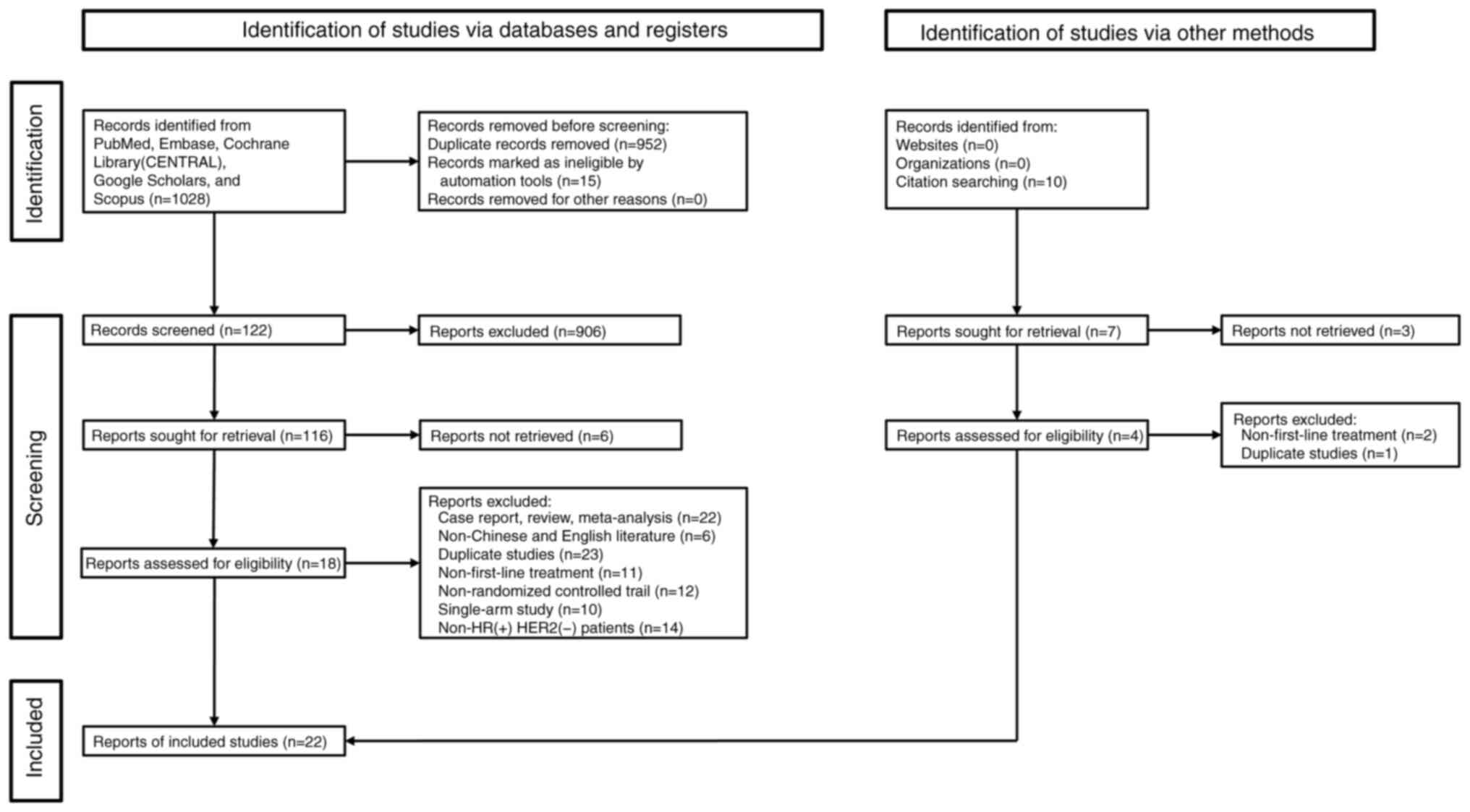
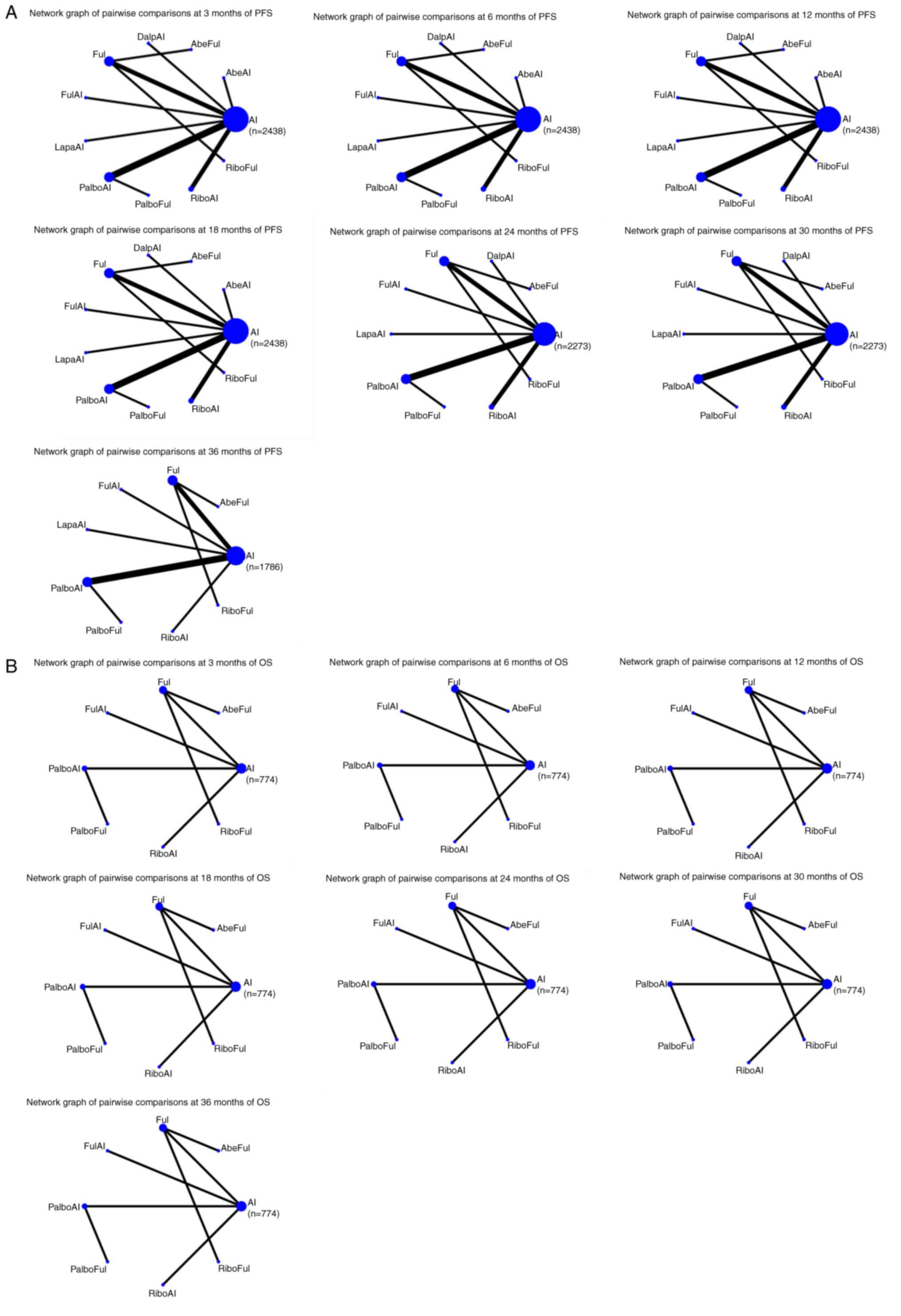
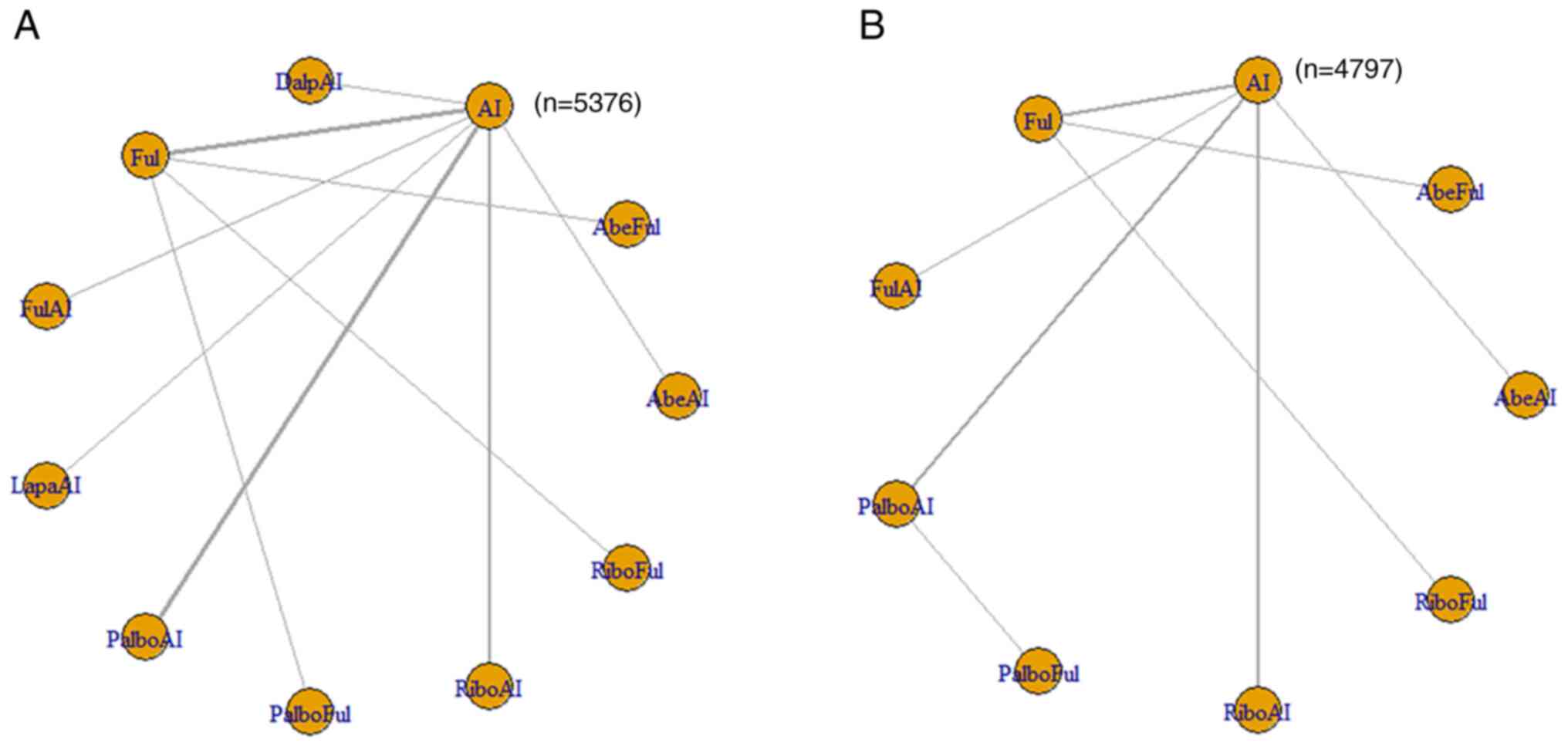
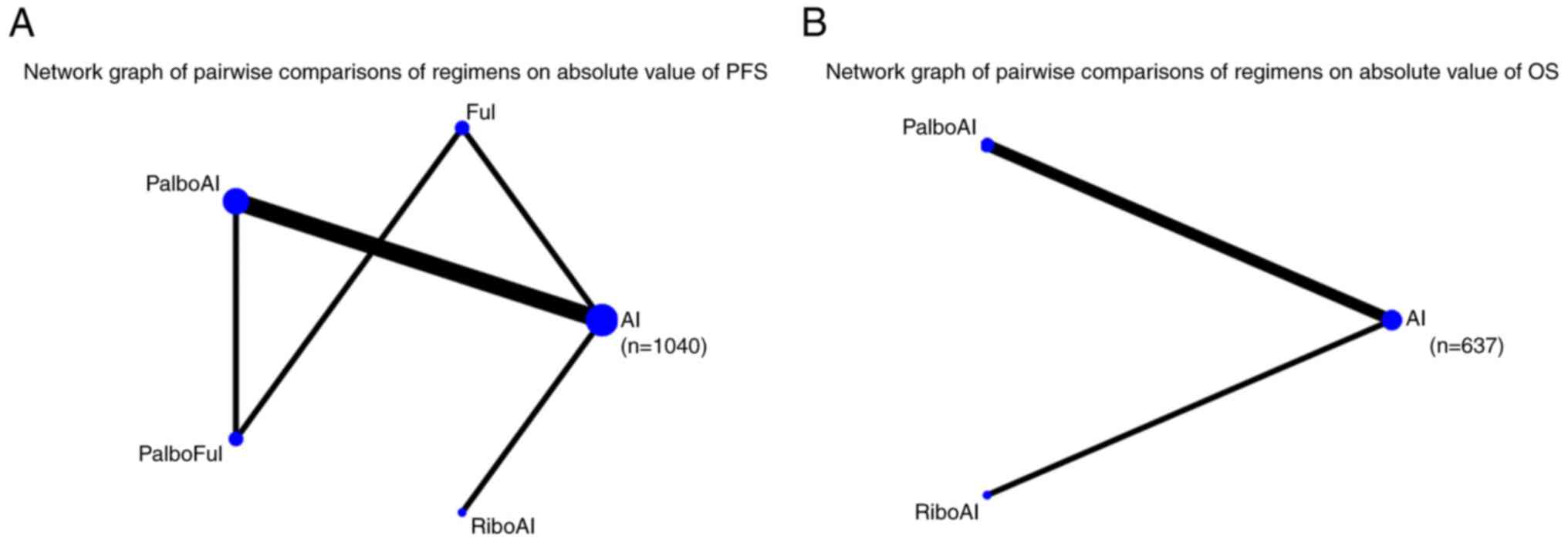
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 2:7317–48. 2023.
|
|
2
|
Trogdon JG, Baggett CD, Gogate A,
Reeder-Hayes KE, Rotter J, Zhou X, Ekwueme DU, Fairley TL and
Wheeler SB: Medical costs associated with metastatic breast cancer
in younger, midlife, and older women. Breast Cancer Res Treat.
181:653–665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moy B and Goss PE: Estrogen receptor
pathway: Resistance to endocrine therapy and new therapeutic
approaches. Clin Cancer Res. 12:4790–4793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ
and Chew H: NCCN Guidelines® Insights: Breast cancer,
version 4.2023. J Natl Compr Canc Netw. 21:594–608. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atkins CD: Tamoxifen versus
medroxyprogesterone acetate for metastatic breast cancer. J Clin
Oncol. 12:2515–2516. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paridaens RJ, Dirix LY, Beex LV, Nooij M,
Cameron DA, Cufer T, Piccart MJ, Bogaerts J and Therasse P: Phase
III study comparing exemestane with tamoxifen as first-line
hormonal treatment of metastatic breast cancer in postmenopausal
women: The European Organisation for research and treatment of
cancer breast cancer cooperative group. J Clin Oncol. 26:4883–4890.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellis MJ, Llombart-Cussac A, Feltl D,
Dewar JA, Jasiówka M, Hewson N, Rukazenkov Y and Robertson JF:
Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line
treatment of advanced breast cancer: Overall survival analysis from
the phase II FIRST study. J Clin Oncol. 33:3781–3787. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Untch M, Augustin D, Ettl J, Haidinger R,
Harbeck N, Lück HJ, Lüftner D, Marmé F, Müller L, Overkamp F, et
al: ABC3 consensus commented from the perspective of the German
guidelines: Third international consensus conference for advanced
breast cancer (ABC3), Lisbon, 07. 11. 2015. Geburtshilfe
Frauenheilkd. 76:156–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al:
Palbociclib in Hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spring LM, Wander SA, Zangardi M and
Bardia A: CDK 4/6 inhibitors in breast cancer: Current
controversies and future directions. Curr Oncol Rep. 21:252019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chirila C, Mitra D, Colosia A, Ling C,
Odom D, Iyer S and Kaye JA: Comparison of palbociclib in
combination with letrozole or fulvestrant with endocrine therapies
for advanced/metastatic breast cancer: Network meta-analysis. Curr
Med Res Opin. 33:1457–1466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu S, Sun X, Xu X and Lin F: Comparison
of endocrine therapies in hormone Receptor-Positive and human
epidermal growth factor receptor 2-Negative locally advanced or
metastatic breast cancer: A network Meta-Analysis. J Breast Cancer.
23:460–483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Syst Rev. 10:892021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sbidian E, Chaimani A, Garcia-Doval I, Do
G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, et
al: Systemic pharmacological treatments for chronic plaque
psoriasis: A network meta-analysis. Cochrane Database Syst Rev.
1:CD011535. 2020.
|
|
15
|
Wang J, Xu B, Cai L, Song Y, Kang L, Sun
T, Teng Y, Tong Z, Li H, Ouyang Q, et al: 235P Efficacy and safety
of first-line therapy with fulvestrant or exemestane for
postmenopausal ER+/HER2-advanced breast cancer patients after
adjuvant nonsteroidal aromatase inhibitor treatment: A randomized,
open-label, multicenter study. Ann Oncol. 32 (Suppl 5):S461–S462.
2021. View Article : Google Scholar
|
|
16
|
Zhang P, Zhang Q, Tong Z, Sun T, Li W,
Ouyang Q, Hu X, Cheng Y, Yan M, Pan Y, et al: Dalpiciclib plus
letrozole or anastrozole versus placebo plus letrozole or
anastrozole as first-line treatment in patients with hormone
receptor-positive, HER2-negative advanced breast cancer (DAWNA-2):
A multicentre, randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 24:646–657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robertson JFR, Bondarenko IM, Trishkina E,
Dvorkin M, Panasci L, Manikhas A, Shparyk Y, Cardona-Huerta S,
Cheung KL, Philco-Salas MJ, et al: Fulvestrant 500 mg versus
anastrozole 1 mg for hormone receptor-positive advanced breast
cancer (FALCON): An international, randomised, double-blind, phase
3 trial. Lancet. 388:2997–3005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robertson JF, Lindemann JP,
Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Emerson L, Dean A
and Ellis MJ: Fulvestrant 500 mg versus anastrozole 1 mg for the
first-line treatment of advanced breast cancer: Follow-up analysis
from the randomized ‘FIRST’ study. Breast Cancer Res Treat.
136:503–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Llombart-Cussac A, Pérez-García JM, Bellet
M, Dalenc F, Gil-Gil M, Ruíz-Borrego M, Gavilá J, Sampayo-Cordero
M, Aguirre E, Schmid P, et al: Fulvestrant-Palbociclib vs
Letrozole-Palbociclib as initial therapy for Endocrine-sensitive,
hormone Receptor-positive, ERBB2-negative advanced breast cancer: A
randomized clinical trial. JAMA Oncol. 7:1791–1799. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goetz MP, Toi M, Huober J, Sohn J, Tredan
O, Park H, Campone M, Chen SC, Sanchez LM, Shahir A, et al: LBA15
MONARCH 3: Interim overall survival (OS) results of abemaciclib
plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts)
with HR+, HER2-advanced breast cancer (ABC). Ann Oncol. 33 (Suppl
7):S13842022. View Article : Google Scholar
|
|
22
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neven P, Johnston SRD, Toi M, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Subgroup analysis of patients receiving abemaciclib plus
fulvestrant as first-line and second-line therapy for HR+,
HER2-advanced breast cancer. Clin Cancer Res. 27:5801–5809. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Boer K, Bondarenko I, Patel R,
Pinter T, Schmidt M, Shparyk YV, Thummala A, Voitko N, Bananis E,
et al: Overall survival results from the randomized phase 2 study
of palbociclib in combination with letrozole versus letrozole alone
for first-line treatment of ER+/HER2-advanced breast cancer
(PALOMA-1, TRIO-18). Breast Cancer Res Treat. 183:419–428. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, et
al: Overall survival with ribociclib plus letrozole in advanced
breast cancer. N Engl J Med. 386:942–950. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu B, Hu X, Li W, Sun T, Shen K, Wang S,
Cheng Y, Zhang Q, Cui S, Tong Z, et al: Palbociclib plus letrozole
versus placebo plus letrozole in Asian postmenopausal women with
oestrogen receptor-positive/human epidermal growth factor receptor
2-negative advanced breast cancer: Primary results from PALOMA-4.
Eur J Cancer. 175:236–245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albanell J, Martínez MT, Ramos M, O'Connor
M, de la Cruz-Merino L, Santaballa A, Martínez-Jañez N, Moreno F,
Fernández I, Alarcón J, et al: Randomized phase II study of
fulvestrant plus palbociclib or placebo in endocrine-sensitive,
hormone receptor-positive/HER2-advanced breast cancer:
GEICAM/2014-12 (FLIPPER). Eur J Cancer. 161:26–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tripathy D, Im SA, Colleoni M, Franke F,
Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, et al:
Ribociclib plus endocrine therapy for premenopausal women with
hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A
randomised phase 3 trial. Lancet Oncol. 19:904–915. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slamon DJ, Neven P, Chia S, Jerusalem G,
De Laurentiis M, Im S, Petrakova K, Valeria Bianchi G, Martín M,
Nusch A, et al: Ribociclib plus fulvestrant for postmenopausal
women with hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer in the phase III
randomized MONALEESA-3 trial: Updated overall survival. Ann Oncol.
32:1015–1024. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2–negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finn RS, Rugo HS, Dieras VC, Harbeck N, Im
SA, Gelmon KA, Walshe JM, Martin M, Mac Gregor MC, Bananis E, et
al: Overall survival (OS) with first-line palbociclib plus
letrozole (PAL+ LET) versus placebo plus letrozole (PBO+ LET) in
women with estrogen receptor-positive/human epidermal growth factor
receptor 2-negative advanced breast cancer (ER+/HER2-ABC): Analyses
from PALOMA-2. Am Soc Clin Oncol. 402022.
|
|
33
|
Rugo HS, Finn RS, Diéras V, Ettl J,
Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, et al:
Palbociclib plus letrozole as first-line therapy in estrogen
receptor-positive/human epidermal growth factor receptor 2-negative
advanced breast cancer with extended follow-up. Breast Cancer Res
Treat. 174:719–729. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu YS, Im SA, Colleoni M, Franke F, Bardia
A, Cardoso F, Harbeck N, Hurvitz S, Chow L, Sohn J, et al: Updated
overall survival of ribociclib plus endocrine therapy versus
endocrine therapy alone in Pre- and Perimenopausal patients with
HR+/HER2-advanced breast cancer in MONALEESA-7: A phase III
randomized clinical trial. Clin Cancer Res. 28:851–859. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bergh J, Jönsson PE, Lidbrink EK, Trudeau
M, Eiermann W, Brattström D, Lindemann JP, Wiklund F and Henriksson
R: FACT: An open-label randomized phase III study of fulvestrant
and anastrozole in combination compared with anastrozole alone as
first-line therapy for patients with receptor-positive
postmenopausal breast cancer. J Clin Oncol. 30:1919–1925. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Skibinski A and Kuperwasser C: The origin
of breast tumor heterogeneity. Oncogene. 34:5309–5316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lainetti PF, Leis-Filho AF, Laufer-Amorim
R, Battazza A and Fonseca-Alves CE: Mechanisms of resistance to
chemotherapy in breast cancer and possible targets in drug delivery
systems. Pharmaceutics. 12:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parisi S, Ruggiero R, Gualtieri G, Volpe
ML, Rinaldi S, Nesta G, Bogdanovich L, Lucido FS, Tolone S,
Parmeggiani D, et al: Combined LOCalizer™ and intraoperative
ultrasound localization: First experience in localization of
Non-palpable breast cancer. In Vivo. 35:1669–1676. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parisi S, Gambardella C, Conzo G, Ruggiero
R, Tolone S, Lucido FS, Iovino F, Fisone F, Brusciano L,
Parmeggiani D and Docimo L: Advanced localization technique for
Non-Palpable breast cancer: Radiofrequency alone VS combined
technique with ultrasound. J Clin Med. 12:50762023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gambardella C, Clarizia G, Patrone R, Offi
C, Mauriello C, Romano R, Filardo M, Conzo A, Sanguinetti A,
Polistena A, et al: Advanced hemostasis in axillary lymph node
dissection for locally advanced breast cancer: New technology
devices compared in the prevention of seroma formation. BMC Surg.
18 (Suppl 1):S1252019. View Article : Google Scholar
|
|
41
|
Spring LM, Wander SA, Andre F, Moy B,
Turner NC and Bardia A: Cyclin-dependent kinase 4 and 6 inhibitors
for hormone receptor-positive breast cancer: Past, present, and
future. Lancet. 395:817–827. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam
H, Bergqvist S, Solowiej J, Diehl W, He YA, et al: Spectrum and
degree of CDK drug interactions predicts clinical performance. Mol
Cancer Ther. 15:2273–2281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marra A and Curigliano G: Are all
cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast
Cancer. 5:272019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spring LM, Zangardi ML, Moy B and Bardia
A: Clinical management of potential toxicities and drug
interactions related to Cyclin-dependent kinase 4/6 inhibitors in
breast cancer: Practical considerations and recommendations.
Oncologist. 22:1039–1048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang QY, Sun T, Yin YM, Li HP, Yan M,
Tong ZS, Oppermann CP, Liu YP, Costa R, Li M, et al: MONARCH plus:
Abemaciclib plus endocrine therapy in women with HR+/HER2-advanced
breast cancer: The multinational randomized phase III study. Ther
Adv Med Oncol. 12:17588359209639252020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,
Winer EP, et al: Ribociclib as first-line therapy for HR-positive,
advanced breast cancer. N Engl J Med. 375:1738–1748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roncato R, Angelini J, Pani A, Cecchin E,
Sartore-Bianchi A, Siena S, De Mattia E, Scaglione F and Toffoli G:
CDK4/6 inhibitors in breast cancer treatment: Potential
interactions with drug, gene, and pathophysiological conditions.
Int J Mol Sci. 21:63502020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Horie T, Kijima T, Yamaguchi M, Honda S,
Horie M, Ishitobi K, Yamagata S, Sakano S and Kurokohchi K: Severe
hypoglycaemia under abemaciclib administration in a patient with
breast cancer: A case report. Mol Clin Oncol. 14:612021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lagampan C, Poovorawan N and
Parinyanitikul N: Lactic acidosis, a potential toxicity from
drug-drug interaction related to concomitant ribociclib and
metformin in preexisting renal insufficiency: A case report. Cancer
Rep (Hoboken). 5:e15752022. View Article : Google Scholar : PubMed/NCBI
|