Introduction
Gastric cancer is the third most common type of
tumor in China, with ~480,000 new cases annually (1), and the disease was estimated to
account for 4.9% of cancer cases worldwide in 2022 (2). Surgical resection is the mainstay and
gold-standard treatment for gastric cancer (3). However, post-operative complications
and unplanned readmissions are prevalent among patients with
gastric cancer (4). With the advent
of enhanced recovery protocols, hospital stays for patients
undergoing surgery have been shortened. However, this raises
concerns that the discharge of vulnerable patients could lead to
unplanned readmissions. According to a previous meta-analysis,
individuals with gastric cancer experience readmissions at a higher
rate (6.6–8.7%) than those with other types of malignancies
(5). Unplanned readmissions have a
negative impact on the quality of life and post-operative recovery
of patients, and also markedly raise the economic burden of
healthcare on patients and their families (6). It was estimated that the medical costs
associated with post-operative hospital readmission in the US
totaled $17.4 billion in 2004 (7).
Clearly, unplanned readmissions impose a high financial burden and
increased strain on health insurance and health care providers. It
has been reported that unplanned readmission is also associated
with increased mortality in patients who undergo gastrectomy
(8). Therefore, it is important to
identify the risk factors for readmission to enable the rate of
readmission after gastrectomy to be reduced.
In 2019, two meta-analyses of original literature
were performed to explore the risk predictors of 30-day unplanned
readmission after surgery for gastric cancer, both of which
included ≤10 articles (5,9). Furthermore, the outcomes of
multivariate regression analyses were used to investigate the risk
predictors, but these analyses were based on only one to three
studies for each predictor (5).
Over the subsequent 5 years, updated data and novel articles have
emerged. Therefore, an updated systematic review and meta-analysis
is required to determine the risk variables for an unplanned 30-day
readmission after gastric cancer surgery.
Materials and methods
Search strategy
Relevant studies were found mainly by searching the
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Central
Register of Controlled Trials (https://www.cochranelibrary.com), Web of Science
(https://clarivate.com) and Embase databases
(https://www.embase.com/). The main search terms
were (‘gastric carcinoma’ OR ‘gastric cancer’ OR ‘gastrectomy’) AND
(‘readmission’ OR ‘secondary admission’). The final search was
performed on March 30, 2024. Two authors independently reviewed the
title and abstract of each citation and obtained the full text of
potentially eligible studies. Disagreements were resolved through
discussion or with the involvement of the third author.
Additionally, the reference lists of all retrieved studies were
reviewed to identify any additional eligible studies. The study
protocol was registered at PROSPERO (https://www.crd.york.ac.uk/PROSPERO/)
(CRD42024537213).
Inclusion and exclusion criteria
Inclusion criteria for these studies were as
follows: i) Case-control studies, randomized controlled trials or
cohort studies in humans; ii) information on 30-day unplanned
readmissions following gastric cancer surgery was included; iii)
potential causes and risk factors of multivariate analysis were
available; and iv) no limitation on surgical methods was imposed.
Exclusion criteria: i) Reviews, letters, case reports and
conference abstracts; ii) animal experiments, in vitro
studies and ongoing trials; and iii) required data were
lacking.
Data extraction and quality
assessment
The data were independently retrieved from the
included studies by two authors. The information encompassed trial
name, publication date, country, study period, number of patients,
tumor stage, surgical procedure, surgery plan, and potential causes
and risk factors for 30-day readmission. Only causes identified in
two or more studies and risk factors reported in the multivariate
analysis of two or more articles were included.
Two authors conducted independent assessments.
Differences between these two authors were settled by conversation
or by seeking the advice of the third author. No exclusions were
made based on this. The risk of bias in the eligible studies was
comprehensively assessed using the Newcastle-Ottawa Scale (NOS)
(10). The NOS has a total of 8
items and a maximum score of 9. Studies with a score <6 were
deemed moderate- or low-quality studies.
Statistical analysis
The PRISMA statement methodology (11) was utilized to report the systematic
reviews and perform the meta-analysis. All categorical variables
were non-continuous. Forest plots were automatically generated
using Review Manager 5.4 software (Cochrane Collaboration). Odds
ratios (ORs), P-values and 95% confidence intervals (CIs) were
utilized to assess the significance of differences. P<0.05 was
considered to indicate a statistically significant result.
Heterogeneity evaluation was performed using I2 for the
included studies: I2 <50% indicated no significant
heterogeneity, while I2 ≥50% denoted heterogeneity. The
random effects model was used to analyze and merge the data in
forest plots. In addition, ORs with a 95% CI based on multivariate
analysis were used to determine the potential risk predictors for
30-day readmission. To clarify the underlying source of
heterogeneity, subgroup analyses were performed according to
grouping by country, study design and surgical procedure. A
‘leave-one-out’ influence analysis was also performed to assess the
influence of individual studies of the risk predictors by removing
one study at a time. Publication bias was evaluated with a funnel
plot and Egger's test. The influence analysis and publication bias
evaluation were conducted using STATA 16.0 (StataCorp LP).
Results
Study selection
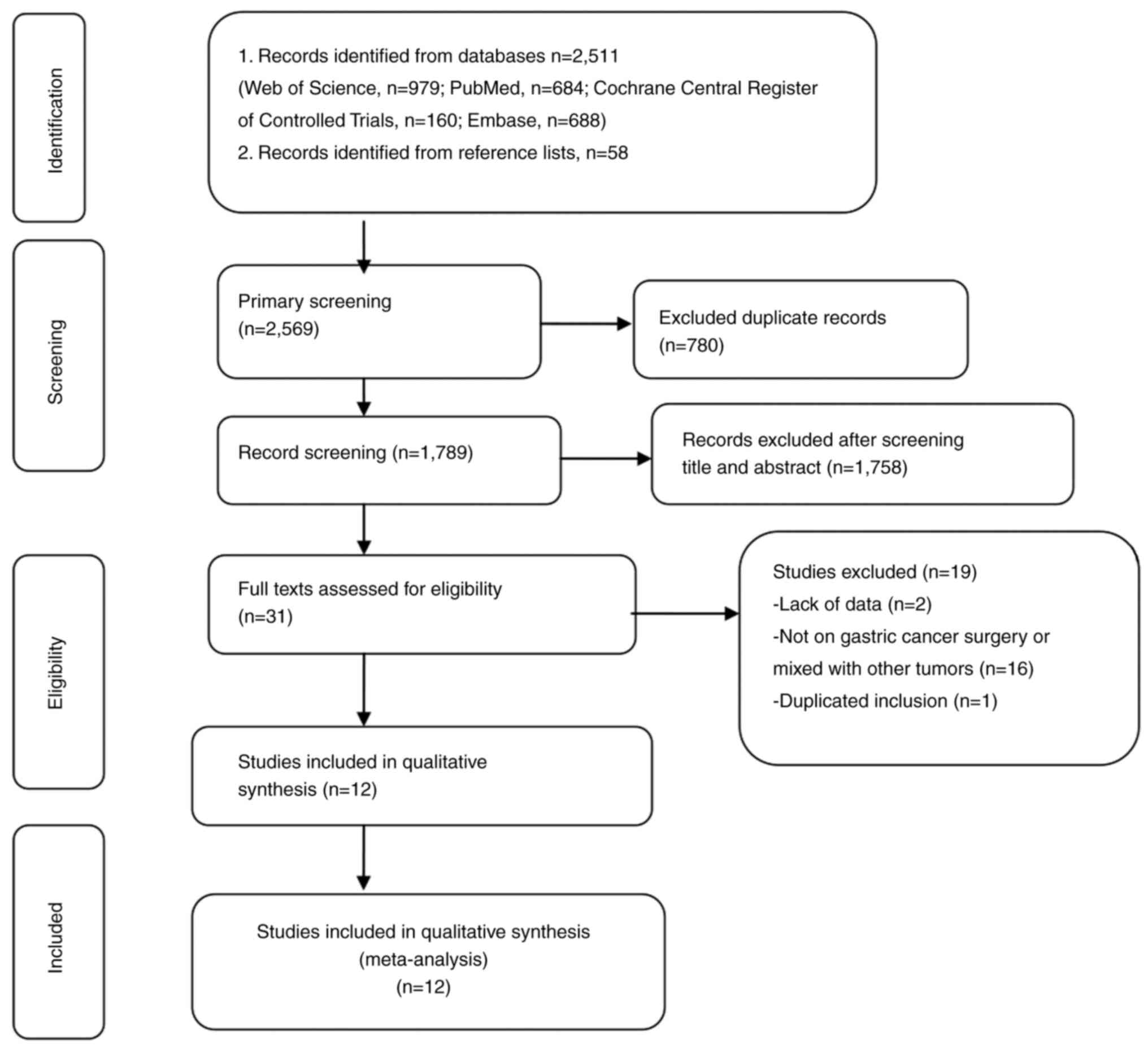
Through a systematic literature search process, a
total of 2,569 articles were initially retrieved. After the removal
of duplicates followed by the screening of individual titles and
abstracts, the full texts of 31 articles were read to assess their
eligibility. In total, 19 studies were excluded due to insufficient
data, not being on gastric cancer surgery, the gastric cancer being
mixed with other tumors, and duplication. Finally, 12 eligible
studies (12–23) remained and were included in the
present meta-analysis. A flow chart showing the selection of
literature according to PRISMA guidelines is shown in Fig. 1.
Characteristics of studies
The included studies comprised 10 retrospective
case-control studies (12,13,16–23)
and two prospective case-control studies (14,15).
Two of the retrospective studies (13,16)
were multicenter case-control studies from the United States. One
study (17) included cases of early
gastric cancer only. The present meta-analysis included a total of
16,154 patients, of whom 1,736 were readmitted and 14,418 were not
readmitted. Table I displays key
characteristics of the included studies. The quality of each study
was assessed using the NOS scoring system, and was found to range
from 6 to 8, as presented in Table
I. Detailed NOS scores are presented in Table SI).
 | Table I.Key parameters extracted from the
included studies. |
Table I.
Key parameters extracted from the
included studies.
| First author,
year | Country | Study design | Study period | R/T, n | Stage | Surgical
procedure | Surgery plan | NOS score | (Refs.) |
|---|
| Kim et al,
2012 | Korea | Retro-S | 2003-2008 | 69/461 | I–II | Radical subtotal +
G | O + L | 6 | (12) |
| Ahmad et al,
2014 | USA | Retro-M | 1995-2001 | 61/418 | I–IV | Curative + G | NA | 7 | (13) |
| Kim et al,
2014 | Korea | Pro-S | 2002-2009 | 22/102 | I–II | Radical total +
G | O + L | 7 | (14) |
| Zhuang et
al, 2015 | China | Pro-S | 2013-2014 | 27/376 | I–IV | Curative + G | O + L | 7 | (15) |
| Acher et al,
2016 | USA | Retro-M | 2000-2012 | 121/855 | I–IV | Curative + G | NA | 8 | (16) |
| Honda et al,
2016 | Japan | Retro-S | 2007-2011 | 21/1461 | I | G | O + L | 6 | (17) |
| Ammori et
al, 2018 | USA | Retro-S | 2005-2011 | 1195/6985 | NA | G | NA | 6 | (18) |
| Asaoka et
al, 2019 | Japan | Retro-S | 2010-2016 | 52/1929 | I–IV | Curative + G | O + L | 6 | (19) |
| Ma et al,
2019 | China | Retro-M | 2014-2017 | 37/585 | I–II | Curative + G | O + L | 6 | (20) |
| Xiao et al,
2018 | China | Retro-S | 2010-2017 | 60/2023 | I–IV | Curative + G | O + L | 7 | (21) |
| Cai et al,
2022 | China | Retro-S | 2016-2017 | 43/657 | I–III | Curative + G | O + L | 8 | (22) |
| Chen et al,
2023 | China | Retro-S | 2014-2018 | 28/302 | I–III | Curative + G | O + L | 7 | (23) |
Preoperative clinical characteristics
associated with 30-day readmission
A total of 12 preoperative clinical characteristics
were identified for inclusion in the current meta-analysis
(Table II). Of these, five
characteristics were found to have a statistically significant
association with 30-day readmission, namely, age ≥70 years (OR,
1.58; 95% CI, 1.40–1.79; P<0.00001), cardiovascular comorbidity
(OR, 2.00; 95% CI, 1.50–2.66; P<0.00001), a Nutritional Risk
Screening (NRS) 2002 score of ≥3 (OR, 2.02; 95% CI, 1.02–3.98;
P=0.04), respiratory diseases (OR, 1.46; 95% CI, 1.07–1.99; P=0.02)
and male sex (OR, 1.17; 95% CI, 1.03–1.33; P=0.02). By contrast,
seven characteristics were found not to have a statistically
significant association with 30-day readmission, namely, anemia
(OR, 1.06; 95% CI, 0.93–1.21; P=0.40), body mass index (BMI) ≥25
kg/m2 (OR, 1.01; 95% CI, 0.57–1.79; P=0.98), comorbidity
(OR, 1.35; 95% CI, 0.86–2.11; P=0.20), diabetes (OR, 1.10; 95% CI,
0.67–1.83; P=0.70), hypertension (OR, 1.23; 95% CI, 0.99–1.52;
P=0.06), sarcopenia (OR, 2.08; 95% CI, 0.60–7.20; P=0.25) and
smoking history (OR, 1.17; 95% CI, 0.62–2.20; P=0.64). Forest plots
for these characteristics are presented in Figs. S1, S2, S3A
and S3B.
 | Table II.Meta-analysis of clinical
characteristics between patients with and without post-operative
30-day readmission. |
Table II.
Meta-analysis of clinical
characteristics between patients with and without post-operative
30-day readmission.
| A,
Pre-operative |
|---|
|
|---|
|
Characteristics | No. of studies | Readmitted, n | Not readmitted,
n | Heterogeneity,
I2 % | Meta-analysis | Forest plot, Fig.
no. | (Refs.) |
|---|
|
|---|
| OR | 95% CI | P-value |
|---|
| Age ≥70 years | 3 | 1,240 | 8,097 | 0 | 1.58 | 1.40–1.79 | <0.00001 | S1A | (18,21,23) |
| Anemia | 3 | 1,236 | 7,493 | 0 | 1.06 | 0.93–1.21 | 0.40 | S1B | (17,18,23) |
| BMI ≥25
kg/m2 | 2 | 88 | 2,237 | 0 | 1.01 | 0.57–1.79 | 0.98 | S1C | (21,23) |
| Cardiovascular
comorbidity | 5 | 1,442 | 10,204 | 42 | 2.00 | 1.50–2.66 | <0.00001 | S1D | (13,16–19) |
| Comorbidity | 3 | 151 | 2,504 | 35 | 1.35 | 0.86–2.11 | 0.20 | S1E | (12,14,21) |
| Diabetes | 4 | 1,287 | 9,445 | 55 | 1.10 | 0.67–1.83 | 0.70 | S2A | (15,17–19) |
| Hypertension | 4 | 1,401 | 9,830 | 33 | 1.23 | 0.99–1.52 | 0.06 | S2B | (16–19) |
| NRS 2002 score
≥3 | 3 | 98 | 1,237 | 60 | 2.02 | 1.02–3.98 | 0.04 | S2C | (15,2,23) |
| Respiratory
diseases | 4 | 1,321 | 9,470 | 17 | 1.46 | 1.07–1.99 | 0.02 | S2D | (13,17–19) |
| Sarcopenia | 3 | 108 | 1,434 | 82 | 2.08 | 0.60–7.20 | 0.25 | S2E | (20,22,23) |
| Sex
(male/female) | 11 | 1,680 | 13,866 | 4 | 1.17 | 1.03–1.33 | 0.02 | S3A | (12–19,21–23) |
| Smoking
history | 2 | 181 | 2,697 | 74 | 1.17 | 0.62–2.20 | 0.64 | S3B | (16,21) |
|
| B,
Peri-operative |
|
|
Characteristics | No. of
studies | Readmitted,
n | Not readmitted,
n | Heterogeneity,
I2 % |
Meta-analysis | Forest plot,
Fig. no. | (Refs.) |
|
| OR | 95% CI | P-value |
|
| ASA score ≥3 | 6 | 335 | 5,319 | 0 | 1.64 | 1.23–2.20 | 0.0009 | S3C | (15–17,21–23) |
| Combined
multi-organ resection | 3 | 1,333 | 8,530 | 23 | 1.78 | 1.32–2.41 | 0.0002 | S3D | (16,1,21) |
| Complication due to
the tumor | 2 | 116 | 3,269 | 68 | 1.76 | 0.86–3.60 | 0.12 | S3E | (17,21) |
| Depth of invasion:
(T3-4/T1-2) | 3 | 149 | 2,611 | 64 | 2.05 | 1.08–3.88 | 0.03 | S4A | (13,21,23) |
| Discharge to home
with provision of care services | 2 | 1,818 | 4,432 | 0 | 1.51 | 1.30–1.75 | <0.0001 | S4B | (15,18) |
| Extent of gastric
resection (total/subtotal) | 8 | 1,474 | 12,502 | 77 | 1.25 | 0.85–1.82 | 0.25 | S4C | (13,15,17–19,21–23) |
| Extent of lymph
node dissection ≥D2 | 3 | 177 | 3,674 | 52 | 1.14 | 0.73–1.79 | 0.57 | S4D | (12,17,19) |
| History of
abdominal surgery | 4 | 158 | 3,200 | 21 | 1.29 | 0.74–2.26 | 0.37 | S4E | (15,21–23) |
| Neoadjuvant
therapy | 3 | 172 | 4,214 | 0 | 1.65 | 1.03–2.67 | 0.04 | S5A | (13,19,21) |
| Operation method
(laparoscopy) | 8 | 357 | 6,995 | 79 | 0.78 | 0.45–1.35 | 0.38 | S5B | (12,14–15,17,19,21–23) |
| Operation time ≥210
min | 2 | 55 | 683 | 61 | 1.63 | 0.67–3.96 | 0.28 | S5C | (15,23) |
| Post-operative
complications | 8 | 1,546 | 11,790 | 91 | 2.70 | 1.54–4.74 | 0.0005 | S5D | (12,14–16,18,19,21,22) |
| pTNM stage
(III–IV/I–II) | 5 | 210 | 5,071 | 8 | 1.11 | 0.81–1.52 | 0.54 | S5E | (15,19,21–23) |
| Receipt of
blood | 2 | 1,212 | 7,796 | 0 | 1.27 | 1.11–1.44 | 0.0003 | S6A | (18,21) |
| Recurrence | 2 | 91 | 541 | 0 | 0.99 | 0.21–4.63 | 0.98 | S6B | (12,14) |
Perioperative clinical characteristics
associated with 30-day readmission
Seven factors were found to be significantly
different between the readmission and non-readmission groups in the
present meta-analysis (Table II).
A higher proportion of patients who had an American Society of
Anesthesiologists (ASA) score ≥3 (OR, 1.64; 95% CI, 1.23–2.20;
P=0.0009), had undergone combined multi-organ resection (OR, 1.78;
95% CI: 1.32–2.41; P=0.0002) had a high depth of invasion
(T3-4/T1-2; OR, 2.05; 95% CI, 1.08–3.88; P=0.03) or were discharged
to home with the provision of care services (OR, 1.51; 95% CI,
1.31–1.75; P<0.00001) were identified in the readmission group
compared with that in the non-readmission group. In addition, the
proportions of patients who received neoadjuvant therapy (OR, 1.65;
95% CI, 1.03–2.67; P=0.04), experienced post-operative
complications (OR, 2.70; 95% CI, 1.54–4.74; P=0.0005) or had
received blood (OR, 1.27; 95% CI, 1.11–1.44; P=0.0003) were also
observed to be higher in the readmission group compared with the
non-readmission group. However, no difference between the
readmission and non-readmission groups was identified in the
following nine characteristics: Complication due to the tumor (OR,
1.76; 95% CI, 0.86–3.60; P=0.12), gastric resection
(total/subtotal; OR, 1.25; 95% CI, 0.85–1.82; P=0.25), extent of
lymph node dissection ≥D2 (OR, 1.14; 95% CI, 0.73–1.79; P=0.57),
history of abdominal surgery (OR, 1.29; 95% CI, 0.74–2.26; P=0.37),
laparoscopic surgery (OR, 0.78; 95% CI, 0.45–1.35; P=0.38),
duration of surgery ≥210 min (OR, 1.63; 95% CI, 0.67–3.96; P=0.28),
pTNM stage (III–IV/I–II; OR, 1.11; 95% CI, 0.81–1.52; P=0.54) and
recurrence (OR, 0.99; 95% CI, 0.21–4.63; P=0.98). Forest plots for
these analyses are presented in Figs.
S3C-E, S4, S5, S6A
and S6B).
Risk predictors for post-operative
30-day unplanned readmission
Data from multi-factor analyses were incorporated
into a meta-analysis to determine independent risk predictors. The
results indicated that seven factors were associated with the
highest risk of 30-day unplanned readmission. Specifically,
cardiovascular comorbidity (OR, 2.57; 95% CI, 1.77–3.71:
P<0.00001), NRS 2002 score ≥3 (OR, 2.94; 95% CI, 1.72–5.03;
P<0.0001), pancreatectomy (OR, 1.62; 95% CI, 1.17–2.25; P=0.004)
and post-operative complications (OR, 2.66; 95% CI, 1.34–5.27;
P=0.005) were found to be significant risk factors (Table III). Forest plots for these
analyses are presented in Figs.
S4, S6C, S6E and S7).
 | Table III.Meta-analysis of risk predictors for
post-operative 30-day readmission. |
Table III.
Meta-analysis of risk predictors for
post-operative 30-day readmission.
|
|
|
| Meta-analysis |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Risk factor | No. of studies | Heterogeneity,
I2 % | OR | 95% CI | P-value | Forest plot, Fig.
no. | (Refs.) |
|---|
| BMI (≥25
kg/m2) | 2 | 81 | 0.99 | 0.32–3.07 | 0.98 | S6C | (19,22) |
| Sex
(male/female) | 2 | 0 | 0.95 | 0.83–1.09 | 0.48 | S6D | (13,18) |
| Cardiovascular
comorbidity | 2 | 0 | 2.57 | 1.77–3.71 | <0.00001 | S6E | (13,16) |
| Pulmonary
comorbidity | 2 | 0 | 1.08 | 0.91–1.28 | 0.38 | S7A | (13,18) |
| NRS 2002 score
≥3 | 3 | 0 | 2.94 | 1.72–5.03 | <0.0001 | S7B | (16,22,23) |
| Pancreatectomy | 2 | 11 | 1.62 | 1.17–2.25 | 0.004 | S7C | (16,18) |
| Post-operative
complications | 4 | 92 | 2.66 | 1.34–5.27 | 0.005 | S7D | (13,16,18,21) |
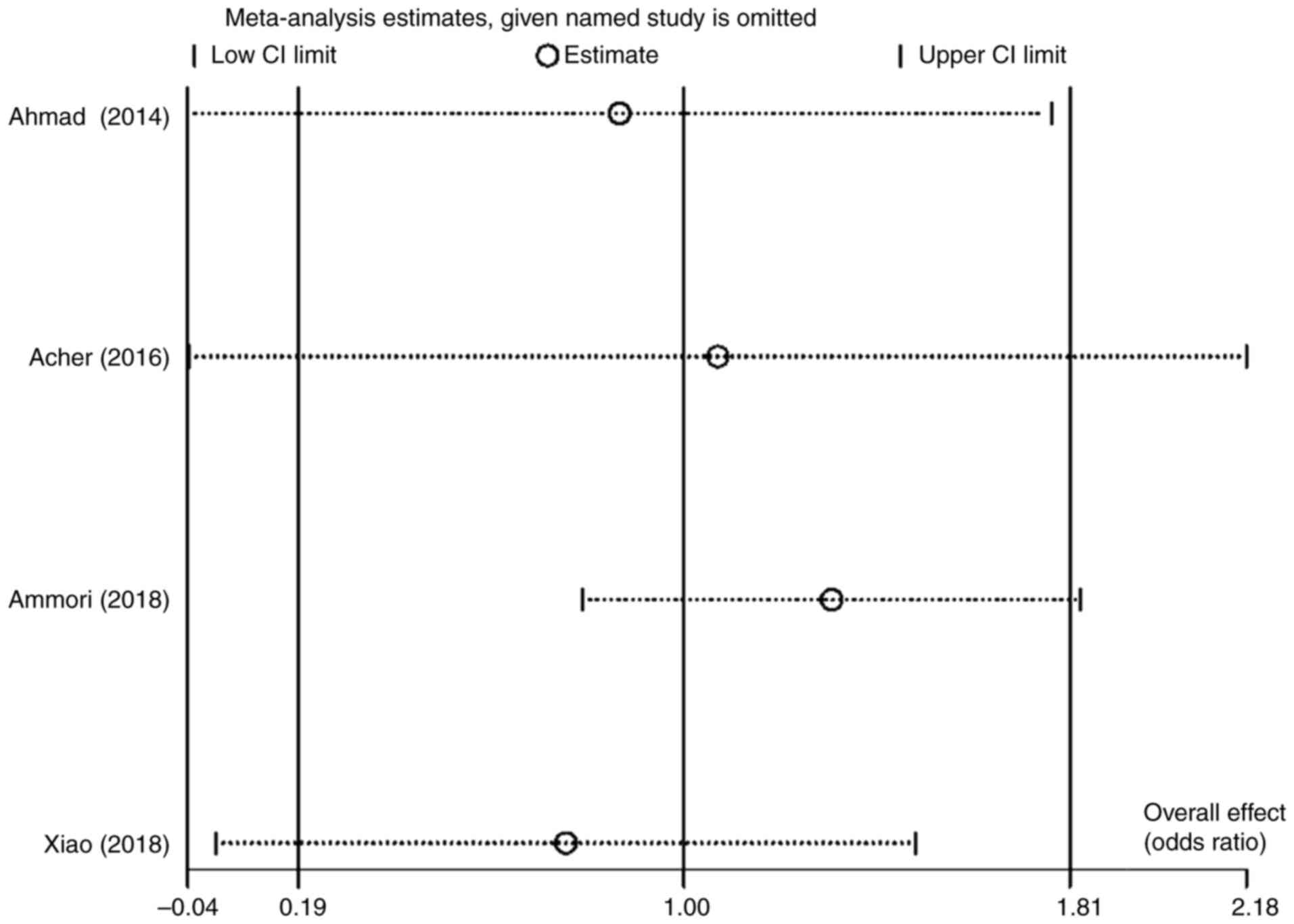
A substantial degree of heterogeneity
(I2=92) was observed in the analysis of the
post-operative complications risk factor. Nevertheless, no
meta-regression analysis was performed, as the meta-analysis only
included four trials. Instead, a sensitivity analysis and random
effects model analysis were used (Fig.
2). The results of the meta-analysis appear to be markedly
impacted by one study by Ammori et al (18), which based on the influence analysis
plot, is a source of heterogeneity manifested by the use of various
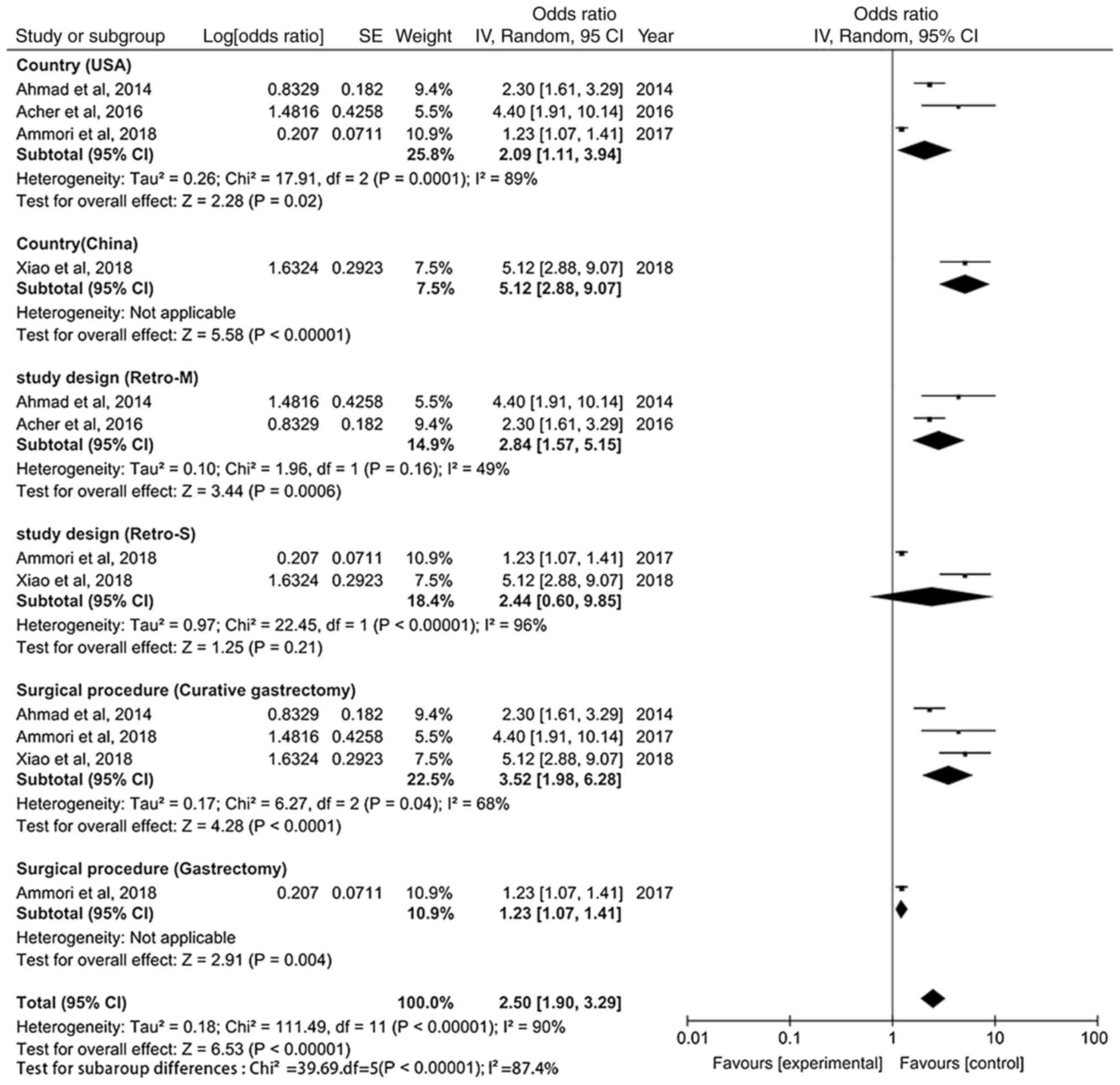
surgical techniques. Therefore, subgroup analyses were performed
based on different countries, study designs and surgical procedures
(Fig. 3). The forest plots show
that heterogeneity decreased most significantly according to study
design, followed by surgical procedure. This indicates that
differences in study designs could be significant contributors to
heterogeneity. Notably, BMI ≥25 kg/m2 (OR, 0.99; 95% CI,
0.32–3.07; P=0.98), male sex (OR, 0.95; 95% CI, 0.83–1.09; P=0.48)
and pulmonary comorbidity (OR, 1.08; 95% CI, 0.91–1.28; P=0.38)
were not found to be risk factors for the prediction of
post-operative 30-day unplanned readmission (Table III).
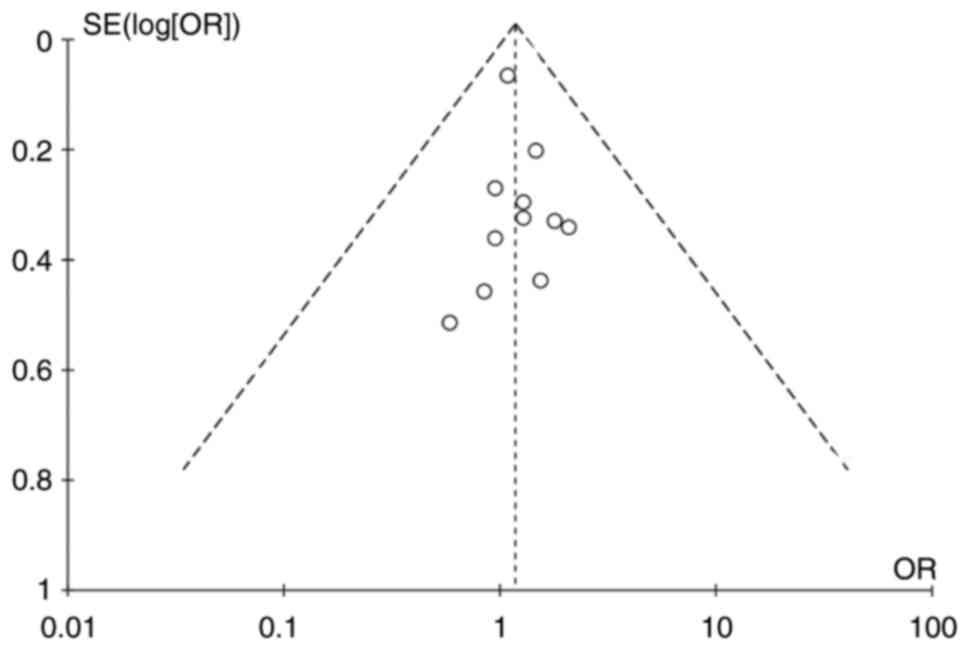
Publication bias
A funnel plot (Fig.
4) was constructed to estimate publication bias, and no obvious
bias was observed. In addition, no significant publication bias was
identified using the Egger test (P=0.394).
Discussion
The present meta-analysis exhibits both similarities
and differences with the two previously published meta-analyses on
the risk factors for readmission after gastric cancer surgery
(5,9). Compared with one of the previous
meta-analyses (5), the present
meta-analysis includes four additional studies (18,20,22,23),
and has others in common (12–17,19),
while compared with the other (9),
the present meta-analysis covers six additional studies (12,14,17,20,22,23),
with others in common (13,15,16,18,19,21).
Also, only univariate regression analysis results from the original
literature were incorporated in one of the prior meta-analyses
(9); while the other (5) included data from multivariate
regression analyses, some of the risk factors identified by it were
only derived from one item of original literature, including longer
length of hospital stay, NRS 2002 score ≥3, combined multi-organ
resection, laparoscopic vs. open surgery and CRP on post-operative
3-day ≥12. The present meta-analysis differs in that at least two
multivariate analysis results from the original literature were
included when identifying the risk predictors for readmission. In
common with the previous meta-analyses, the present study analyzed
age, BMI, cardiovascular comorbidity, diabetes, respiratory
diseases, sex, combined multi-organ resection, operation method and
post-operative complications. However, it also analyzed other
clinical characteristics, including anemia, hypertension, NRS 2002
score, smoking history, sarcopenia, ASA score, tumor-associated
complications, depth of invasion, discharge to home with service,
the extent of gastric resection, the extent of lymph node
dissection, history of abdominal surgery, neoadjuvant therapy, the
duration of surgery, pTNM stage, receipt of blood and
recurrence.
Post-operative 30-day unplanned readmission after
gastric cancer surgery is a concern for patients due to the high
mortality rate and additional medical expenses (16,24).
Malnutrition is frequent in individuals with gastric cancer, with
an incidence of 36–43% (25,26).
In patients undergoing major abdominal surgery, preoperative
malnutrition is generally associated with an increased risk of
post-operative complications, which commonly manifest as anemia,
sarcopenia and an NRS 2002 score of ≥3 (27,28).
The present meta-analysis confirmed that the proportion of patients
with an NRS 2002 score of ≥3 was higher in the readmission group
compared with that in the non-readmission group, but no difference
between the groups was observed for anemia or sarcopenia. Multiple
studies have indicated that post-operative complications are a risk
factor for unplanned readmission (12,13,29).
Comorbidities can increase the post-operative complication rate,
delay discharge time and increase the readmission rate (10,30,31).
In the present study, cardiovascular comorbidity and respiratory
disease rates were more common in the readmission group compared
with those in the non-readmission group. The association between
obesity and readmission was also examined. Obesity is frequently
associated with diabetes, heart disease and hypertension (32,33).
However, the present meta-analysis detected no distinction in the
prevalence of obesity, defined as a BMI ≥25, between the
readmission and non-readmission groups. Age and sex characteristics
were also examined in the current analysis. Readmissions were more
common than non-readmissions in ≥70-year-old and male patients.
This implies that older male patients, particularly those with
respiratory disorders, an NRS 2002 score ≥3 or cardiovascular
disease, require more attention, both at home and in the
hospital.
In colorectal cancer, an association between the TNM
stage and readmission has been reported (34). The prognosis of gastric cancer is
strongly associated with its stage; however, the association
between the stage and 30-day readmission after surgery has not yet
been established. The results of the present meta-analysis revealed
that there was no difference in pTNM stage (III–IV/I–II), but a
significant difference in depth of invasion (T3-4/T1-2), between
the readmission group and the non-readmission group, which appears
to be a paradoxical finding. Additionally, patients in the
readmission group were more likely to have undergone combined
multi-organ resection. However, no difference between the
readmission and non-readmission groups was detected in terms of
whether total or subtotal gastric resection was performed, the
extent of lymph node dissection, history of abdominal surgery,
operation method and the duration of surgery. Therefore, it is
speculatively hypothesized that post-operative readmission is not
associated with the gastrectomy and lymph node dissection methods,
but is associated with combined multi-organ resection. Patients
with stage IV gastric cancer are the primary candidates for
gastrectomy combined with organectomy, and these patients typically
have a poor nutritional status, pyloric blockage, ascites, anemia,
more post-operative problems and a worse prognosis. Therefore, it
is imperative that patients with stage IV gastric cancer are
provided with increased attention and care in order to lower their
readmission rates.
Meyer et al (35) concluded that patients with higher
ASA scores had an elevated risk of cardiovascular complications, as
the ASA score represents the preoperative baseline status of the
patient. Similarly, in the present study, patients in the
readmission group had higher ASA scores than those in the
non-readmission group, consistent with findings for cardiovascular
complications. Previous studies have indicated that
gastrointestinal complications followed by post-operative
infectious complications are the most frequent causes of
readmission (12,36), and post-operative complications
account for a greater proportion of readmissions than
tumor-associated complications. It has been reported that being
discharged to a non-home facility doubles the probability of
readmission, even after adjusting for confounding factors (9). This illustrates the importance of care
in the home after discharge from hospital. The current study
analyzed patients who were discharged to their own home with or
without support services, and the findings indicate that a greater
percentage of patients were discharged home with support services
in the readmission group compared with the non-readmission group.
This outcome is most likely due to patients who are released from
the hospital and sent home without any services being sufficiently
healed after surgery and in a good physical condition. Patients who
have received a blood transfusion are prone to post-operative
malnutrition, high post-operative complications and negative
survival effects (37). In the
present study, blood transfusion was confirmed to be associated
with post-operative 30-day unplanned readmission, as was
neoadjuvant therapy. A study conducted by Beaudart et al
(38) found a substantial
connection between post-operative 30-day unplanned readmission with
malignancy and sarcopenia. However, the present study did not find
such an association, which could be since fewer studies were
included. Thus, additional clinical research on the association
between sarcopenia and readmission is warranted in the future.
The results from multivariate regression analyses in
the original literature were incorporated into the present
meta-analysis, and the outcomes showed that cardiovascular
comorbidity, NRS 2002 score ≥3 and post-operative complications
were significant risk predictors of 30-day unplanned readmission
following post-operative gastric cancer. These predictors were also
found in the two previously published meta-analyses (5,9).
Moreover, with the inclusion of additional literature, the present
meta-analysis was the first to analyze whether pancreatectomy, BMI,
sex and pulmonary comorbidity are risk predictors for readmission.
Among these, only pancreatectomy was identified to be a risk
predictor. Patients with malnutrition experience higher mortality
and complication rates following pancreatectomy (39), and patients with gastric cancer are
more likely to suffer from malnutrition than those with other types
of cancer. This is because, in addition to factors such as
increased protein consumption due to the tumor, cytokines such as
TNF-α are released by tumor cells, which can negatively affect
certain metabolic pathways in the body (40). Higher metabolic demands and faster
tumor cell replication in advanced gastric cancer often translate
into increased energy metabolism and a poor nutritional status
(41). Malnutrition may affect ~80%
of patients with upper digestive tract cancer, and there is
sufficient evidence to show that, in patients with cancer, it is an
independent risk factor for increased mortality, prolonged hospital
stays, post-operative complications and
radiotherapy/chemotherapy-induced toxicity (42). Thus, a good nutritional status is
highly associated with lower 30-day readmission rates, improved
patient recovery and fewer surgical complications in patients with
gastric cancer. This indicates that it is crucial to thoroughly
analyze the nutritional status of patients before and after
surgery, using the NRS 2002 score as a tool for nutritional
assessment, to reduce the risk of complications and the 30-day
readmission rate.
Ito et al (43) demonstrated that intraoperative blood
loss (IBL) negatively impacts post-operative complications and
long-term survival in patients with gastric cancer who have
undergone gastrectomy, and is an independent risk factor for
long-term prognosis. Three possible causes of such adverse effects
have been proposed: Antitumor immunosuppression, unfavorable
post-operative conditions and peritoneal recurrence due to the
spillage of cancer cells into the pelvis (44). By contrast, post-operative
chemotherapy and radiotherapy have positive effects on the
long-term prognosis of patients with gastric cancer after
gastrectomy, and both are independent prognostic factors (45,46).
It would have been interesting to investigate the relationships of
IBL, post-operative chemotherapy and radiotherapy with
post-operative 30-day unplanned readmissions for gastric cancer in
the present meta-analysis. However, among the 12 studies included
in the present meta-analysis, only one study (21) examined the relationship between IBL
and 30-day unplanned readmission, and the multivariate analysis
results showed that patients with an IBL ≥300 ml exhibited a trend
towards a higher incidence of readmission (OR, 1.679; 95% CI,
0.948–2.974), but without statistical significance (P=0.076).
Similarly, only the study by Ahmad et al (13) reported on the association of
post-operative chemotherapy and radiotherapy with 30-day planned
readmission, and it found that chemotherapy (P=0.454) and
radiotherapy (P=0.113) are not independent risk factors by
multivariate analysis. Therefore, in the future, more studies are
required to explore the relationships of IBL, post-operative
chemotherapy and radiotherapy with 30-day unplanned readmissions
after post-operative gastric cancer.
In conclusion, during the perioperative period,
patients who are at a high risk of 30-day unplanned readmission
following post-operative gastric cancer may be screened out using
the identified risk predictors. It is then recommended that these
patients should be the focus of additional attention and support to
reduce their rate of readmission, improve their prognosis, reduce
economic expenditure and lighten the burden on health insurance
providers. However, there are several limitations to the current
study. First, most of the 12 studies included in the study were
retrospective, and selection bias and quality deviation in these
studies are likely to affect the results of the meta-analysis.
Second, heterogeneity was consistently detected during the
examination of post-operative complications as a risk predictor,
and no significant source of heterogeneity was identified, despite
influence and subgroup analyses being performed. It is proposed
that some heterogeneity may result from variations in the criteria
for post-operative complications, the decision-making process for
post-operative readmission and the quality of judgment for
readmission in the studies. Third, the analysis of risk predictors
includes only a small number of publications. A larger number and
higher quality of studies are necessary in the future. Fourth, some
possible risk factors were not analyzed in this meta-analysis, such
as post-operative chemotherapy (13), post-operative radiotherapy (13), renal failure (18) and IBL (21).
In conclusion, the present meta-analysis revealed
that cardiovascular comorbidity, NRS 2002 score ≥3, pancreatectomy
and post-operative complications are independent risk predictors
for 30-day unplanned readmission following gastric cancer surgery.
Extra attention and support should be given to those patients with
high-risk predictors during the perioperative period.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JL and SZ were responsible for conceptualization.
JL, SZ and XH contributed to data curation, formal analysis,
methodology and software. SZ supervised the study. JL and XH wrote
the original draft of the manuscript, and SZ reviewed and edited
the manuscript. JL and SZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartlett EK, Roses RE, Kelz RR, Drebin JA,
Fraker DL and Karakousis GC: Morbidity and mortality after total
gastrectomy for gastric malignancy using the American College of
surgeons national surgical quality improvement program database.
Surgery. 156:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Özer İ, Bostancı EB, Ulaş M, Özoğul Y and
Akoğlu M: Changing trends in gastric cancer surgery. Balkan Med J.
34:10–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dan Z, YiNan D, ZengXi Y, XiChen W, JieBin
P and LanNing Y: Thirty-day readmission after radical gastrectomy
for gastric cancer: A meta-analysis. J Surg Res. 243:180–188. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu X, Gong K, Hong L, Yu X, Tu H, Lan Y,
Yao J, Ye S, Weng H, Li Z, et al: The burden and predictors of
30-day unplanned readmission in patients with acute liver failure:
A national representative database study. BMC Gastroenterol.
24:1532024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sellers MM, Merkow RP, Halverson A, Hinami
K, Kelz RR, Bentrem DJ and Bilimoria KY: Validation of new
readmission data in the American College of surgeons national
surgical quality improvement program. J Am Coll Surg. 216:420–427.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju MR, Blackwell JM, Zeh HJ, Yopp AC, Wang
SC and Porembka MR: Redefining high-volume gastric cancer centers:
The impact of operative volume on surgical outcomes. Ann Surg
Oncol. 28:4839–4847. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu WW, Zhang WH, Zhang WY, Yang L, Deng XQ
and Zhu T: Risk factors of the postoperative 30-day readmission of
gastric cancer surgery after discharge: A PRISMA-compliant
systematic review and meta-analysis. Medicine (Baltimore).
98:e146392019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Int J Surg. 88:1059062021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MC, Kim KH and Jung GJ: A 5 year
analysis of readmissions after radical subtotal gastrectomy for
early gastric cancer. Ann Surg Oncol. 19:2459–2464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad R, Schmidt BH, Rattner DW and Mullen
JT: Factors influencing readmission after curative gastrectomy for
gastric cancer. J Am Coll Surg. 218:1215–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YD, Kim MC, Kim KH, Kim YM and Jung
GJ: Readmissions following elective radical total gastrectomy for
early gastric cancer: A case-controlled study. Int J Surg.
12:200–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuang CL, Wang SL, Huang DD, Pang WY, Lou
N, Chen BC, Chen XL, Yu Z and Shen X: Risk factors for hospital
readmission after radical gastrectomy for gastric cancer: a
prospective study. PLoS One. 10:e01255722015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Acher AW, Squires MH, Fields RC,
Poultsides GA, Schmidt C, Votanopoulos KI, Pawlik TM, Jin LX, Ejaz
A, Kooby DA, et al: Readmission following gastric cancer resection:
Risk factors and survival. J Gastrointest Surg. 20:1284–1294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honda M, Hiki N, Nunobe S, Ohashi M, Mine
S, Watanabe M, Kamiya S, Irino T, Sano T and Yamaguchi T: Unplanned
admission after gastrectomy as a consequence of fast-track surgery:
A comparative risk analysis. Gastric Cancer. 19:1002–1007. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ammori JB, Navale S, Schiltz N and
Koroukian SM: Predictors of 30-day readmissions after gastrectomy
for malignancy. J Surg Res. 224:176–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asaoka R, Kawamura T, Makuuchi R, Irino T,
Tanizawa Y, Bando E and Terashima M: Risk factors for 30-day
hospital readmission after radical gastrectomy: A single-center
retrospective study. Gastric Cancer. 22:413–420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma BW, Chen XY, Fan SD, Zhang FM, Huang
DD, Li B, Shen X, Zhuang CL and Yu Z: Impact of sarcopenia on
clinical outcomes after radical gastrectomy for patients without
nutritional risk. Nutrition. 61:61–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao H, Quan H, Pan S, Yin B, Luo W, Tang
M, Ouyang Y and Tang W: Incidence, causes and risk factors for
30-day readmission after radical gastrectomy for gastric cancer: A
retrospective study of 2,023 patients. Sci Rep. 8:105822018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai Y, Chen S, Chen X, Chen W, Wang P, Zhu
G and Jin J: Association of sarcopenia and low nutritional status
with unplanned hospital readmission after radical gastrectomy in
patients with gastric cancer: A case-control study. J Healthc Eng.
2022:72468482022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen WS, Huang ZX, Zhang HH, Chen XD, Cai
YQ, Chen WJ, Zhu GB and Huang YS: Lactate dehydrogenase and risk of
readmission with gastric cancer: A propensity score matching
analysis. J Invest Surg. 36:21724882023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams EI and Fitton F: Factors
affecting early unplanned readmission of elderly patients to
hospital. BMJ. 297:784–787. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gavazzi C, Colatruglio S, Sironi A,
Mazzaferro V and Miceli R: Importance of early nutritional
screening in patients with gastric cancer. Br J Nutr.
106:1773–1778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryu SW and Kim IH: Comparison of different
nutritional assessments in detecting malnutrition among gastric
cancer patients. World J Gastroenterol. 16:3310–3317. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jie B, Jiang ZM, Nolan MT, Zhu SN, Yu K
and Kondrup J: Impact of preoperative nutritional support on
clinical outcome in abdominal surgical patients at nutritional
risk. Nutrition. 28:1022–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Funk Debleds P, Chambrier C and Slim K:
Postoperative nutrition in the setting of enhanced recovery
programmes. Eur J Surg Oncol. 50:1068662024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang SL, Zhuang CL, Huang DD, Pang WY, Lou
N, Chen FF, Zhou CJ, Shen X and Yu Z: Sarcopenia adversely impacts
postoperative clinical outcomes following gastrectomy in patients
with gastric cancer: A prospective study. Ann Surg Oncol.
23:556–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Damle RN and Alavi K: Risk factors for
30-d readmission after colorectal surgery: A systematic review. J
Surg Res. 200:200–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park P, Nerenz DR, Aleem IS, Schultz LR,
Bazydlo M, Xiao S, Zakaria HM, Schwalb JM, Abdulhak MM, Oppenlander
ME and Chang VW: Risk factors associated with 90-day readmissions
after degenerative lumbar fusion: An examination of the michigan
spine surgery improvement collaborative (MSSIC) registry.
Neurosurgery. 85:402–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shah NP, Lu R, Haddad F, Shore S, Schaack
T, Mega J, Pagidipati NJ, Palaniappan L, Mahaffey K, Shah SH, et
al: Relationship between body mass index and cardiometabolic health
in a multi-ethnic population: A project baseline health study. Am J
Prev Cardiol. 18:1006462024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Antza C, Grassi G, Weber T, Persu A,
Jordan J, Nilsson PM, Redon J, Stabouli S, Kreutz R and Kotsis V:
Assessment and management of patients with obesity and hypertension
in European society of hypertension excellence centres. A survey
from the ESH working group on diabetes and metabolic risk factors.
Blood Press. 33:23172562024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quintana JM, Anton-Ladislao A, Lázaro S,
Gonzalez N, Bare M, de Larrea NF, Redondo M, Briones E, Escobar A,
Sarasqueta C, et al: Predictors of readmission and reoperation in
patients with colorectal cancer. Support Care Cancer. 28:2339–2350.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meyer AC, Eklund H, Hedström M and Modig
K: The ASA score predicts infections, cardiovascular complications,
and hospital readmissions after hip fracture-a nationwide cohort
study. Osteoporos Int. 32:2185–2192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emick DM, Riall TS, Cameron JL, Winter JM,
Lillemoe KD, Coleman J, Sauter PK and Yeo CJ: Hospital readmission
after pancreaticoduodenectomy. J Gastrointest Surg. 10:1243–1253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Zhao L, Niu P, Zhang X, Luan X,
Zhao D and Chen Y: Effects of perioperative blood transfusion in
gastric cancer patients undergoing gastrectomy: A systematic review
and meta-analysis. Front Surg. 9:10110052023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beaudart C, Drost RMWA, Evers SMAA, Paulus
ATG and Hiligsmann M: Associations between muscle mass/strength and
healthcare costs/use for patients with cancer: A systematic
literature review. Cancer Treat Res Commun. 33:1006332022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee DU, Hastie DJ, Fan GH, Addonizio EA,
Lee KJ, Han J and Karagozian R: Effect of malnutrition on the
postoperative outcomes of patients undergoing pancreatectomy for
pancreatic cancer: Propensity score-matched analysis of 2011-2017
US hospitals. Nutr Clin Pract. 37:117–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanda M, Tanaka H, Shimizu D, Miwa T,
Umeda S, Tanaka C, Kobayashi D, Hattori N, Suenaga M, Hayashi M, et
al: SYT7 acts as a driver of hepatic metastasis formation of
gastric cancer cells. Oncogene. 37:5355–5366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang HM, Wang TJ, Huang CS, Liang SY, Yu
CH, Lin TR and Wu KF: Nutritional status and related factors in
patients with gastric cancer after gastrectomy: A cross-sectional
study. Nutrients. 14:26342022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deftereos I, Kiss N, Isenring E, Carter VM
and Yeung JM: A systematic review of the effect of preoperative
nutrition support on nutritional status and treatment outcomes in
upper gastrointestinal cancer resection. Eur J Surg Oncol.
46:1423–1434. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito Y, Kanda M, Ito S, Mochizuki Y,
Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H,
et al: Intraoperative blood loss is associated with shortened
postoperative survival of patients with stage II/III gastric
cancer: Analysis of a multi-institutional dataset. World J Surg.
43:870–877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakanishi K, Kanda M and Kodera Y:
Long-lasting discussion: Adverse effects of intraoperative blood
loss and allogeneic transfusion on prognosis of patients with
gastric cancer. World J Gastroenterol. 25:2743–2751. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma X, Zhou X, Guo J, Feng X, Zhao M, Zhang
P, Zhang C, Gong S, Wu N, Zhang Y, et al: CA19-9 is a significant
prognostic factor in stage III gastric cancer patients undergoing
radical gastrectomy. BMC Surg. 24:312024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Che K, Wang Y, Wu N, Liu Q, Yang J, Liu B
and Wei J: Prognostic nomograms based on three lymph node
classification systems for resected gastric adenocarcinoma: A large
population-based cohort study and external validation. Ann Surg
Oncol. 28:8937–8949. 2021. View Article : Google Scholar : PubMed/NCBI
|