Introduction
Low-grade appendiceal mucinous neoplasm (LAMN) is an
epithelial, non-invasive tumor with any of the following features:
loss of muscularis mucosae, fibrosis of submucosa and/or ‘pushing
invasion’ (expansile or diverticulum-like growth) (1–3). LAMN
is detected in 0.7–1.7% of all appendectomies. In particular, the
‘pushing invasion’ feature of LAMNs may increase the possibility of
ovarian involvement. Ovarian metastases are found in ~50% of female
patients with appendiceal tumors (4). Symptoms of this disease are
non-specific, although abdominal pain in the right lower quadrant
is the most common complaint (3).
If ovarian metastasis occurs, a pelvic mass may become palpable
during gynecological examination (2). The most common clinical manifestation
of LAMN is an acute appendicitis combined with a perforation of the
appendiceal wall (3).
Although preoperative diagnosis can be made by
computed tomography (CT) examination (5–7), LAMN
is mostly frequently identified intraoperatively or even
postoperatively incidentally. This issue particularly concerns
female patients, since metastatic ovarian mucinous neoplasms also
share similar atypical clinical manifestations and imaging findings
(4). The most effective
differential diagnostic technique known for LAMN is
immunohistochemical examination. The most common
immunohistochemistry (IHC) markers applied for diagnostic purposes
include cytokeratin (CK)7, CK20, caudal type homeobox 2 (CDX2),
paired box gene 8 (PAX8) and special AT-rich sequence-binding
protein 2 (SATB2) (8–10).
The treatment method for LAMN typically depends on
the tumor stage, whether perforation of the appendiceal wall has
occurred, presence of metastases and the existence of peritoneal
mucin spread. For localized LAMN, appendectomy is generally
sufficient. In cases of metastases to the abdominal organs or the
pseudomyxoma peritonei (PMP), cytoreductive surgery followed by
hyperthermic intraperitoneal chemotherapy (HIPEC) is highly
recommended (1,3,11–13).
In particular, the most dangerous complication among the
aforementioned is PMP, when the mucin from the appendix spreads to
the peritoneum (1,14,15).
The risk of developing PMP increases significantly when spontaneous
perforation of the appendiceal wall has occurred (16).
There are only a few articles concerning this topic
worldwide, and they generally focus on different aspects of this
tumor from the surgical point of view. The lack of articles from
the perspective of gynecologists indicates that further exploration
of this topic is required. Therefore, the present report documents
the case of the 61-year-old female patient who was diagnosed
postoperatively with LAMN metastasizing to the right ovary and
omentum. Diagnostic difficulties during the clinical course of this
patient were summarized before differentiation between metastatic
LAMN in the ovary and primary ovarian mucinous cancer (POMC).
Case report
A 61-year-old female patient was admitted to the
Second Department of Gynecological Surgery and Gynecological
Oncology, University Clinical Hospital no. 4, Lublin Medical
University (Lublin, Poland) with the primary diagnosis of a right
ovarian tumor in May, 2021. The condition manifested as chronic
pelvic pain and pain after defecation lasting several weeks. The
patient denied having other symptoms, illnesses or medicaments.
According to the medical history of the patient, the last
menstruation occurred 10 years ago, and the patient underwent two
vaginal deliveries. The last cervical cytological examination was
performed 3 years ago and was normal. The patient also suffered
from hepatitis B at the age of 15 years old and chronic varices in
both legs for >10 years.
On gynecological examination, the external
genitalia, vagina and uterine cervix all revealed normal results.
However, a palpable pathological mass in the lower-right abdomen
was detected. The body of the uterus had an uneven surface and was
painful on palpation. The left ovary was not palpable. Biochemical
examination revealed cancer antigen 125 levels of 20.2 U/ml
[reference range (RR) <35 U/ml], carcinoembryonic antigen levels
of 7.7 ng/ml (RR <2.5 ng/ml in non-smoking patients) and a Risk
of Ovarian Malignancy Algorithm index of 12.4% (RR <29.9% in
postmenopausal women). On transvaginal ultrasound examination, a
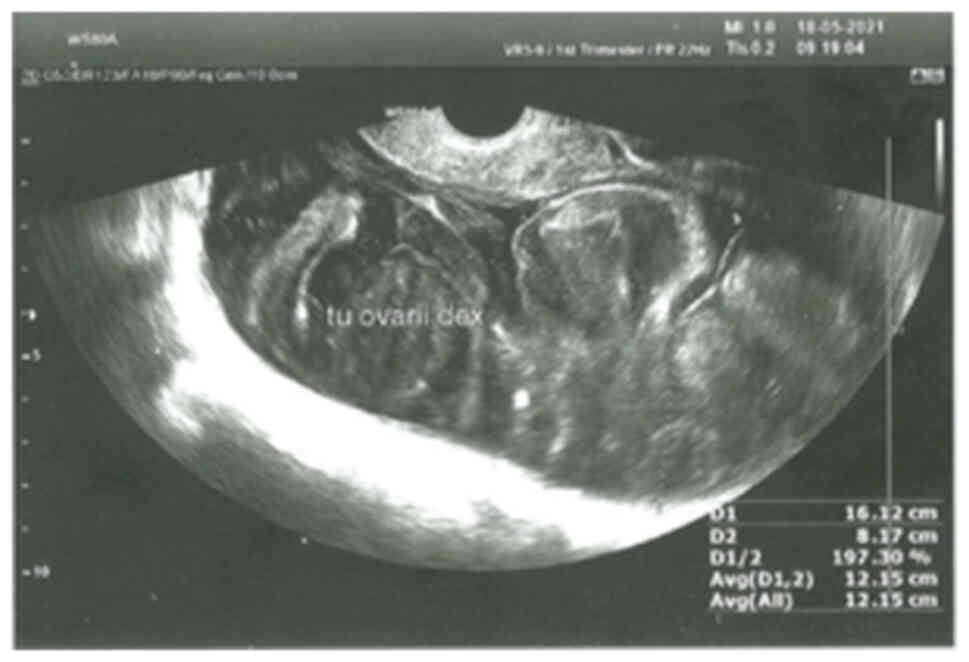
16-cm-wide tumor in the right ovary with heterogenous echogenicity
was observed (Fig. 1). Between the
tumor and circumfluent lesions, a noticeable border was confirmed.
The uterus and the left ovary were normal, where the endometrium
thickness was 4 mm. A small quantity of ascites fluid was detected
in the Douglas pouch, but distant metastases or pathological
regional lymph nodes could not be identified.
Abdominal CT scans revealed a large polycystic,
pathological mass in the lesser pelvis. The size of the tumor was
17.4×11.7×9.9 cm. No other abnormalities were found in the pelvis
minor. The patient was then recommended for explorative laparotomy.
In the abdominal cavity, a wide litho-cystic tumor (10×17 cm)
originating from the right adnexa was found. The mass in the right
ovary was in continuity with the appendiceal neoplasm, the
appendiceal walls were thickened and the lesion was swollen. The
size of the uterus was normal. Numerous lesions were found to be
localized on the left ovary, Douglas peritoneum, greater omentum
and both diaphragmatic of the domes. The patient underwent total
abdominal hysterectomy, bilateral salpingo-oophorectomy, total
omentectomy, appendectomy and resection of the Douglas peritoneum.
The postoperative period was uneventful, and 5 days after the
surgery the patient was discharged from the hospital in good
condition.
Postoperative histopathological examination revealed
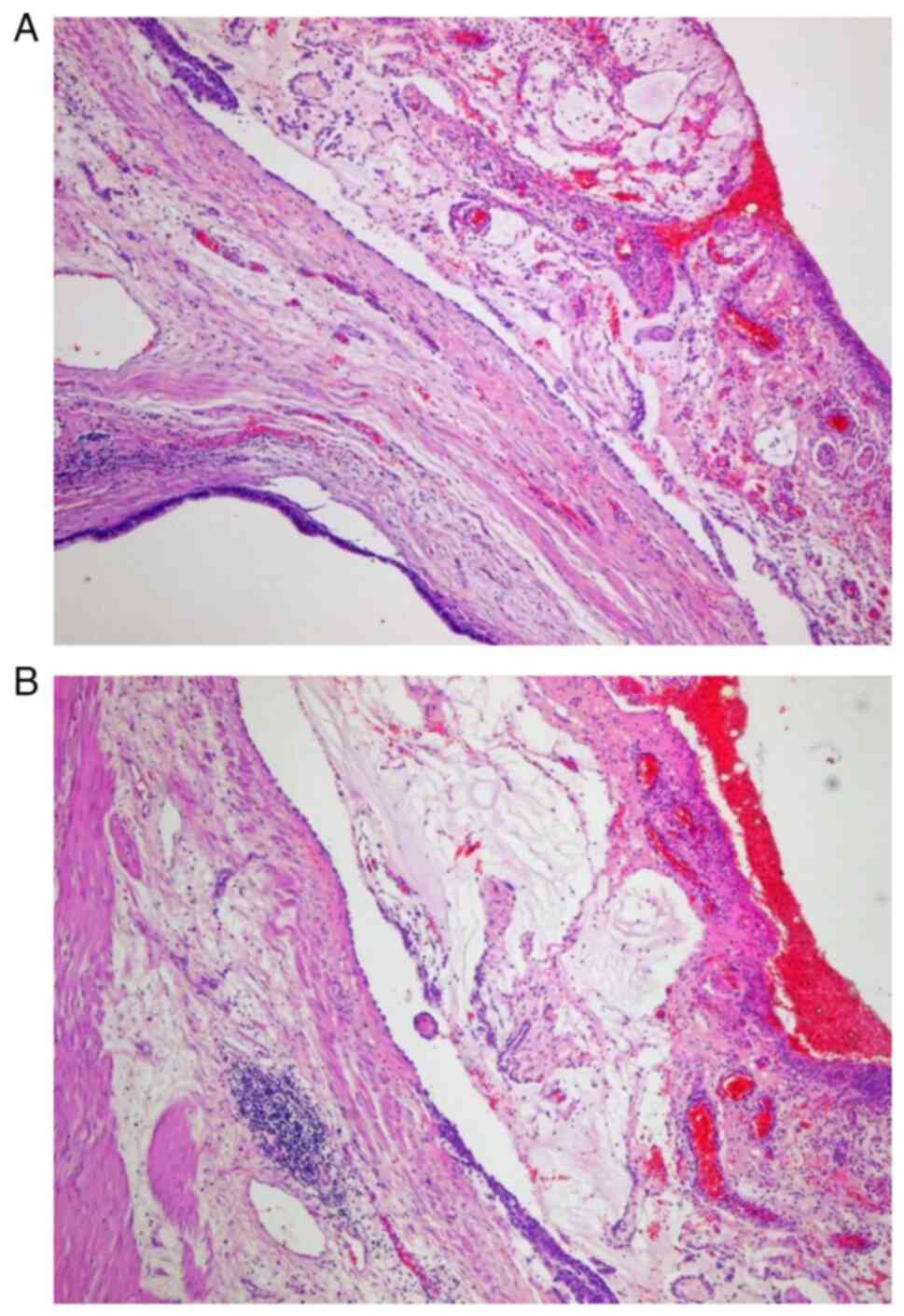
a LAMN with metastases to the right ovary and omentum. Mucinous
tumors were found in the Douglas peritoneum and in the ‘free end’
of the appendix (0.7 cm; Fig. 2).
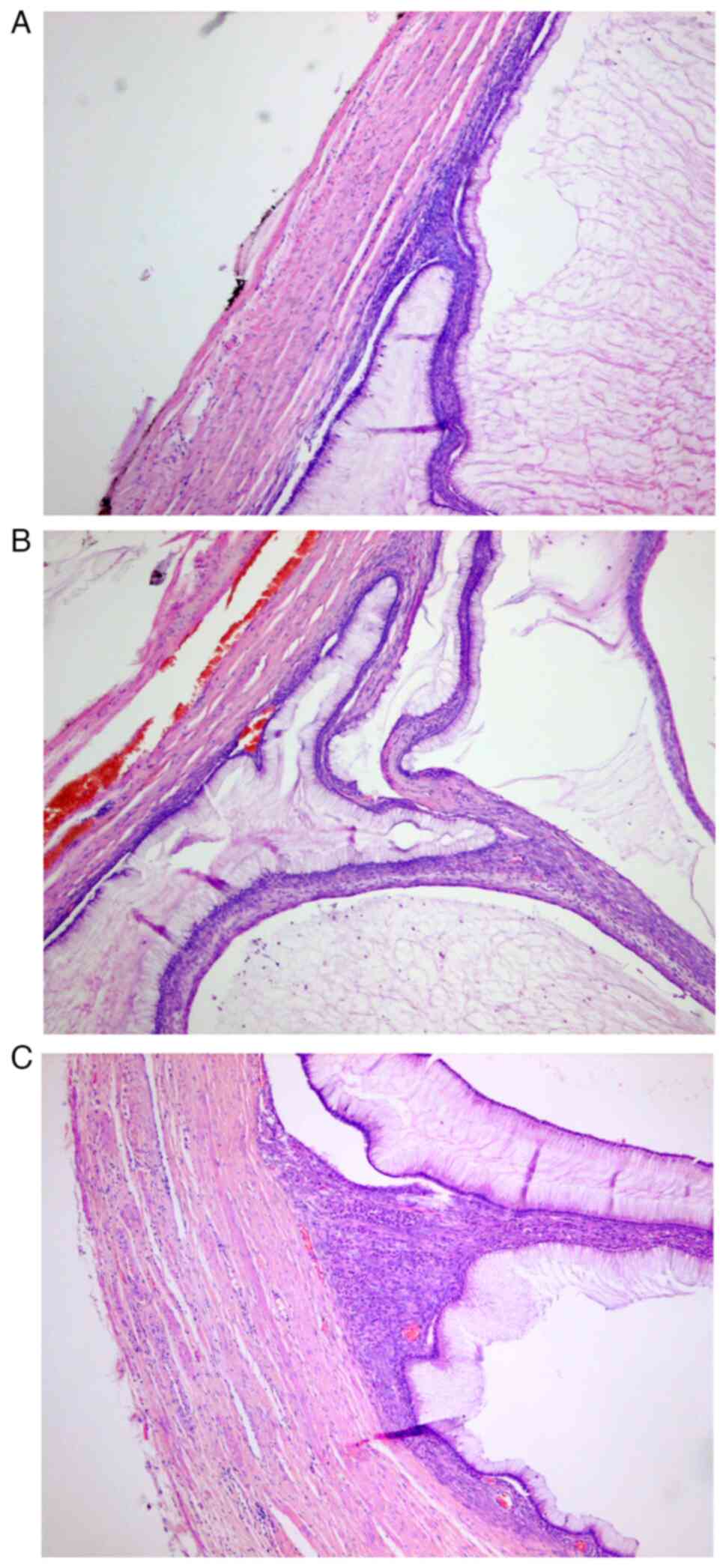
Wall perforation and neoplastic infiltration of the appendiceal
mucosa were also observed (Fig. 3).
For postoperative histopathological examination, the tissues were
fixed with 10% formalin and embedded into paraffin blocks for 24–48
h at room temperature. Then, 5-µm sections were deparaffinized and
rehydrated using routine techniques. Antigen retrieval by microwave
and blocking of endogenous peroxidase activity (by 1% hydrogen
peroxide in distillated water for 10 min at room temperature) was
conducted before incubation with the following primary antibodies:
CK20 (monoclonal mouse anti-human antibody; ready-to-use; cat. no.
GA777; DAKO; Agilent Technologies, Inc.), CDX2 (monoclonal mouse
anti-human antibody; ready-to-use; cat. no. GA080; DAKO; Agilent
Technologies, Inc.) and PAX8 (monoclonal mouse anti-human antibody;
ready-to-use; cat. no. 760-4618; Roche Diagnostics) at 4°C
overnight. The primary antibodies were then removed, and the slides
were incubated for 30 min at room temperature with a biotin-free
horseradish peroxidase enzyme-labelled polymer of the DAKO RealTM
EnVisionTM/HRP detection system (DAKO; Agilent Technologies, Inc.).
Next, following reaction with 3,3′-diaminobenzidine, the sections
were counterstained with hematoxylin for 2 h at room temperature,
dehydrated and cover-slipped. Appropriate positive and negative
controls were also applied. Stained slides were carefully examined
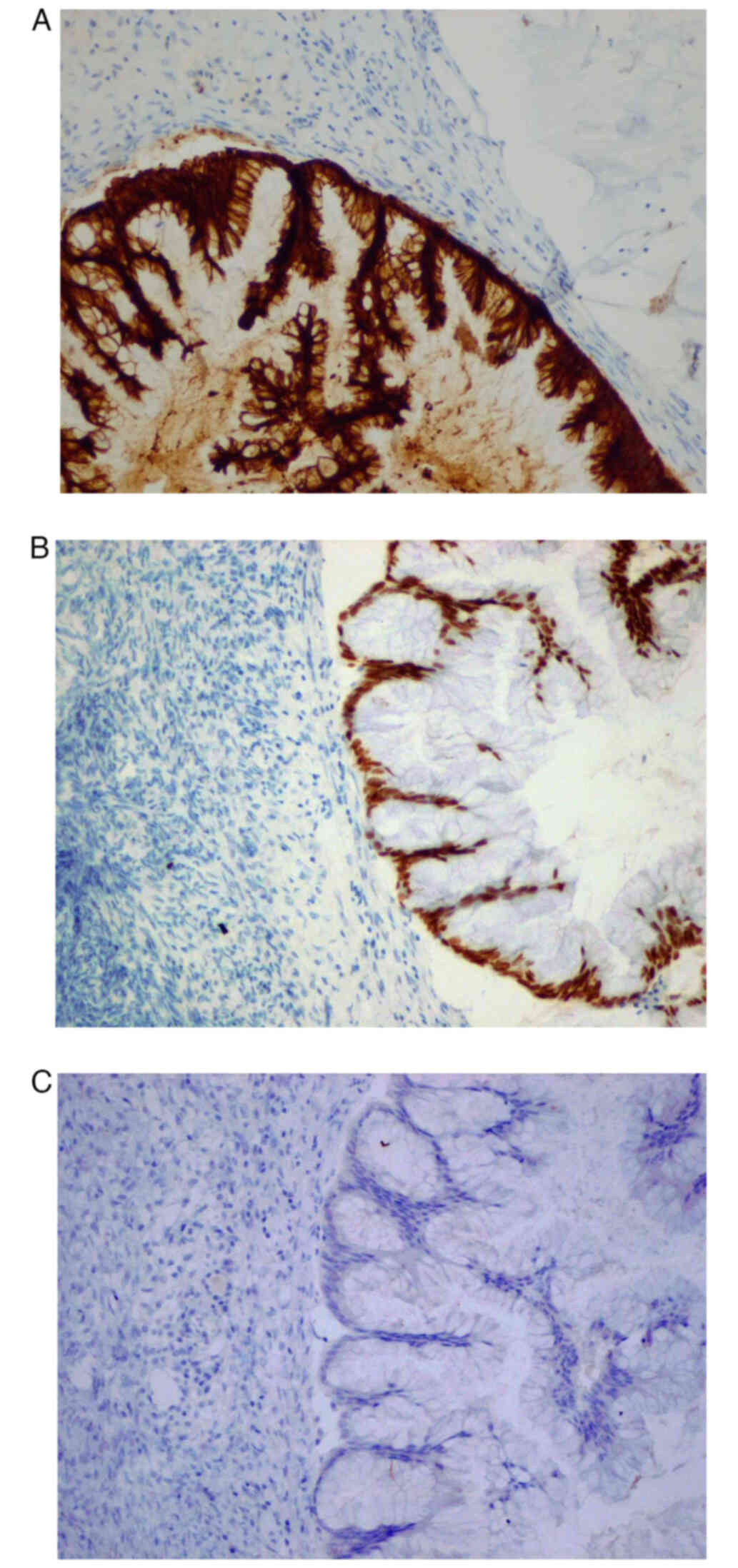
by two investigators by light microscopy. The results indicated
CK20 (Fig. 4A) and CDX2 (Fig. 4B) positive staining in the right
ovary and appendix, whereas PAX8 (Fig.
4C) staining was negative.The patient was then referred to the
Department of Surgical Oncology, Lublin Medical University, where
the patient underwent a one-time HIPEC procedure with 30 mg
mitomycin C for 1 h at 43°C. After HIPEC, the patient was
discharged in good condition. The recurrence of LAMN was not
reported during the 1-year follow-up. After 1 year, a CT scan was
performed, which revealed no evidence of recurrent disease. The
patient remains in the follow-up program devised by the
gynecological oncology specialists and remains asymptomatic.
Discussion
One of the main difficulties with mucinous ovarian
neoplasms is differentiating them from other types of metastatic
tumor, particularly those originating from the gastrointestinal
tract. POMCs represent 3% of all primary ovarian tumors, whereas
metastatic ovarian tumors account for 5–30% of all ovarian
malignancies. The majority of metastases to the ovaries arise from
organs in the gastrointestinal tract, where those of appendiceal
origin pertain to 13% of such cases (17–19).By
gross morphology, ovarian metastases are generally smaller compared
with those of primary neoplasms, with sizes typically ≤10 cm in
diameter (Table I) (2,13,16,19,20).
Histologically, the growth pattern in secondary mucinous tumors is
mostly infiltrative and may present as a nodular growth with or
without single-cell invasion. In addition, metastases frequently
show signs of lymphovascular space invasion, surface and hilar
involvements, whilst primary tumors are generally characterized by
the presence of microscopic cystic glands and expansive invasion
patterns (2,18,19).
Differentiating between POMC and ovarian metastases of
gastrointestinal tract tumors involves the immunohistochemical
assessment of several valuable markers, including SATB2, CDX2, CK7,
CK20 and PAX8 (Table II) (17,19).
POMC was found to express CK7, CK20 and PAX8 in 90, 65–70 and 35%
of all cases assessed, respectively. In addition, the concomitant
expression of CK7 and CK20 is present in ~67% of all cases
(9,17,20).
By contrast, CDX2 expression is found in <50% of all cases of
POMC, whereas expression in metastases of lower gastrointestinal
tract origin is markedly more common, occurring in 90% of all
tumors (17,20). SATB2 is a recently described marker
that has been demonstrated to be a novel tool for the diagnosis of
the gastrointestinal neoplasms. SATB2 positivity has been reported
in 85–90% of all appendiceal tumors, where its expression is
stronger and more specific compared with CDX2 (9,17,20–22).
Generally, for POMC, CK7 is considered to be the most sensitive
marker, whereas PAX8 is the most specific. Furthermore, CDX2 is
considered to be the most sensitive marker for ovarian metastases
of lower gastrointestinal tract origin, whilst SATB2 would appear
to be the most specific (17,23).
Although the aforementioned markers are invaluable diagnostic
tools, they cannot be interpreted separately since tumors typically
exhibit concomitant expression patterns of each of the individual
markers. We recommended the analysis of expression of at least two
independent immunohistochemical markers (Table III) (8,9,19,21–28).
 | Table I.A comparison between primary ovarian
mucinous tumors and mucinous ovarian metastases. |
Table I.
A comparison between primary ovarian
mucinous tumors and mucinous ovarian metastases.
| Feature | Primary tumor | Metastatic
tumor |
|---|
| Laterality | Unilateral | Bilateral |
| Size, cm | >10 | <10 |
| Age of patients,
years | <50 | >50 |
| Presence of signet
ring cells | Absent | Present |
| Type of
invasion | Expansile | Infiltrative,
vascular |
 | Table II.Expression patterns of the most
common immunohistochemistry markers depending on the origin of the
tumor. |
Table II.
Expression patterns of the most
common immunohistochemistry markers depending on the origin of the
tumor.
| Marker | POMN, % | APE, % |
|---|
| CK7 | 90 | 26 |
| CK20 | 65-70 | 92 |
| PAX8 | 35 | <5 |
| CDX2 | <50 | 97 |
| SATB2 | 8 | 85-90 |
 | Table III.Expression patterns of concomitant
IHC markers in POMN and APE. |
Table III.
Expression patterns of concomitant
IHC markers in POMN and APE.
| IHC markers | POMN, % | APE, % |
|---|
| CK7/CK20 |
|
|
|
(+)/(+) | 67 | 22 |
|
(−)/(+) | 7 | 78 |
|
(+)/(−) | 26 | - |
| SATB2/CK20 |
|
|
|
(+)/(+) | - | 80 |
| (+)/(−)
or (−)/(+) | - | 20 |
| CDX2/CK20 |
|
|
|
(+)/(+) | - | 90 |
| (+)/(−)
or (−)/(+) | - | 10 |
The molecular mechanism underlying the growth of
POMC and AMNs remains unresolved, since experimental data on these
particular malignancies are still limited. KRAS and TP53 genetic
alterations are the most frequent in POMCs, with their incidence
accounting for 33–46 and 26–55% of cases, respectively (26–27,29).
The treatment strategy for LAMN depends on the
clinical stage, histological grade, tumor invasion, the presence of
metastases and the careful surgical management (3,28,30).
There is an association between the expression profile of the IHC
markers and histopathological parameters of the tumor. CDX2
expression has been found to be associated with histological grade
and depth of tumor invasion, whilst loss of CK20 positivity has
been reported to be associated with a higher histological grade of
colorectal carcinoma (23). An
appendectomy is typically sufficient for treating localized LAMN,
whereas right-sided hemicolectomy should be considered if the
positive margins persist after appendectomy (31). Metastases or PMP are generally
treated with cytoreductive surgery and HIPEC, including oxaliplatin
and mitomycin C. During cytoreductive operations, any suspicious
lesions should be removed. However, if the tumor extends to the
base of the appendix, then there would be a necessity to perform a
caecal wedge resection. Peritoneal surfaces that must be inspected
include the right iliac fossa, right paracolic gutter, right
diaphragmatic surface, greater omentum and pelvic peritoneum
(15). Ovarian metastases of
appendiceal origin are reported in ~50% of patients, where they are
generally metachronous. Both ovaries should be carefully
investigated, in cases where malignancies are discovered, bilateral
salpingo-oophorectomy is highly recommended, even in pre-menopausal
patients (32–34).
In terms of the prognosis of patients with LAMN,
namely overall and disease-free survival, it remains uncertain and
depends on the clinical stage, presence of metastases, age and
general health condition. However, the 5-year survival rate has
been estimated to be 80%. Localized LAMN or PMP appear to have
favorable clinical outcomes after complete resection of the primary
tumor during early-stage disease, but caution must be taken due to
data scarcity (35,36). By contrast, the prognosis for POMC
depends on tumor staging and the subtype of cancer. Early-stage
POMC has a 90% 5-year overall survival rate, though patients with
metastatic mucinous ovarian cancer generally will not survive
beyond 30 months (28). An
abbreviated comparison between LAMN and POMC is shown in Table IV (4,15,28,31,35,36).
 | Table IV.Comparison between POMC and LAMN. |
Table IV.
Comparison between POMC and LAMN.
| Feature | POMC | LAMN |
|---|
| Prevalence | 3-12% of ovarian
malignancies | 1% of all
appendectomies |
| Treatment | Staging
surgery/cytoreductive surgery, appendectomy, consider HIPEC with
cisplatin. | Appendectomy in
localized LAMN, cytoreductive surgery and HIPEC with oxaliplatin
and mitomycin C. In advanced stage, consider right-sided
hemicolectomy. |
| Survival rate | 90% in early-stage
disease, 12–30 months in advance-stage. | 80% in early-stage
disease. |
In conclusion, observations from the present case
suggest that clinical specialists of gynecological oncology should
remain conscious of the possibility of ovarian tumors of
gastrointestinal origin in addition to POMC. If the mucinous mass
involves the base of the appendix or if there is a suspicion of
positive margins, detailed cytoreductive surgery combined with
right-sided hemicolectomy is highly recommended. Differentiation of
the origin of mucinous tumor in the area of the right ovary and/or
the appendix requires histopathological and immunohistochemical
examination using a panel of protein markers. In addition,
molecular studies into LAMN and POMC are warranted to facilitate
the development of novel diagnostic procedures in the future.
Acknowledgements
Not applicable.
Funding
The study was supported by a grant from Lublin Medical
University, Lublin, Poland (grant no. Dz. St. 326/24).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WK made substantial contributions to conception and
design, searched the articles and wrote the manuscript; AAG
performed the surgery, collected medical records and wrote the
manuscript; DL, as a pathologist, diagnosed the case and prepared
the histopathological images; KU analyzed and interpreted the data
and searched the articles; AS made substantial contribution to
conception and design and edited the manuscript. All authors
participated in the drafting of the manuscript. DL and AS confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of anonymized data and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMN
|
appendiceal mucinous neoplasm
|
|
LAMN
|
low-grade appendiceal mucinous
neoplasm
|
|
CT
|
computed tomography
|
|
IHC
|
immunohistochemistry
|
|
PMP
|
pseudomyxoma peritonei
|
|
HIPEC
|
hyperthermic intraperitoneal
chemotherapy
|
|
POMC
|
primary ovarian mucinous cancer
|
References
|
1
|
Legué LM, Creemers GJ, de Hingh IHJT,
Lemmens VEPP and Huysentruyt CJ: Pathologyand its clinical
relevance of mucinous appendiceal neoplasms and pseudomyxoma
peritonei. Clin Colorectal Cancer. 18:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carr NJ, Bibeau F, Bradley RF, Dartigues
P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J,
et al: The histopathological classification, diagnosis and
differential diagnosis of mucinous appendiceal neoplasms,
appendiceal adenocarcinomas and pseudomyxoma peritonei.
Histopathology. 71:847–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaib WL, Assi R, Shamseddine A, Alese OB,
Staley C III, Memis B, Adsay V, Bekaii-Saab T and El-Rayes BF:
Appendiceal mucinous neoplasms: Diagnosis and management.
Oncologist. 22:1107–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perivoliotis K, Christodoulidis G, Samara
AA, Sgantzou IK, Floros T, Volakakis G, Karasavvidou F and Tepetes
K: Low-grade appendiceal mucinous neoplasm (LAMN) primarily
diagnosed as an ovarian mucinous tumor. Case Rep Surg.
22:55237362021.PubMed/NCBI
|
|
5
|
Arshi J, Peter AD, Liang Y and Hao Y:
Confined low grade appendiceal mucinous neoplasm with coexisting
distant metastasis. In Vivo. 38:295–298. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guzman GA, Montealegre I and Obando AM:
Incidental diagnosis of a low-grade mucinous appendicular neoplasm:
A case report. Int J Surg Case Rep 105998. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu X-R, Mao J, Tang W, Meng X-Y, Tian Y
and Du Z-L: Low-grade appendiceal mucinous neoplasms confined to
the appendix: Clinical manifestations and CT findings. J Invest
Med. 68:75–81. 2020. View Article : Google Scholar
|
|
8
|
Schmoeckel E, Kirchner T and Mayr D: SATB2
is a supportive marker for the differentiation of a primary
mucinous tumor of the ovary and an ovarian metastasis of a
low-grade appendiceal mucinous neoplasm (LAMN): A series of seven
cases. Pathol Res Pract. 214:426–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aldaoud N, Erashdi M, Alkhatib S, Abdo N,
Al-Mohtaseb A and Graboski-Bauer A: The utility of PAX8 and SATB2
immunohistochemical stains in distinguishing ovarian mucinous
neoplasms from colonic and appendiceal mucinous neoplasm. BMC Res
Notes. 12:7702019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borges AL, Reis-de-Carvalho C, Chorao M,
Pereira H and Djokovic D: Low-grade mucinous appendiceal neoplasm
mimicking an ovarian lesion: A case report and review of
literature. World J Clin Cases. 9:2334–2343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang W, Nie P, Liu X and Peng J:
Comparative effectiveness of prophylactic hyperthermic
intraperitoneal chemotherapy (HIPEC) for resected low-grade
appendiceal ucinous neoplasm (LAMN). A protocol for systematic
review and network meta-analysis. Medicine (Baltimore).
99:e220712020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guaglio M, Sinukumar S, Kusamura S,
Milione M, Pietrantonio F, Battaglia L, Guadagni S, Baratti D and
Deraco M: Clinical surveillance after macroscopically complete
surgery forlow-grade appendiceal mucinous neoplasms (LAMN) with or
without limited peritoneal spread: Long-term results in a
prospective series. Ann Surg Oncol. 25:878–884. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Istl AC, Gage MM, Esquivel J, Ahuja N,
Greer JB and Johnston FM: Management of low-grade appendiceal
mucinous neoplasms (LAMN): An international survey of surgeons
performing CRS and HIPEC. Ann Surg Oncol. 28:3831–3837. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watanabe H, Miyasaki Y, Watanabe K,
Sakamoto I, Nakagomi H, Takano A, Ikegame K, Yamamoto A, Nakada H,
Yosutome M, et al: Pseudomyxoma peritonei due to low-grade
appendiceal mucinous neoplasm with symptoms of inguinal hernia and
uterine prolapse: A case report and review of the literature. Int
Cancer Conf J. 6:158–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ceelen W, Man MD, Willaert W, H van
Ramshorst G, Geboes K and Hoorens A: Incidentally found mucinous
epithelial tumors of the appendix with or without pseudomyxoma
peritonei: Diagnostic and therapeutic algorithms based on current
evidence. Acta Chir Belg. 121:225–234. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hegg KS, Mack LA, Bouchard-Fortier A,
Temple WJ and Gui X: Macroscopic and microscopic characteristics of
low grade appendiceal mucinous neoplasms (LAMN) on appendectomy
specimens and correlations with pseudomyxoma peritonei development
risk. Ann Diagn Pathol. 48:1516062020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dundr P, Singh N, Nozickova B, Nemejcova
K, Bartu M and Struzinska I: Primary mucinous ovarian tumors vs.
ovarian metastases from gastrointestinal tract, pancreas and
biliary tree: A review of current problematics. Diagn Pathol.
16:202021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mills AM and Shanes ED: Mucinous ovarian
tumors. Surg Pathol Clin. 12:565–585. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubecek O, Laco J, Spacek J, Petera J,
Kopecky J, Kubeckova A and Filip S: The pathogenesis, diagnosis,
and management of metastatic tumors to the ovary: A comprehensive
review. Clin Exp Metastasis. 34:295–307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vang R, Gown AM, Wu L-S-F, Barry TS,
Wheeler DT, Yemelyanova A, Seidman JD and Ronnett BM:
Immunohistochemical expression of CDX2 in primary ovarian mucinous
tumors and metastatic mucinous carcinomas involving the ovary:
Comparison with CK20 and correlation with coordinate expression of
CK7. Mod Pathol. 19:1421–1428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Rock JB, Roth R, Lehman A, Marsh WL,
Suarez A and Frankel WL: Dual stain with SATB2 and CK20/Villin is
useful to distinguish colorectal carcinomas from other tumors. Am J
Clin Pathol. 149:241–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meagher NS, Wang L, Rambau PF, Intermaggio
MP, Huntsman DG, Wilkens LR, El-Bahrawy MA, Ness RB, Odunsi K,
Steed H, et al: A combination of the immunohistochemical markers
CK7 and SATB2 is highly sensitive and specific for distinguishing
primary ovarian mucinous tumors from colorectal and appendiceal
metastases. Mod Pathol. 32:1834–1846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ilieva N, Tashkova D, Staykov D, Serteva
D, Feodorova Y, Mehterov N, Mollova A and Bachurska S:
Immunohistochemical expression of CK20, CK7, and CDX2 in colorectal
carcinoma in correlation with pathomorphological characteristics.
Folia Med (Plovdiv). 64:214–220. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bassiouny D, Ismiil N, Dube V, Han G,
Cesari M, Lu FI, Slodkowska E, Parra-Herran C, Chiu HF, Naeim M, et
al: Comprehensive clinicopathologic and updated immunohistochemical
characterization of primary ovarian mucinous carcinoma. Int J Surg
Pathol. 26:306–317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Roth R, Rock JB, Lehman A, Marsh WL,
Suarez A and Frankel WL: Dual immunostain with SATB2 and CK20
differentiates appendiceal mucinous neoplasms from ovarian mucinous
neoplasms. Am J Clin Pathol. 147:484–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Genestie C, Auguste A, Al Battal M,
Scoazec JY, Gouy S, Lacroix L, Morice P, Pautier P, Leary A and
Devouassoux-Shisheboran M: Histological classification of mucinous
ovarian tumors: Inter-observer reproducibility, clinical relevance,
and role of genetic biomarkers. Virchows Arch. 478:885–891. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanai Y, Saito T, Hayashi T, Akazawa Y,
Yatagai N, Tsuyama S, Tomita S, Hirai S, Ogura K, Matsumoto T, et
al: Molecular and clinicopathological features of appendiceal
mucinous neoplasms. Virchows Arch. 478:413–426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gorringe KL, Cheasley D, Wakefield MJ,
Ryland GL, Allan PE, Alsop K, Amarasinghe KC, Ananda S, Bowtell
DDL, Christie M, et al: Therapeutic options for mucinous ovarian
carcinoma. Gynecol Oncol. 156:552–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Babaier A and Ghatage P: Mucinous cancer
of the ovary: Overview and current status. Diagnostics (Basel).
10:522020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Misdraji J: Mucinous epithelial neoplasms
of the appendix and pseudomyxoma peritonei. Mod Pathol. 28
(Suppl):S67–S79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ciarrocchi A, Rindi G and Pietroletti R:
Diagnosis and treatment of primary tumors of the appendix: A
critical review. J Gastrointest Cancer. 52:471–475. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Govaerts K, Lurvink RJ, De Hingh IHJT, Van
der Speeten K, Villeneuve L, Kusamura S, Kepenekian V, Deraco M,
Glehen O and Moran BJ: PSOGI: Appendiceal tumours and pseudomyxoma
peritonei: Literature review with PSOGI/EURACAN clinical
practiceguidelines for diagnosis and treatment. Eur J Surg Oncol.
2021.47:11–35. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Auer RC, Sivajohanathan D, Biagi J, Conner
J, Kennedy E and May T: Indications for hyperthermic
intraperitoneal chemotherapy with cytoreductive surgery: A
systematic review. Eur J Cancer. 127:76–95. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Zhou J, Dong M and Yang L:
Management and prognosis of low-grade appendiceal mucinous
neoplasms: A clinicopathologic analysis of 50 cases. Eur J Surg
Oncol. 44:1640–1645. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen I, Liu X, Kovar-Peltz S, Conrad SJ,
Chen HH and Liao X: Clinicopathological spectrums and prognosis of
primary appendiceal adenocarcinoma, goblet cell adenocarcinoma, and
low-grade appendiceal mucinous neoplasms. Pathology. 55:375–382.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kohler F, Reese L, Hendricks A, Kastner C,
Muller S, Lock JF, German C-T and Wiegering A: Low-grade mucinous
neoplasms (LAMN) of the appendix in Germany between2011 and 2018: A
nationwide analysis based on data provided by the German Center for
Cancer Registry Data (ZfKD) at the Robert Koch Institute (RKI).
Langenbecks Arch Surg. 407:3615–3622. 2022. View Article : Google Scholar : PubMed/NCBI
|