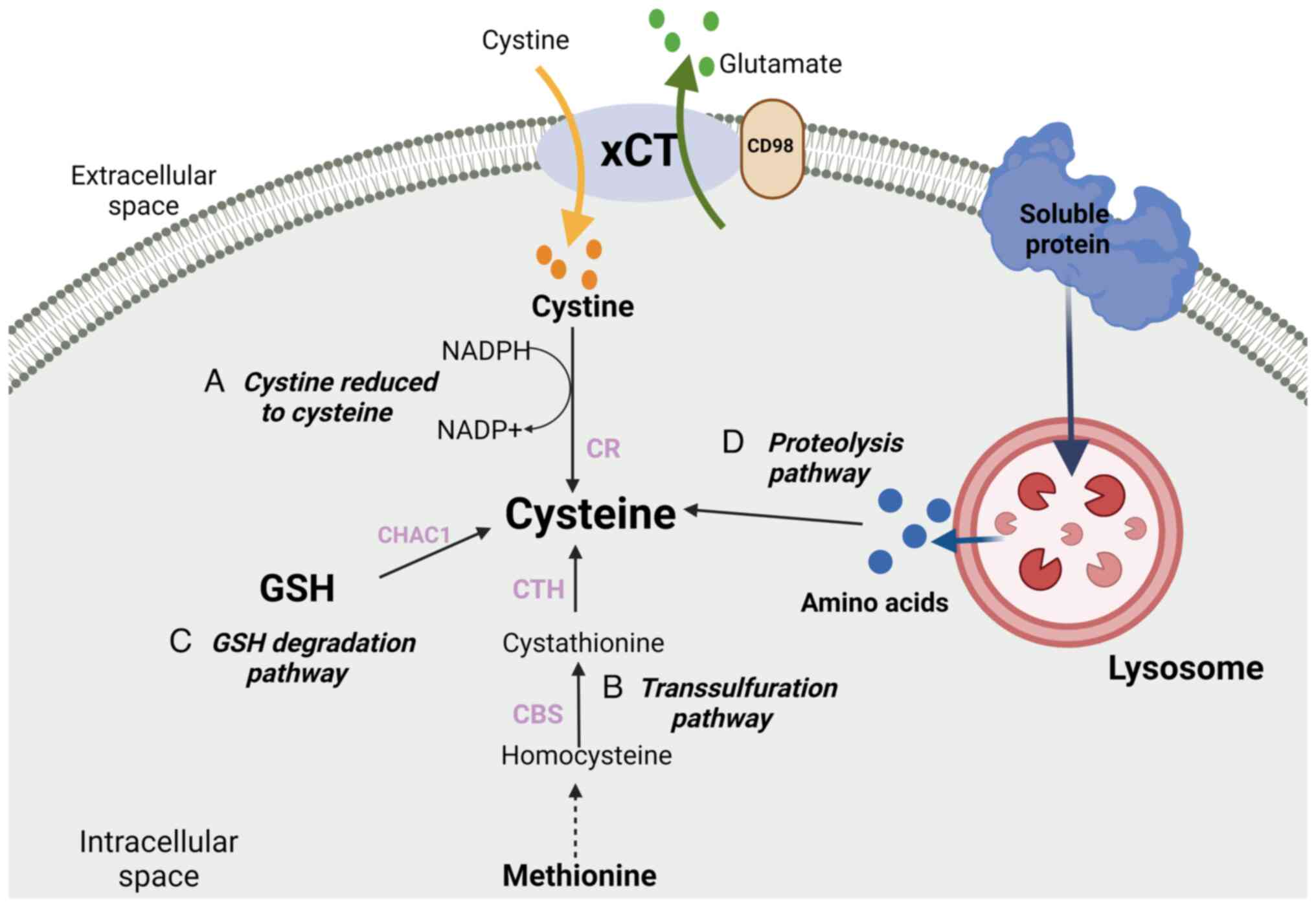
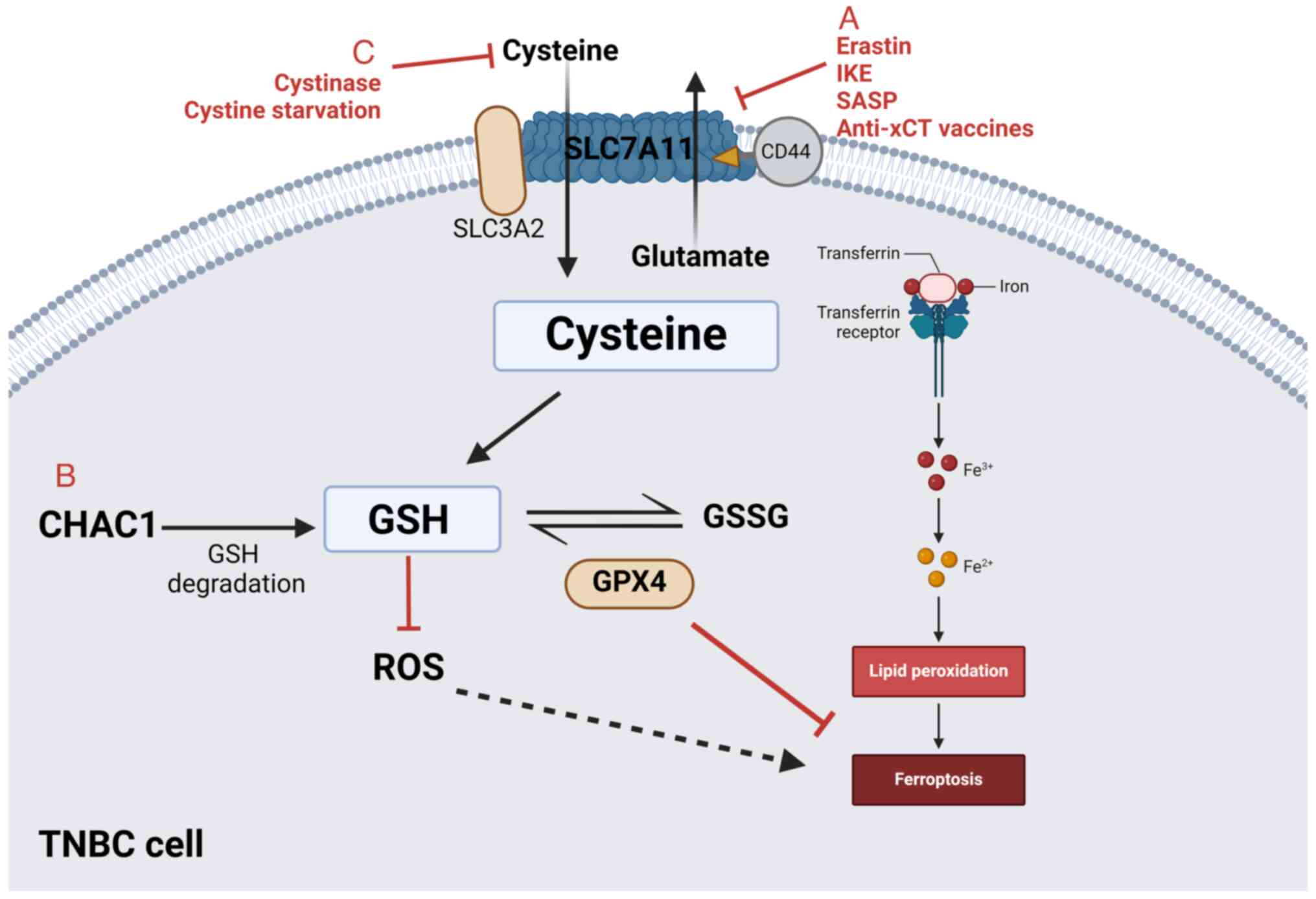
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morris GJ, Naidu S, Topham AK, Guiles F,
Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K and
Mitchell EP: Differences in breast carcinoma characteristics in
newly diagnosed African-American and Caucasian patients: A
single-institution compilation compared with the National Cancer
Institute's Surveillance, Epidemiology, and End Results database.
Cancer. 110:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh DD and Yadav DK: TNBC: Potential
targeting of multiple receptors for a therapeutic breakthrough,
nanomedicine, and immunotherapy. Biomedicines. 9:8762021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietze EC, Sistrunk C, Miranda-Carboni G,
O'Regan R and Seewaldt VL: Triple-negative breast cancer in
African-American women: Disparities versus biology. Nat Rev Cancer.
15:248–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhi N and Das GM: Metabolic
reprogramming in breast cancer and its therapeutic implications.
Cells. 8:892019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germline BRCA mutation. N Engl J Med. 379:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Jiang Q and Dong C: Metabolic
reprogramming in triple-negative breast cancer. Cancer Biol Med.
17:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nedeljković M and Damjanović A: Mechanisms
of chemotherapy resistance in triple-negative breast cancer-how we
can rise to the challenge. Cells. 8:9572019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang X, Lin CC, Spasojevic I, Iversen ES,
Chi JT and Marks JR: A joint analysis of metabolomics and genetics
of breast cancer. Breast Cancer Res. 16:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Z, Liu X, Cheng C, Yu W and Yi P:
Metabolism of amino acids in cancer. Front Cell Dev Biol.
8:6038372021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheen JH, Zoncu R, Kim D and Sabatini DM:
Defective regulation of autophagy upon leucine deprivation reveals
a targetable liability of human melanoma cells in vitro and in
vivo. Cancer Cell. 19:613–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang X, Wu J, Ding CK, Lu M, Keenan MM,
Lin CC, Lin CA, Wang CC, George D, Hsu DS and Chi JT: Cystine
deprivation triggers programmed necrosis in VHL-Deficient renal
cell carcinomas. Cancer Res. 76:1892–1903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iglehart J, York RM, Modest AP, Lazarus H
and Livingston D: Cystine requirement of continuous human lymphoid
cell lines of normal and leukemic origin. J Biol Chem.
252:7184–7191. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edwards DN, Ngwa VM, Raybuck AL, Wang S,
Hwang Y, Kim LC, Cho SH, Paik Y, Wang Q, Zhang S, et al: Selective
glutamine metabolism inhibition in tumor cells improves antitumor T
lymphocyte activity in triple-negative breast cancer. J Clin
Invest. 131:e1401002021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Trachootham D, Liu J, Chen G,
Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W,
et al: Stromal control of cystine metabolism promotes cancer cell
survival in chronic lymphocytic leukaemia. Nat Cell Biol.
14:276–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daher B, Vučetić M and Pouysségur J:
Cysteine depletion, a key action to challenge cancer cells to
ferroptotic cell death. Front Oncol. 10:7232020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stipanuk MH: Sulfur amino acid metabolism:
Pathways for production and removal of homocysteine and cysteine.
Annu Rev Nutr. 24:539–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HF, Klein Geltink RI, Parker SJ and
Sorensen PH: Transsulfuration, minor player or crucial for cysteine
homeostasis in cancer. Trends Cell Biol. 32:800–814. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Combs JA and DeNicola GM: The
non-essential amino acid cysteine becomes essential for tumor
proliferation and survival. Cancers (Basel). 11:6782019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Berisa M, Schwörer S, Qin W, Cross
JR and Thompson CB: Transsulfuration activity can support cell
growth upon extracellular cysteine limitation. Cell Metab.
30:865–876.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lien EC, Ghisolfi L, Geck RC, Asara JM and
Toker A: Oncogenic PI3K promotes methionine dependency in breast
cancer cells through the cystine-glutamate antiporter xCT. Sci
Signal. 10:eaao66042017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pajares MA and Perez-Sala D: Mammalian
sulfur amino acid metabolism: A nexus between redox regulation,
nutrition, epigenetics, and detoxification. Antioxid Redox Signal.
29:408–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Ding CK, Wu J, Sjol J, Wardell S,
Spasojevic I, George D, McDonnell DP, Hsu DS, Chang JT and Chi JT:
Cystine addiction of triple-negative breast cancer associated with
EMT augmented death signaling. Oncogene. 36:4235–4242. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS,
Lee HC and Tseng LM: CHAC1 degradation of glutathione enhances
cystine-starvation-induced necroptosis and ferroptosis in human
triple negative breast cancer cells via the GCN2-eIF2α-ATF4
pathway. Oncotarget. 8:114588–114602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Lee IM, Song Y, Cook NR, Selhub J,
Manson JE, Buring JE and Zhang SM: Plasma homocysteine and cysteine
and risk of breast cancer in women. Cancer Res. 70:2397–2405. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu LL and Wu JT: Hyperhomocysteinemia is a
risk factor for cancer and a new potential tumor marker. Clin Chim
Acta. 322:21–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun CF, Haven TR, Wu TL, Tsao KC and Wu
JT: Serum total homocysteine increases with the rapid proliferation
rate of tumor cells and decline upon cell death: A potential new
tumor marker. Clin Chim Acta. 321:55–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue F and Michels KB: Diabetes, metabolic
syndrome, and breast cancer: A review of the current evidence. Am J
Clin Nutr. 86:S823–S835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
La Vecchia C, Giordano SH, Hortobagyi GN
and Chabner B: Overweight, obesity, diabetes, and risk of breast
cancer: Interlocking pieces of the puzzle. Oncologist. 16:726–729.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The Cystine/Glutamate Antiporter System xc-in Health and
disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signa. 18:522–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kandasamy P, Gyimesi G, Kanai Y and
Hediger MA: Amino acid transporters revisited: New views in health
and disease. Trends Biochem Sci. 43:752–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyazaki I, Murakami S, Torigoe N,
Kitamura Y and Asanuma M: Neuroprotective effects of levetiracetam
target xCT in astrocytes in parkinsonian mice. J Neurochem.
136:194–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugano K, Maeda K, Ohtani H, Nagahara H,
Shibutani M and Hirakawa K: Expression of xCT as a predictor of
disease recurrence in patients with colorectal cancer. Anticancer
Res. 35:677–682. 2015.PubMed/NCBI
|
|
41
|
Robert SM, Buckingham SC, Campbell SL,
Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid
MA, Eschbacher JM, et al: SLC7A11 expression is associated with
seizures and predicts poor survival in patients with malignant
glioma. Sci Transl Med. 7:289ra862015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruiu R, Rolih V, Bolli E, Barutello G,
Riccardo F, Quaglino E, Merighi IF, Pericle F, Donofrio G, Cavallo
F and Conti L: Fighting breast cancer stem cells through the
immune-targeting of the xCT cystine-glutamate antiporter. Cancer
Immunol Immunother. 68:131–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lanzardo S, Conti L, Rooke R, Ruiu R,
Accart N, Bolli E, Arigoni M, Macagno M, Barrera G, Pizzimenti S,
et al: Immunotargeting of antigen xCT attenuates Stem-like cell
behavior and metastatic progression in breast cancer. Cancer Res.
76:62–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Timmerman LA, Holton T, Yuneva M, Louie
RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van't Veer LJ, et
al: Glutamine sensitivity analysis identifies the xCT antiporter as
a common triple-negative breast tumor therapeutic target. Cancer
Cell. 24:450–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hasegawa M, Takahashi H, Rajabi H, Alam M,
Suzuki Y, Yin L, Tagde A, Maeda T, Hiraki M, Sukhatme VP, et al:
Functional interactions of the cystine/glutamate antiporter, CD44v
and MUC1-C oncoprotein in triple-negative breast cancer cells.
Oncotarget. 7:11756–11769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc- and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fotiadis D, Kanai Y and Palacín M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang Y, Cao Y, Wang Y, Li W, Liu X, Lv Y,
Li X and Mi J: Cysteine transporter SLC3A1 promotes breast cancer
tumorigenesis. Theranostics. 7:1036–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jeong JY, Oh KJ, Sohn JS, Jun DY, Shin JI,
Lee KH and Lee JY: Clinical course and mutational analysis of
patients with cystine stone: A Single-Center experience.
Biomedicines. 11:27472023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chio C II and Tuveson DA: ROS in cancer:
The burning question. Trends Mol Med. 23:411–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haraguchi N, Inoue H, Tanaka F, Mimori K,
Utsunomiya T, Sasaki A and Mori M: Cancer stem cells in human
gastrointestinal cancers. Hum Cell. 19:24–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tarragó-Celada J, Foguet C,
Tarrado-Castellarnau M, Marin S, Hernández-Alias X, Perarnau J,
Morrish F, Hockenbery D, Gomis RR, Ruppin E, et al: Cysteine and
folate metabolism are targetable vulnerabilities of metastatic
colorectal cancer. Cancers (Basel). 13:4252021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bao WEI, Han Q, Guan X, Wang Z and Gu MIN:
Solute carrier-related signature for assessing prognosis and
immunity in patients with clear-cell renal cell carcinoma. Oncol
Res. 31:181–192. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cha YJ, Kim ES and Koo JS: Amino acid
transporters and glutamine metabolism in breast cancer. Int J Mol
Sci. 19:9072018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Todorova VK, Kaufmann Y, Luo S and
Klimberg VS: Tamoxifen and raloxifene suppress the proliferation of
estrogen receptor-negative cells through inhibition of glutamine
uptake. Cancer Chemother Pharmacol. 67:285–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bernhardt S, Bayerlová M, Vetter M,
Wachter A, Mitra D, Hanf V, Lantzsch T, Uleer C, Peschel S, John J,
et al: Proteomic profiling of breast cancer metabolism identifies
SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res.
19:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeon YJ, Khelifa S, Ratnikov B, Scott DA,
Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, et al:
Regulation of glutamine carrier proteins by RNF5 determines breast
cancer response to ER Stress-Inducing chemotherapies. Cancer Cell.
27:354–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sayin VI, Ibrahim MX, Larsson E, Nilsson
JA, Lindahl P and Bergo MO: Antioxidants accelerate lung cancer
progression in mice. Sci Transl Med. 6:221ra152014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MCU, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Weigelt B and Reis-Filho JS: Histological
and molecular types of breast cancer: Is there a unifying taxonomy?
Nat Rev Clin Oncol. 6:718–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sato R, Nakano T, Hosonaga M, Sampetrean
O, Harigai R, Sasaki T, Koya I, Okano H, Kudoh J, Saya H and Arima
Y: RNA sequencing analysis reveals interactions between breast
cancer or melanoma cells and the tissue microenvironment during
brain metastasis. Biomed Res Int. 2017:80329102017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hosonaga M, Saya H and Arima Y: Molecular
and cellular mechanisms underlying brain metastasis of breast
cancer. Cancer Metastasis Rev. 39:711–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ruiu R, Cossu C, Iacoviello A, Conti L,
Bolli E, Ponzone L, Magri J, Rumandla A, Calautti E and Cavallo F:
Cystine/glutamate antiporter xCT deficiency reduces metastasis
without impairing immune system function in breast cancer mouse
models. J Exp Clin Cancer Res. 42:2542023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hu J, Li G, Zhang P, Zhuang X and Hu G: A
CD44v+ subpopulation of breast cancer stem-like cells with enhanced
lung metastasis capacity. Cell Death Dis. 8:e26792017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lollini PL, Cavallo F, Giovanni CD and
Nanni P: Preclinical vaccines against mammary carcinoma. Expert Rev
Vaccines. 12:1449–1463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nagano O, Okazaki S and Saya H: Redox
regulation in stem-like cancer cells by CD44 variant isoforms.
Oncogene. 32:5191–5198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kinoshita H, Okabe H, Beppu T, Chikamoto
A, Hayashi H, Imai K, Mima K, Nakagawa S, Ishimoto T, Miyake K, et
al: Cystine/glutamic acid transporter is a novel marker for
predicting poor survival in patients with hepatocellular carcinoma.
Oncol Rep. 29:685–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Molecular mechanisms of ferroptosis and its role in cancer
therapy. J Cell Mol Med. 23:4900–4912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng YW, Miao XY, Xiong L, Chen B, Kong
FH, Zhou JJ, Liu ZT, Wen Y, Zhang ZJ and Zou H: Sulfasalazine
sensitizes polyhematoporphyrin-mediated photodynamic therapy in
cholangiocarcinoma by targeting xCT. Front Pharmacol.
12:7234882021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu H, Hu K, Zhang T and Ren H:
Identification of target genes related to sulfasalazine in
triple-negative breast cancer through Network pharmacology. Med Sci
Monit. 26:e9265502020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gout P, Buckley A, Simms C and Bruchovsky
N: Sulfasalazine, a potent suppressor of lymphoma growth by
inhibition of the x(c)-cystine transporter: A new action for an old
drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guan J, Lo M, Dockery P, Mahon S, Karp CM,
Buckley AR, Lam S, Gout PW and Wang YZ: The × c-cystine/glutamate
antiporter as a potential therapeutic target for small-cell lung
cancer: Use of sulfasalazine. Cancer Chemother Pharmacol.
64:463–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Doxsee DW, Gout PW, Kurita T, Lo M,
Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR and Wang YZ:
Sulfasalazine-induced cystine starvation: Potential use for
prostate cancer therapy. Prostate. 67:162–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shitara K, Doi T, Nagano O, Imamura CK,
Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka
S, et al: Dose-escalation study for the targeting of CD44v+ cancer
stem cells by sulfasalazine in patients with advanced gastric
cancer (EPOC1205). Gastric Cancer. 20:341–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shitara K, Doi T, Nagano O, Fukutani M,
Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, et al:
Phase 1 study of sulfasalazine and cisplatin for patients with
CD44v-positive gastric cancer refractory to cisplatin (EPOC1407).
Gastric Cancer. 20:1004–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Otsubo K, Nosaki K, Imamura CK, Ogata H,
Fujita A, Sakata S, Hirai F, Toyokawa G, Iwama E, Harada T, et al:
Phase I study of salazosulfapyridine in combination with cisplatin
and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci.
108:1843–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dai Z, Huang Y, Sadee W and Blower P:
Chemoinformatics analysis identifies cytotoxic compounds
susceptible to chemoresistance mediated by glutathione and
cystine/glutamate transport system xc-. J Med Chem. 50:1896–1906.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Narang VS, Pauletti GM, Gout PW, Buckley
DJ and Buckley AR: Sulfasalazine-induced reduction of glutathione
levels in breast cancer cells: Enhancement of growth-inhibitory
activity of doxorubicin. Chemotherapy. 53:210–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Barutello G, Di Lorenzo A, Gasparetto A,
Galiazzi C, Bolli E, Conti L and Cavallo F: Immunotherapy against
the Cystine/Glutamate Antiporter xCT improves the efficacy of
APR-246 in preclinical breast cancer models. Biomedicines.
10:28432022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Conti L, Bolli E, Di Lorenzo A, Franceschi
V, Macchi F, Riccardo F, Ruiu R, Russo L, Quaglino E, Donofrio G
and Cavallo F: Immunotargeting of the xCT Cystine/Glutamate
antiporter potentiates the efficacy of HER2-targeted
immunotherapies in breast cancer. Cancer Immunol Res. 8:1039–53.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu H, Yang C, Jian L, Guo S, Chen R, Li K,
Qu F, Tao K, Fu Y, Luo F and Liu S: Sulfasalazine-induced
ferroptosis in breast cancer cells is reduced by the inhibitory
effect of estrogen receptor on the transferrin receptor. Oncol Rep.
42:826–838. 2019.PubMed/NCBI
|
|
87
|
Sehm T, Rauh M, Wiendieck K, Buchfelder M,
Eyüpoglu IY and Savaskan NE: Temozolomide toxicity operates in a
xCT/SLC7a11 dependent manner and is fostered by ferroptosis.
Oncotarget. 7:746302016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wei CW, Yu YL, Lu JY, Hung YT, Liu HC and
Yiang GT: Anti-cancer effects of sulfasalazine and Vitamin E
succinate in MDA-MB 231 Triple-negative breast cancer cells. Int J
Med Sci. 16:494–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Verbruggen L, Sprimont L, Bentea E,
Janssen P, Gharib A, Deneyer L, De Pauw L, Lara O, Sato H, Nicaise
C and Massie A: Chronic sulfasalazine treatment in mice induces
system xc−-Independent adverse effects. Front
Pharmacol. 12:6256992021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Robe PA, Martin DH, Nguyen-Khac MT, Artesi
M, Deprez M, Albert A, Vanbelle S, Califice S, Bredel M and Bours
V: Early termination of ISRCTN45828668, a phase 1/2 prospective,
randomized study of sulfasalazine for the treatment of progressing
malignant gliomas in adults. BMC Cancer. 9:3722009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Donofrio G, Tebaldi G, Lanzardo S, Ruiu R,
Bolli E, Ballatore A, Rolih V, Macchi F, Conti L and Cavallo F:
Bovine herpesvirus 4-based vector delivering the full length xCT
DNA efficiently protects mice from mammary cancer metastases by
targeting cancer stem cells. Oncoimmunology. 7:e14941082018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang F and Yang Y: Suppression of the
xCT-CD44v antiporter system sensitizes triple-negative breast
cancer cells to doxorubicin. Breast Cancer Res Treat. 147:203–210.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Marin-Acevedo JA, Soyano AE, Dholaria B,
Knutson KL and Lou Y: Cancer immunotherapy beyond immune checkpoint
inhibitors. J Hematol Oncol. 11:82018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ruzzi F, Semprini MS, Scalambra L,
Palladini A, Angelicola S, Cappello C, Pittino OM, Nanni P and
Lollini PL: Virus-like particle (VLP) vaccines for cancer
immunotherapy. Int J Mol Sci. 24:129632023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rolih V, Caldeira J, Bolli E, Salameh A,
Conti L, Barutello G, Riccardo F, Magri J, Lamolinara A, Parra K,
et al: Development of a VLP-based vaccine displaying an xCT
extracellular domain for the treatment of metastatic breast cancer.
Cancers. 12:14922020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bolli E, O'Rourke JP, Conti L, Lanzardo S,
Rolih V, Christen JM, Barutello G, Forni M, Pericle F and Cavallo
F: A Virus-Like-Particle immunotherapy targeting Epitope-specific
anti-xCT expressed on cancer stem cell inhibits the progression of
metastatic cancer in vivo. Oncoimmunology. 7:e14087462018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lopes A, Vandermeulen G and Preat V:
Cancer DNA vaccines: Current preclinical and clinical developments
and future perspectives. J Exp Clin Cancer Res. 38:1462019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/Tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Imai H, Matsuoka M, Kumagai T, Sakamoto T
and Koumura T: Lipid Peroxidation-dependent cell death regulated by
GPx4 and ferroptosis. Curr Top Microbiol Immunol. 403:143–170.
2017.PubMed/NCBI
|
|
101
|
Lv Y, Liang C, Sun Q, Zhu J, Xu H, Li X,
Li X, Li YY, Wang Q, Yuan H, et al: Structural insights into FSP1
catalysis and ferroptosis inhibition. Nat Commun. 14:59332023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Harris IS, Endress JE, Coloff JL, Selfors
LM, McBrayer SK, Rosenbluth JM, Takahashi N, Dhakal S, Koduri V,
Oser MG, et al: Deubiquitinases maintain protein homeostasis and
survival of cancer cells upon glutathione depletion. Cell Metab.
29:1166–1181.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shin D, Lee J, You JH, Kim D and Roh JL:
Dihydrolipoamide dehydrogenase regulates cystine
deprivation-induced ferroptosis in head and neck cancer. Redox
Biol. 30:1014182020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ensink EJT, Medeiros HCD, Thurston G,
Pardal A, Yu L and Lunt SY: Pyruvate kinase activity regulates
cystine starvation induced ferroptosis through malic enzyme 1 in
pancreatic cancer cells. bioRxiv. 2023.doi:
10.1101/2023.09.15.557984. PubMed/NCBI
|
|
107
|
Singh S, Maurya P, Rani S, Mishra N, Nisha
R, Singh P and Saraf SA: Development of doxorubicin
hydrochloride-loaded whey protein nanoparticles and its surface
modification with N-acetyl cysteine for triple-negative breast
cancer. Drug Deliv Transl Res. 12:3047–3062. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole
D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL,
et al: Drug-tolerant persister cancer cells are vulnerable to GPX4
inhibition. Nature. 551:247–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Y, Swanda RV, Nie L, Liu X, Wang C,
Lee H, Lei G, Mao C, Koppula P, Cheng W, et al: mTORC1 couples
cyst(e)ine availability with GPX4 protein synthesis and ferroptosis
regulation. Nat Commun. 12:15892021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lei M, Zhang YL, Huang FY, Chen HY, Chen
MH, Wu RH, Dai SZ, He GS, Tan GH and Zheng WP: Gankyrin inhibits
ferroptosis through the p53/SLC7A11/GPX4 axis in triple-negative
breast cancer cells. Sci Rep. 13:219162023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tajan M and Vousden KH: Dietary approaches
to cancer therapy. Cancer Cell. 37:767–785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu H, Zhang W, Wang K, Wang X, Yin F, Li
C, Wang C, Zhao B, Zhong C, Zhang J, et al: Methionine and cystine
double deprivation stress suppresses glioma proliferation via
inducing ROS/autophagy. Toxicol Lett. 232:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Knott SRV, Wagenblast E, Khan S, Kim SY,
Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL, et al:
Asparagine bioavailability governs metastasis in a model of breast
cancer. Nature. 554:378–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jimenez-Alonso JJ and Lopez-Lazaro M:
Dietary manipulation of amino acids for cancer therapy. Nutrients.
15:28792023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang T, Bauer C, Newman AC, Uribe AH,
Athineos D, Blyth K and Maddocks ODK: Polyamine pathway activity
promotes cysteine essentiality in cancer cells. Nat Metab.
2:1062–1076. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Srivastava MK, Sinha P, Clements VK,
Rodriguez P and Ostrand-Rosenberg S: Myeloid-derived suppressor
cells inhibit T-cell activation by depleting cystine and cysteine.
Cancer Res. 70:68–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cramer SL, Saha A, Liu J, Tadi S, Tiziani
S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al:
Systemic depletion of L-cyst(e)ine with cyst(e)inase increases
reactive oxygen species and suppresses tumor growth. Nat Med.
23:120–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Alothaim T, Charbonneau M and Tang X:
HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast
cancer cells to cysteine deprivation. Sci Rep. 11:109562021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Espinoza IKC, Park CH, Vander Steen T,
Kleer CG, Wiley E, Rademaker A, Cuyàs E, Verdura S, Buxó M,
Reynolds C, et al: Depletion of CCN1/CYR61 reduces
triple-negative/basal-like breast cancer aggressiveness. Am J
Cancer Res. 12:839–851. 2022.PubMed/NCBI
|