcinoma
HGC27 cells (Ningbo Mingzhou Biotechnology Co., Ltd.) were cultured
in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 15% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (Ningbo
Mingzhou Biotechnology Co., Ltd.). The culture environment was
maintained at 37°C with 5% CO2.
The cells were seeded into a 6-well plate at a
density of 2×105 cells/well and were transfected with 2
µg pcDNA3.1 PDGFRB overexpression vector or an empty vector
(NovoPro Bioscience, Inc.) using RFect reagent (Baidai; Changzhou
EMI Biotechnology Co., Ltd.) at 37°C. After transfection for 48 h
at 37°C, the cells were treated with lenvatinib (40 µM; Selleck
Chemicals) for 24 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
MKN45 and HGC27 cells were seeded into 96-well
plates at a density of 3×103 cells/well, and were
incubated in the presence of gradient concentrations of lenvatinib
(0–100 µM) for 24 h at 37°C. Subsequently, 10 µl CCK-8 reagent was
added to the wells. The optical density was measured at a
wavelength of 450 nm using a microplate reader (Tecan Group, Ltd.)
after 1 h of incubation. The percentage cell inhibition rate (%)
was calculated using the following formula: Cell inhibition=(OD
value of control group-OD value of experimental group)/(OD value of
control group-OD value of blank group) ×100.
5-Ethynyl-2′-deoxyuridine (EdU)
staining
MKN45 and HGC27 cells were seeded into 96-well
plates, and cell proliferation was assessed using the EdU staining
kit (Beyotime Institute of Biotechnology). As aforementioned, MKN45
and HGC27 cells were treated with lenvatinib, transfected with
Ov-PDGFRB or Ov-NC, and were then incubated with 10 µM EdU reagent
for 2 h at 37°C. The cells were washed twice with
phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for
15 min at room temperature and the nuclei were stained with DAPI.
Images of the stained cells were captured under a fluorescence
microscope (Olympus Corporation).
Colony formation assay
MKN45 and HGC27 cells treated with lenvatinib and
transfected with Ov-PDGFRB or Ov-NC were inoculated into culture
dishes (500 cells/dish) and evenly dispersed. After culturing the
cells for 14 days, they were fixed with 4% paraformaldehyde for 30
min at room temperature and stained with 0.1% crystal violet
(Selleck Chemicals) for 30 min at room temperature. Images of
visible colonies (≥50 cells) were captured under a light microscope
and colonies were counted using ImageJ software (version 1.8;
National Institutes of Health).
Flow cytometry
Cell apoptosis was analyzed using an Annexin V-FITC
Apoptosis Detection kit (BD Biosciences). After being treated with
lenvatinib and transfected with Ov-PDGFRB or Ov-NC, the MKN45 and
HGC27 cells were digested with 0.25% trypsin and washed twice with
PBS. The cells were then suspended in binding buffer, and were
incubated with 5 µl Annexin V-FITC for 30 min, followed by
incubation with 5 µl propidium iodide for 5 min at room temperature
in the dark. Apoptosis was analyzed using a FACSCalibur flow
cytometer (BD Biosciences) and FlowJo software (version 10.7.2;
FlowJo LLC).
Western blotting
After MKN45 and HGC27 cells were treated with
lenvatinib and transfected with Ov-PDGFRB or Ov-NC, total proteins
were extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology) and were quantified using a Nanodrop
spectrophotometer (Thermo Fisher Scientific, Inc.). Protein samples
(30 µg) were separated by SDS-PAGE (10% gel; Bio-Rad Laboratories,
Inc.). PVDF membranes carrying transferred proteins were then
blocked with 5% skimmed milk for 1 h at room temperature, followed
by an overnight incubation with primary antibodies against Bax
(cat. no. 50599-2-Ig; 1:8,000 dilution; Wuhan Sanying
Biotechnology), Bcl-2 (cat. no. 12789-1-AP; 1:9,000 dilution; Wuhan
Sanying Biotechnology), PDGFRB (cat. no. 3169S; 1,000 dilution;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. 10494-1-AP;
1:20,000 dilution; Wuhan Sanying Biotechnology) at 4°C. Thereafter,
the membranes were incubated with an HRP-conjugated goat
anti-rabbit antibody (cat. no. SA00001-2; 1:5,000 dilution; Wuhan
Sanying Biotechnology) at 37°C for 1 h. Signals were visualized
using an ECL Western Blotting substrate kit (BioVision, Inc.) and
were analyzed using ImageJ software (version 1.8; National
Institutes of Health).
Molecular docking analysis
The structure of Lenvatinib was drawn in the
ChemDraw software (version 18.0) and then imported into OpenBabel
software (version 2.3.1) for hydrogenation, and converted into a
mol2 format file. Subsequently, the structure of PDGFRB (PDB ID:
AF-P09619-F1) was obtained from the RCSB PDB (https://www.rcsb.org/). Thereafter, the protein PDGFRB
file was opened in PyMOL software (version 2.2.0) to remove the
excess water molecules, delete any irrelevant small ligands
originally carried and to keep only the protein structure. As the
downloaded protein structure had ligands, the original ligands were
deleted and the original ligand positions were set as the docking
sites. AutoDock (version 1.5.6) (14) was used to display the specific
docking energy value after running. Finally, the results were
analyzed with the adoption of Protein-Ligand Interaction Profiler
(PLIP; http://plip-tool.biotec.tu-dresden.de/plip-web).
Scratch assay
After being treated with lenvatinib and transfected
with Ov-PDGFRB or Ov-NC, serum-starved MKN45 and HGC27 cells were
grown until cells reached 90% confluence, and the central cells on
the monolayer were scraped away using a 200-µl pipette tip. The
distance of migration within 24 h was analyzed using ImageJ
software (version 1.8) to calculate the migration rate under a
light microscope.
Transwell assay
After being treated with lenvatinib and transfected
with Ov-PDGFRB or Ov-NC, 1×105 MKN45 or HGC27 cells
suspended in fresh serum-free RPMI 1640 medium were seeded into the
upper chamber of Transwell plates (8-µm pore size; Costar; Corning,
Inc.) precoated with Matrigel at 37°C for 30 min, and RPMI 1640
medium containing 20% FBS was added to the lower chamber. After a
24-h incubation at 37°C, cells that passed through the Matrigel
were stained with 0.5% crystal violet at room temperature for 10
min and were captured under a light microscope. The results were
analyzed using ImageJ software (version 1.8).
Bioinformatics analysis
Differentially expressed genes between gastric
cancer tissue and paired normal tissue obtained from the GSE79973
(19) and GSE118916 (20) datasets from the GEO database
(ncbi.nlm.nih.gov/gds) were determined using the Limma package in R
software (version 4.1.2; http://www.bioconductor.org/packages/release/bioc/html/limma.html).
The differentially expressed genes in gastric cancer were acquired
from The Cancer Genome Atlas (https://www.cancer.gov/ccg/research/genome-sequencing/tcga).
The target genes of lenvatinib were analyzed through TargetNet
(http://targetnet.scbdd.com/) and
SuperPred (https://prediction.charite.de/) databases. A Venn
diagram was generated to display intersection genes, and the Gene
Expression Profiling Interactive Analysis (GEPIA) database
(http://gepia.cancer-pku.cn/) was used to
display the association between intersection genes and overall
survival.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 9.5; Dotmatics) and quantitative data are
presented as the mean ± SD of three independent experiments.
One-way ANOVA and Tukey's post hoc test were used to determine
statistical differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of lenvatinib on gastric
cancer cell proliferation and colony formation
Gradient concentrations of lenvatinib (0–100 µM)
were used to treat MKN45 and HGC27 cells, and their cell viability
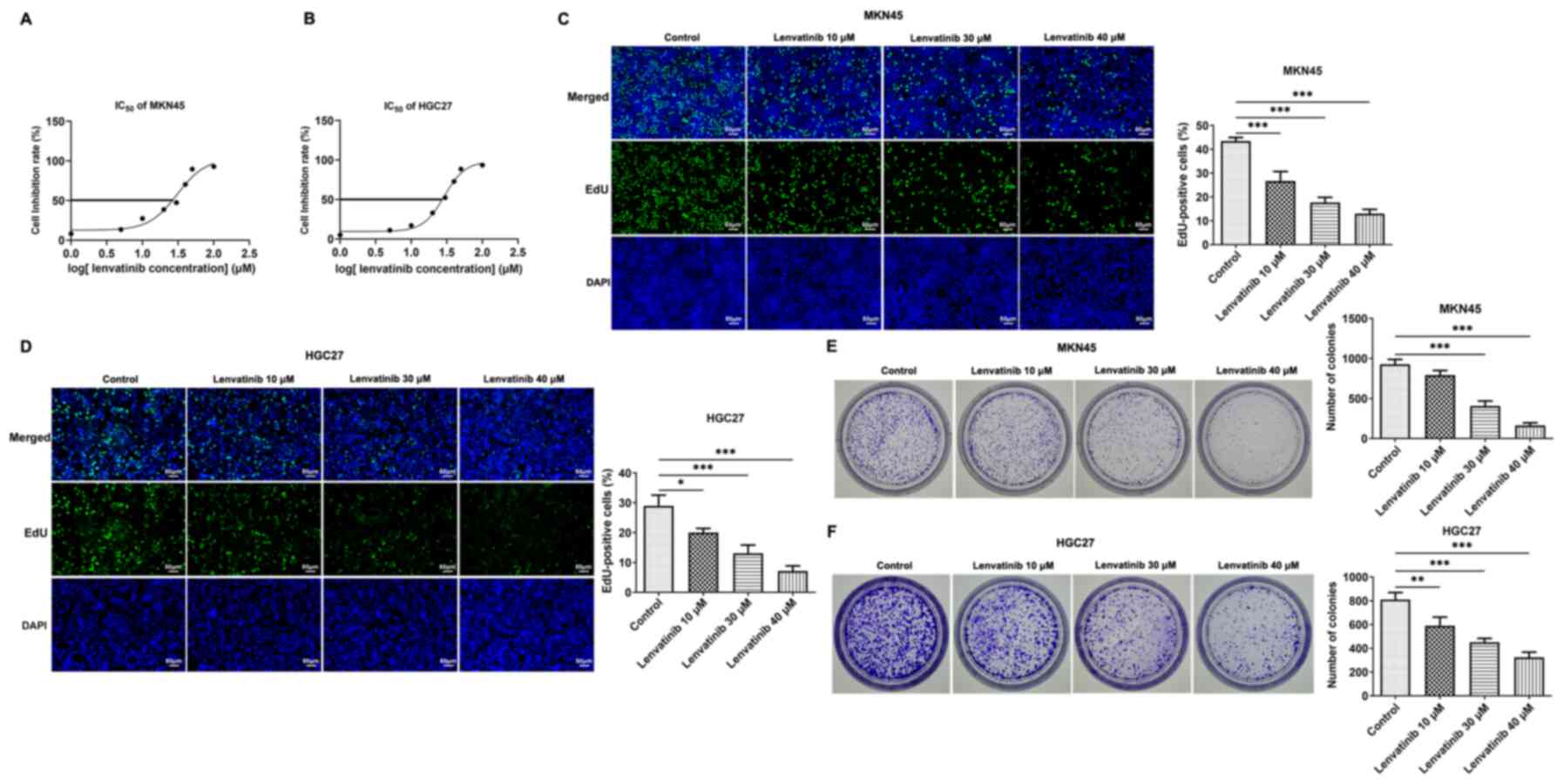
was measured at 24 h. According to the results of the CCK-8 assay,
lenvatinib inhibited the viability of both cells in a
concentration-dependent manner (Fig. 1A
and B). Given that the IC50 values of lenvatinib in
MKN45 and HGC27 cells were 30.75 and 29.03 µM at the 24-h time
point, concentrations of lenvatinib at 0, 10, 30 and 40 µM were
selected for subsequent assays. The effects of lenvatinib on cell
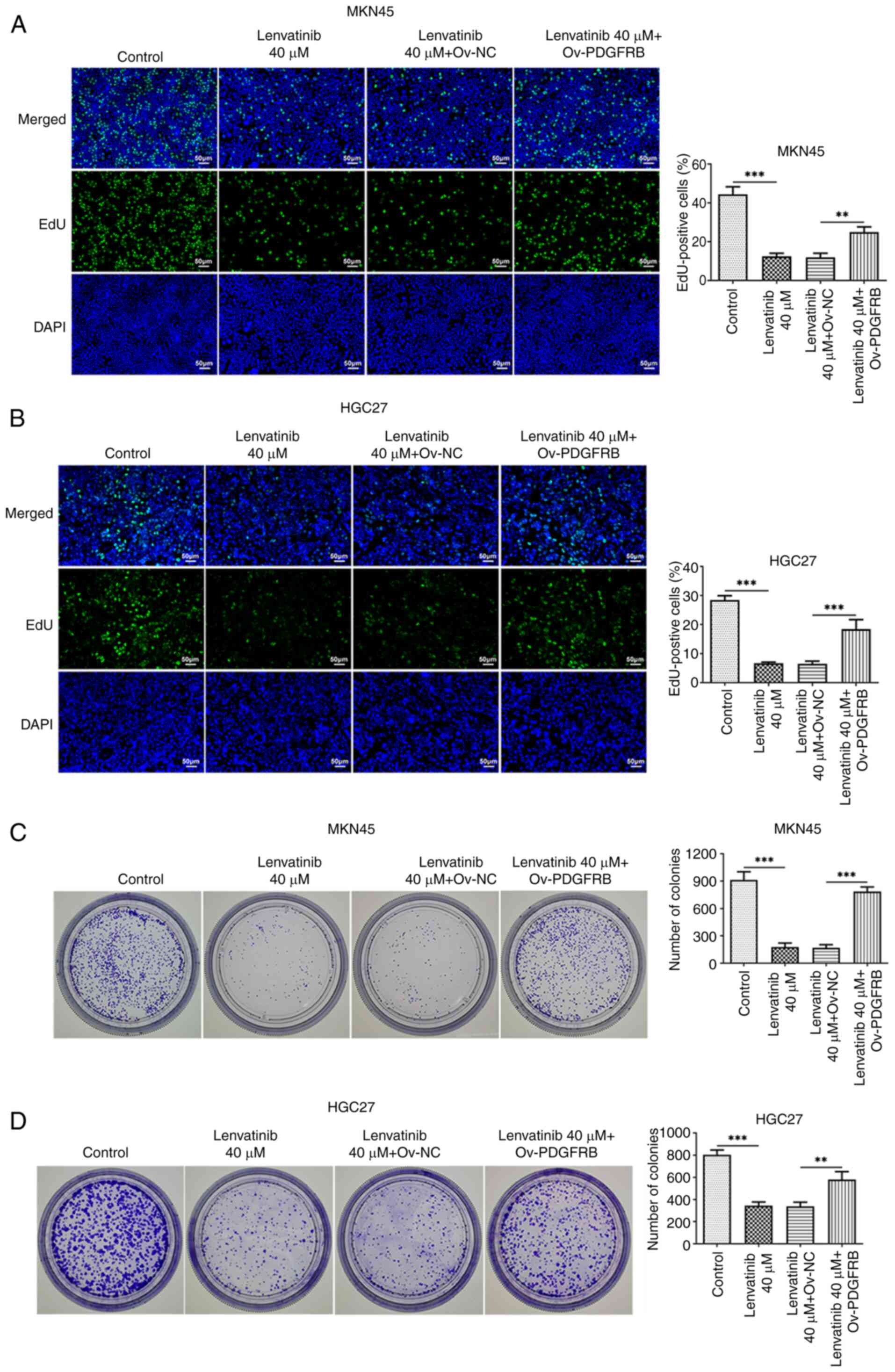
proliferation were determined by EdU staining. Proliferation levels
were quantified by assessing the proportion of EdU-positive cells.
It was discovered that Lenvatinib markedly suppressed the
proliferation of MKN45 and HGC27 cells in a concentration-dependent
manner (Fig. 1C and D). In
addition, lenvatinib inhibited colony formation and the number of
colonies visible to the naked eye was reduced (Fig. 1E and F).
Effects of lenvatinib on gastric
cancer cell apoptosis and invasion
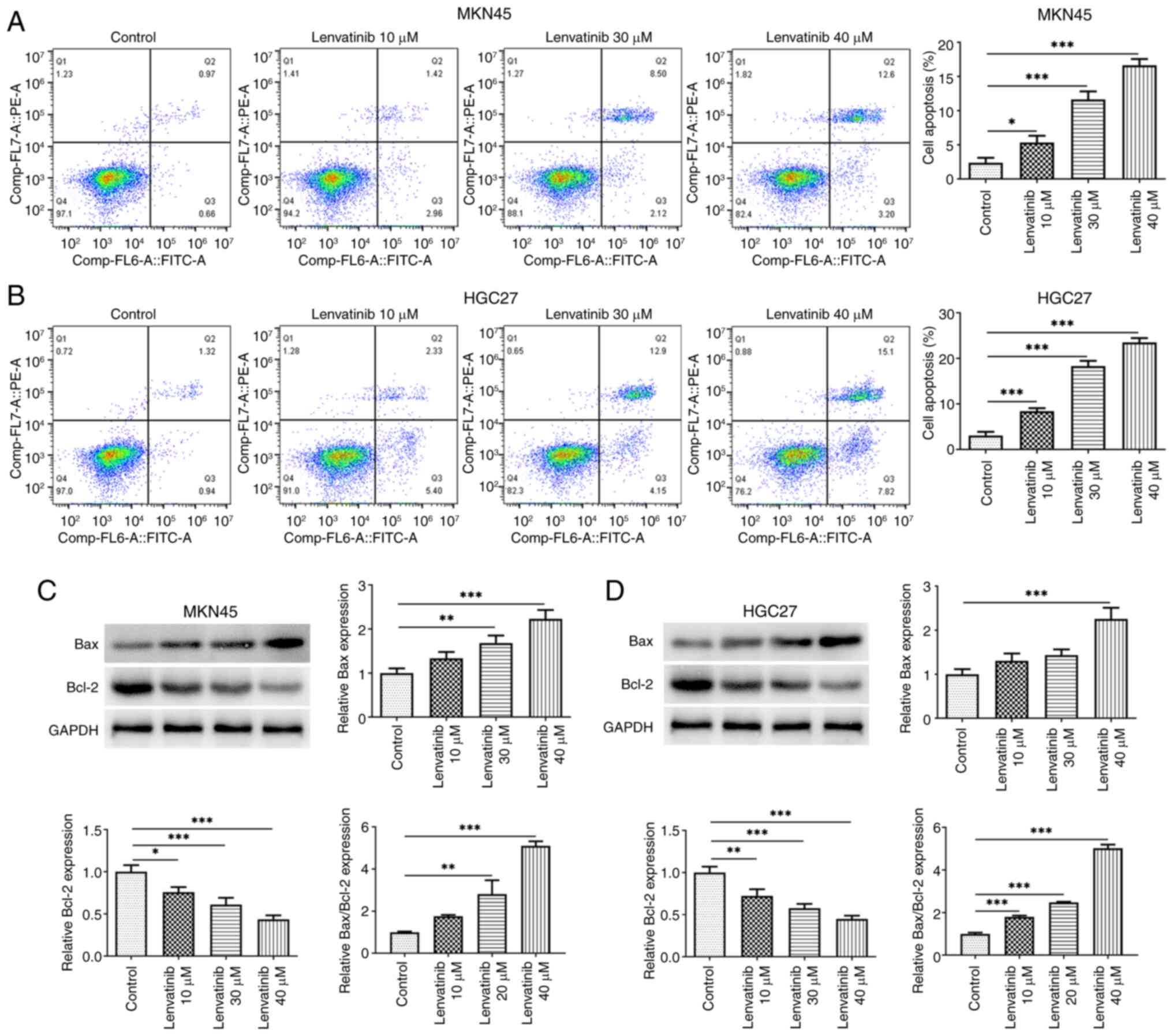
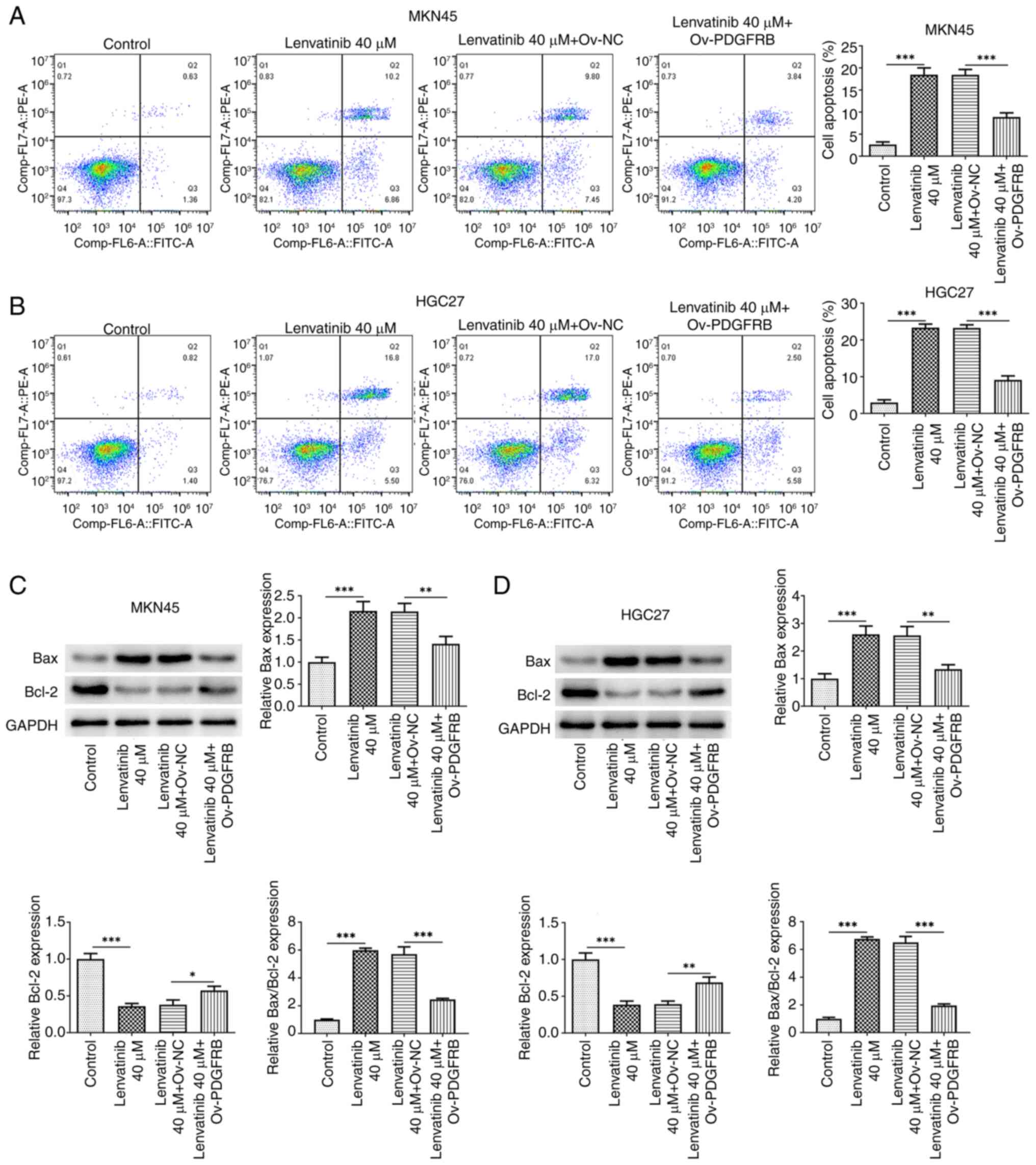
The effect of lenvatinib on MKN45 and HGC27 cell
apoptosis was evaluated by flow cytometry. Lenvatinib increased the
early and late apoptosis of both cells in a concentration-dependent
manner (Fig. 2A and B). According
to the results of western blot analysis, lenvatinib reduced Bcl-2
and increased Bax protein expression levels in cells, supporting
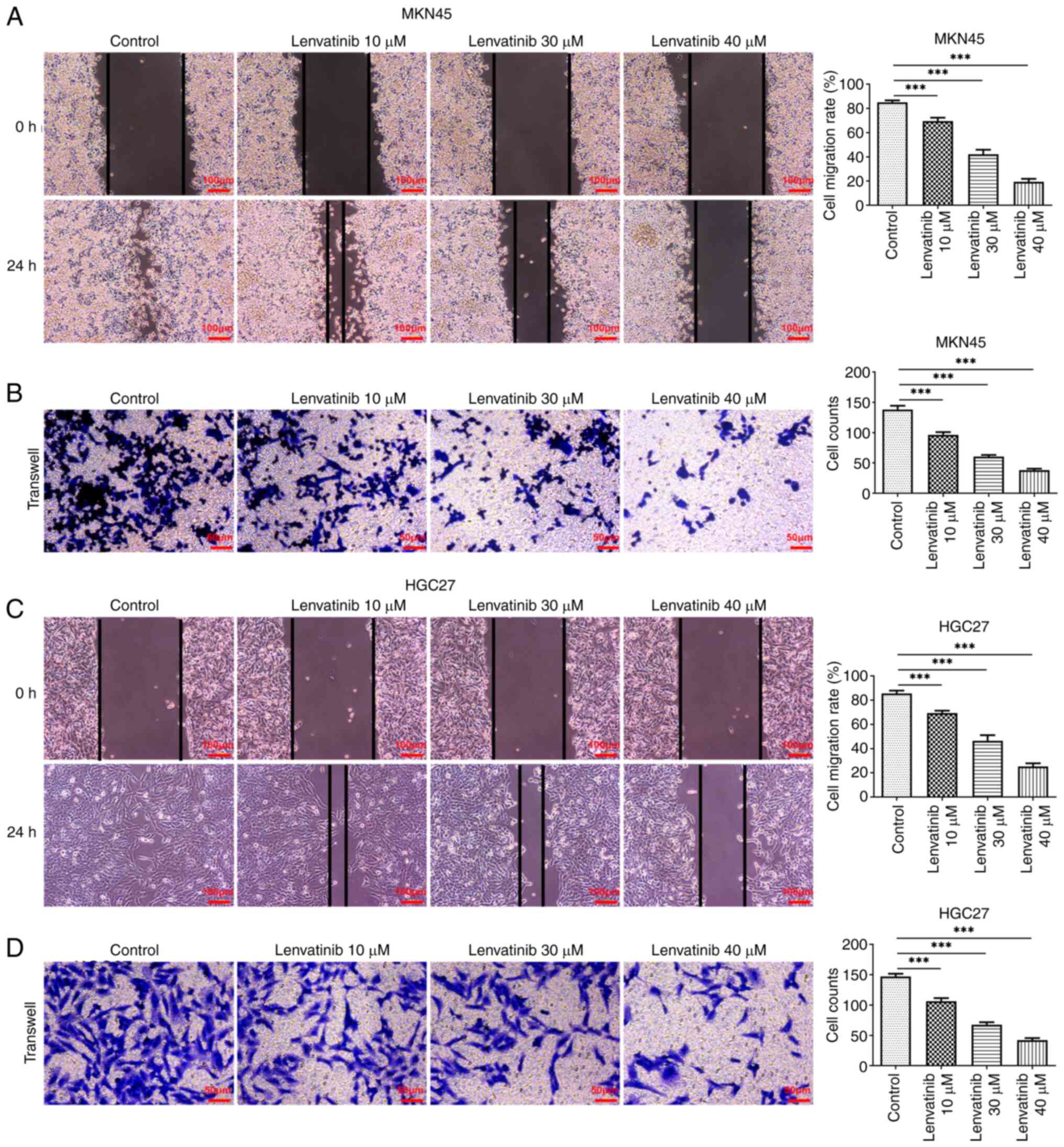
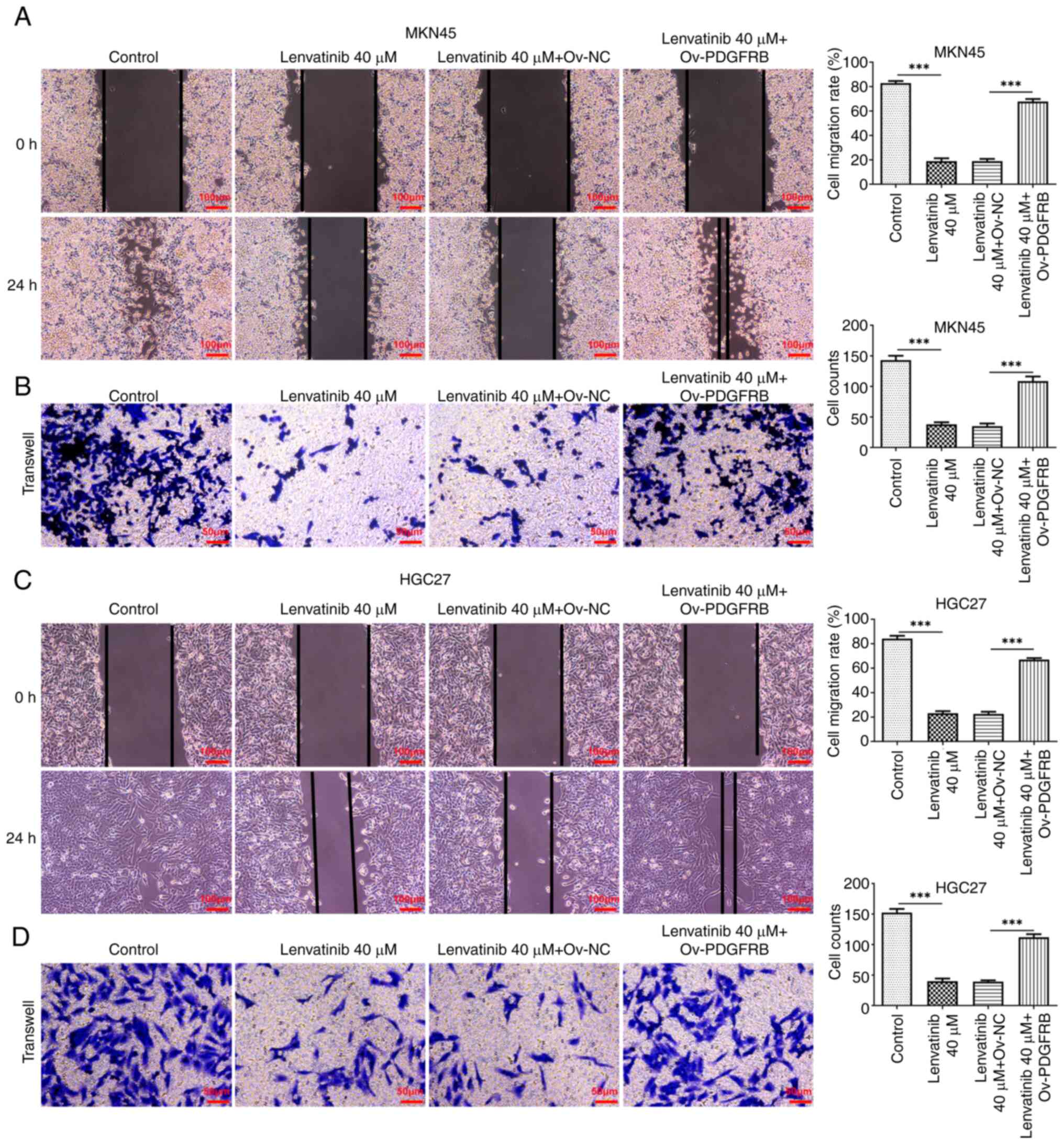
the effect of lenvatinib on apoptosis (Fig. 2C and D). Scratch and Transwell
assays were applied to evaluate the migration and invasion of the
two cell lines, respectively. Lenvatinib significantly reduced the
migration rate and the number of cells that invaded the matrix
membrane, in both MKN45 (Fig. 3A and
B) and HGC27 cells (Fig. 3C and
D).
PDGFRB is a potential target of
lenvatinib
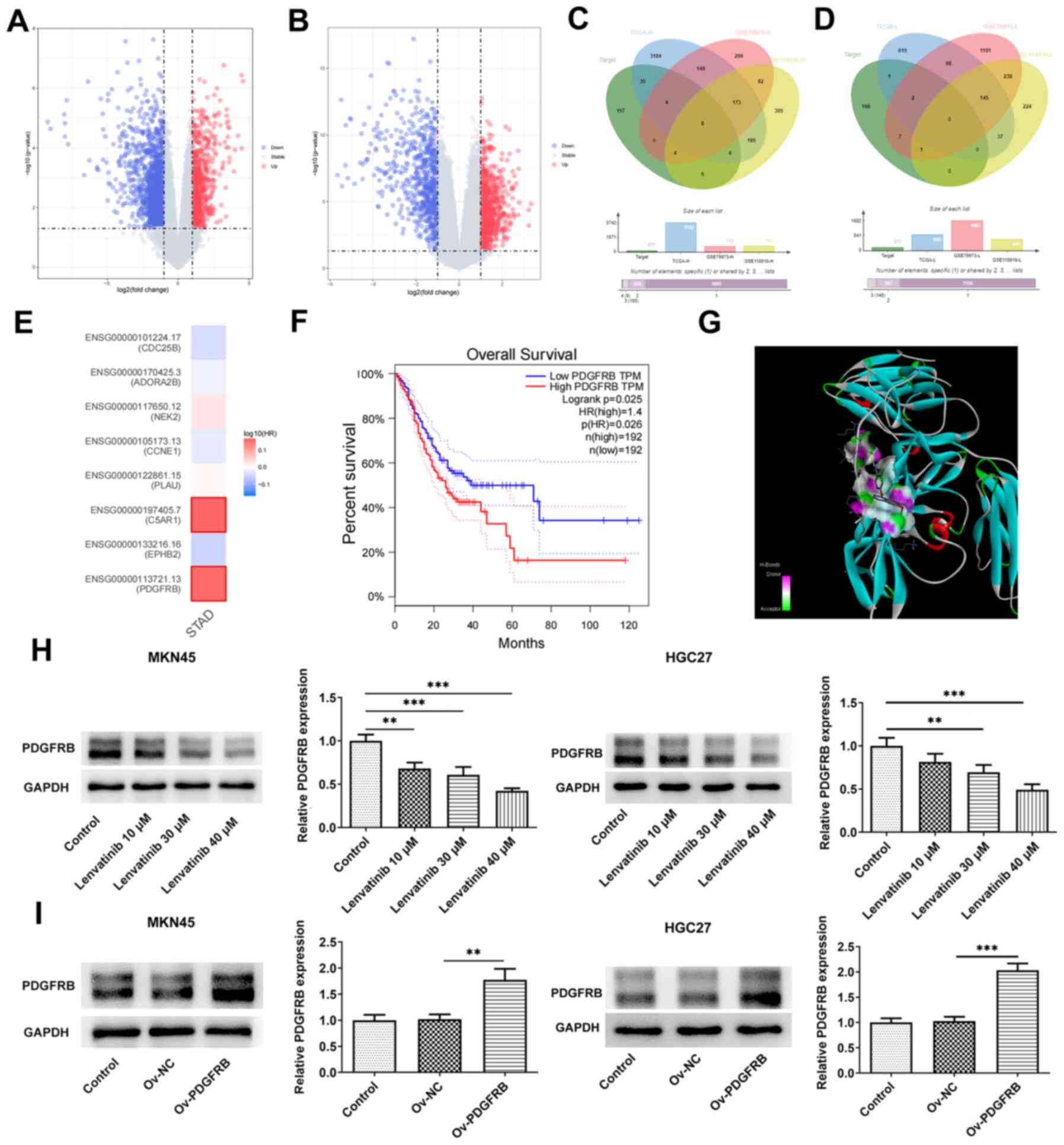
Volcano plots displaying the differentially
expressed genes in gastric cancer are shown in Fig. 4A and B. The Venn diagrams exhibit
the intersection of the predicted targets of lenvatinib and the
differentially upregulated (Fig.
4C) or downregulated (Fig. 4D)
genes in gastric cancer, suggesting that only 8 upregulated genes
in gastric cancer can act as potential targets of lenvatinib. As
depicted in Fig. 4E, the hazard
ratios of these eight intersection genes were exhibited in the
heatmap, and PDGFRB, which had the highest hazard ratio in gastric
cancer, was screened out. Based on GEPIA, low- and high-expression
PDGFRB groups were classified based on the median expression of
PDGFRB, and it was shown that high PDGFRB expression was associated
with poor survival (Fig. 4F);
therefore, the effect of lenvatinib on PDGFRB was subsequently
studied. Molecular docking analysis revealed that lenvatinib formed
multiple hydrogen bonds with PDGFRB (Fig. 4G), indicating that lenvatinib bound
well to amino acids in the protein pocket. Western blot analysis
indicated that the expression levels of PDGFRB in MKN45 and HGC27
cells were reduced in a concentration-dependent manner upon
lenvatinib treatment (Fig. 4H).
Furthermore, PDGFRB was successfully overexpressed by transfection
of MKN45 and HGC27 cells with a pcDNA3.1 PDGFRB overexpression
vector, which was confirmed by western blotting (Fig. 4I).
PDGFRB overexpression reverses the
regulatory effects of lenvatinib
To explore the regulatory effects of lenvatinib on
PDGFRB, cells were induced to overexpress PDGFRB, and the
proliferation and colony formation of the cells were evaluated. The
results demonstrated that PDGFRB overexpression promoted MKN45 and
HGC27 cell proliferation (Fig. 5A and
B) and colony formation (Fig. 5C
and D), and reversed the inhibitory effects of lenvatinib. Flow
cytometry (Fig. 6A and B) and
apoptosis-related protein analysis (Fig. 6C and D) also revealed that PDGFRB
overexpression reduced the proportion of apoptotic cells and the
protein expression levels of Bax, and increased the protein
expression levels of Bcl-2 compared with the lenvatinib 40 µM +
Ov-NC group. The migration and invasion of MKN45 (Fig. 7A and B) and HGC27 cells (Fig. 7C and D) were also enhanced in
response to PDGFRB overexpression, compared with the negative
control group, upon treatment with lenvatinib.
Discussion
The persistently high rates of mortality associated
with gastric cancer underscore the ongoing limitations in treatment
options. Patients with advanced gastric cancer often face
challenges in pursuing radical surgical interventions (21), leaving combination chemotherapy as
the predominant therapeutic option (22,23).
Nonetheless, the existing array of chemotherapeutic agents remains
limited in efficacy, while being markedly associated with toxicity
and side effects (24). By
contrast, molecular targeted therapies offer a promising
alternative characterized by reduced toxicity and enhanced efficacy
(25). Consequently, the aim to
identify novel molecular targeted drugs has emerged as a focal
point in contemporary gastric cancer research. The advent of small
molecule tyrosine kinase inhibitors has heralded advancements in
the management of various types of cancer, including gastric cancer
(26). Within this area, the
present study focused on lenvatinib, aiming to elucidate its
potential in impeding the malignancy of gastric cancer cells. The
experimental findings indicated the capacity of lenvatinib to
inhibit the viability and proliferation of MKN45 and HGC27 cells
while enhancing apoptosis. Furthermore, given the pivotal role of
metastasis in dictating cancer outcomes, the impact of lenvatinib
on the migratory capabilities of these cells was scrutinized.
Notably, lenvatinib treatment exhibited a suppressive effect on the
migration and invasion of both cell lines, underscoring its
potential as a metastasis-inhibiting agent in gastric cancer.
Given the multi-target nature of lenvatinib
(27), bioinformatics analyses were
performed to determine its principal regulatory targets in gastric
cancer. Through an intersectional analysis of lenvatinib-targeted
genes and differentially expressed genes in gastric cancer tissues,
eight genes of interest were identified. Subsequent analyses
implicated PDGFRB as a prominent target, with data from the GEPIA
database corroborating its inverse association with overall
survival rates. Notably, prior research has underscored the pivotal
role of PDGFRB in the metaplasia and dysplasia stages of gastric
carcinogenesis (28). Moreover,
synergistic interactions between PDGFRB blockade and anti-PD-1
immunotherapy have shown promise in impeding tumor growth,
underscoring the interplay between stromal reprogramming and immune
modulation in gastric cancer management (29). Another study has shown that PDGFRB
is closely related to immune cell infiltration in gastric cancer,
especially M2 macrophage infiltration (30). These studies all indicate the
beneficial effects of inhibiting PDGFRB levels on disease
management, supporting the potential use of lenvatinib for the
treatment of gastric cancer. Although this study explored the
potential mechanism of lenvatinib in the context of gastric cancer
using two cell lines, it still has certain limitations, such as the
lack of in vivo experimental data, which will be a part of
future studies.
In conclusion, the present study identified the
ability of lenvatinib to inhibit the malignant phenotype of MKN45
and HGC27 cells, with PDGFRB emerging as a pivotal mediator of its
actions. Coupled with the findings of bioinformatics analyses,
these results highlight PDGFRB as a primary target of lenvatinib in
gastric cancer management. With more clinical research being
performed on lenvatinib, it may have a future role in cancer
therapeutics.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XT, JD and SC contributed to design, and performed
experiments and manuscript drafting. QJ, QW and SZ contributed to
methods and data analysis. XT and SC confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang K, Lu L, Liu H, Wang X, Gao Y, Yang
L, Li Y, Su M, Jin M and Khan S: A comprehensive update on early
gastric cancer: Defining terms, etiology, and alarming risk
factors. Expert Rev Gastroenterol Hepatol. 15:255–273. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conti CB, Agnesi S, Scaravaglio M,
Masseria P, Dinelli ME, Oldani M and Uggeri F: Early gastric
cancer: Update on prevention, diagnosis and treatment. Int J
Environ Res Public Health. 20:21492023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia JY and Aadam AA: Advances in screening
and detection of gastric cancer. J Surg Oncol. 125:1104–1109. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Repetto O, Vettori R, Steffan A,
Cannizzaro R and De Re V: Circulating proteins as diagnostic
markers in gastric cancer. Int J Mol Sci. 24:169312023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farran B, Müller S and Montenegro RC:
Gastric cancer management: Kinases as a target therapy. Clin Exp
Pharmacol Physiol. 44:613–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Röcken C: Predictive biomarkers in gastric
cancer. J Cancer Res Clin Oncol. 149:467–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng Y and Jin RU: Molecular pathogenesis,
targeted therapies, and future perspectives for gastric cancer.
Semin Cancer Biol. 86:566–582. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li AY, McCusker MG, Russo A, Scilla KA,
Gittens A, Arensmeyer K, Mehra R, Adamo V and Rolfo C: RET fusions
in solid tumors. Cancer Treat Rev. 81:1019112019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albarrán V, Villamayor ML, Chamorro J,
Rosero DI, Pozas J, San Román M, Calvo JC, Pérez de Aguado P,
Moreno J, Guerrero P, et al: Receptor tyrosine kinase inhibitors
for the treatment of recurrent and unresectable bone sarcomas. Int
J Mol Sci. 23:137842022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fleuren EDG, Vlenterie M and van der Graaf
WTA: Recent advances on anti-angiogenic multi-receptor tyrosine
kinase inhibitors in osteosarcoma and ewing sarcoma. Front Oncol.
13:10133592023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakraborty E and Sarkar D: Emerging
therapies for hepatocellular carcinoma (HCC). Cancers (Basel).
14:27982022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YC, Hsu FT, Chung JG, Weng MC, Ting
CY, Tsai CJ, Lan A, Wu JY, Lin CC and Lin SS: Lenvatinib induces
AKT/NF-κB inactivation, apoptosis signal transduction and growth
inhibition of non-small cell lung cancer in vivo. Anticancer
Res. 41:2867–2874. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fallahi P, Ferrari SM, Galdiero MR,
Varricchi G, Elia G, Ragusa F, Paparo SR, Benvenga S and Antonelli
A: Molecular targets of tyrosine kinase inhibitors in thyroid
cancer. Semin Cancer Biol. 79:180–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawazoe A, Fukuoka S, Nakamura Y, Kuboki
Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N,
Higuchi T, et al: Lenvatinib plus pembrolizumab in patients with
advanced gastric cancer in the first-line or second-line setting
(EPOC1706): An open-label, single-arm, phase 2 trial. Lancet Oncol.
21:1057–1065. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fei HJ, Chen SC, Zhang JY, Li SY, Zhang
LL, Chen YY, Chang CX and Xu CM: Identification of significant
biomarkers and pathways associated with gastric carcinogenesis by
whole genome-wide expression profiling analysis. Int J Oncol.
52:955–966. 2018.PubMed/NCBI
|
|
19
|
Liu H, Qu Y, Zhou H, Zheng Z, Zhao J and
Zhang J: Bioinformatic analysis of potential hub genes in gastric
adenocarcinoma. Sci Prog. 104:3685042110042602021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karalis JD, Yoon LY, Hammer STG, Hong C,
Zhu M, Nassour I, Ju MR, Xiao S, Castro-Dubon EC, Agrawal D, et al:
Lenvatinib inhibits the growth of gastric cancer patient-derived
xenografts generated from a heterogeneous population. J Transl Med.
20:1162022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sexton RE, Al Hallak MN, Diab M and Azmi
AS: Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metastasis Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li K, Zhang A, Li X, Zhang H and Zhao L:
Advances in clinical immunotherapy for gastric cancer. Biochim
Biophys Acta Rev Cancer. 1876:1886152021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan WL, He Y and Xu RH: Gastric cancer
treatment: Recent progress and future perspectives. J Hematol
Oncol. 16:572023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L,
Liu JY, Pu J and Lv J: Updates on global epidemiology, risk and
prognostic factors of gastric cancer. World J Gastroenterol.
29:2452–2468. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel TH and Cecchini M: Targeted
therapies in advanced gastric cancer. Curr Treat Options Oncol.
21:702020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blay JY, Kang YK, Nishida T and von Mehren
M: Gastrointestinal stromal tumours. Nat Rev Dis Primers. 7:222021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Zhang YN, Wang KT and Chen L:
Lenvatinib for hepatocellular carcinoma: From preclinical
mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer.
1874:1883912020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SH, Contreras Panta EW, Gibbs D, Won
Y, Min J, Zhang C, Roland JT, Hong SH, Sohn Y, Krystofiak E, et al:
Apposition of fibroblasts with metaplastic gastric cells promotes
dysplastic transition. Gastroenterology. 165:374–390. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akiyama T, Yasuda T, Uchihara T,
Yasuda-Yoshihara N, Tan BJY, Yonemura A, Semba T, Yamasaki J,
Komohara Y, Ohnishi K, et al: Stromal reprogramming through dual
PDGFRα/β blockade boosts the efficacy of anti-PD-1 immunotherapy in
fibrotic tumors. Cancer Res. 83:753–770. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Xiao X, Lin Z, Lou Y and Zhao L:
PDGFRB is a potential prognostic biomarker and correlated with
immune infiltrates in gastric cancer. Cancer Biomark. 34:251–264.
2022. View Article : Google Scholar : PubMed/NCBI
|