Introduction
Gallbladder cancer is a frequent malignant tumor of
the biliary tract and shows a poor prognosis (1). It is a highly aggressive malignancy
that is hard to diagnose and has few therapeutic options, with a
5–10% 5-year overall survival (OS) rate (2,3). In
gallbladder cancer, cases diagnosed at the same tumor stage
sometimes show different prognoses because of its heterogeneity
(4). Additionally, distinguishing
benign tumors from malignant tumors is difficult by conventional
imaging modalities (5). Therefore,
accurate and early imaging diagnoses are necessary for selecting
appropriate therapy and improving prognosis.
Although ultrasonography is considered one of the
most reliable imaging modalities for evaluating gallbladder
disease, it is hard to diagnose early lesions because of its low
specificity and sensitivity (6).
Morphological evaluation using computed tomography (CT) is also
widely used. However, it is hard to distinguish gallbladder cancer
from other benign tumors such as chronic cholecystitis (7). In recent years, diffusion-weighted
imaging (DWI) of magnetic resonance imaging (MRI) has shown radical
improvement in the detection of malignant tumors (8). DWI is a functional MRI technique that
can assess diffusion of water molecules and evaluate the
pathological condition of organs and tissue at a high scan speed
without a contrast agent. A quantitative assessment of diffusion
characteristics can be presented by apparent diffusion coefficient
(ADC) values (9); they are
decreased in areas in which diffusion is restricted, such as in
tissue with high cellularity or rich stroma. Previously, it has
been reported that ADC values could estimate the characteristics of
various malignancies (10,11). Our previous study also demonstrated
that ADC values can estimate the prognosis of intrahepatic
cholangiocarcinoma (12).
Previously, DWI was shown to support the diagnosis of gallbladder
malignancy and can also distinguish gallbladder cancers from benign
tumors in gallbladder (6), while
the ADC value can estimate the histological grade of primary
gallbladder carcinoma (13).
The present study examined the utility of ADC values
in the evaluation of malignancies and in the prognostic prediction
of gallbladder tumors.
Materials and methods
Patients and MRI imaging
In the present study, a total of 63 patients (44
female and 19 male patients; median age, 62 years; age range, 25–91
years) who underwent surgical resection for gallbladder tumors at
the Department of Surgery, Tokushima University Hospital, between
January 2011 and December 2023 were enrolled. The patients that
were included: i) Underwent DWI of MRI within 1 month before
surgical resection; ii) showed no previous treatment before
surgical resection, and no extrahepatic metastasis; and iii) had a
gallbladder tumor confirmed pathologically. Cases of non-curative
resection (R2) and patients that underwent neoadjuvant chemotherapy
were not included in the present study. Regarding gallbladder
cancer, morphological and pathological characteristics and the
Japanese Tumor-Node-Metastasis stage were assessed according to the
guidelines of the Japanese Society of Biliary Surgery (14).
MR images were obtained with 1.5-T superconducting
units (Signa HDe/Explorer; GE Healthcare) using 8-channel
phased-array coil. Fast spin-echo T2-weighted images and DWI (b=0,
20, 800 s/mm2) were obtained. Using ADC maps on Synapse
Vincent (ver. 6.8; Fujifilm Healthcare), the mean ADC values
(×10−3 mm2/sec) of tumors were calculated in
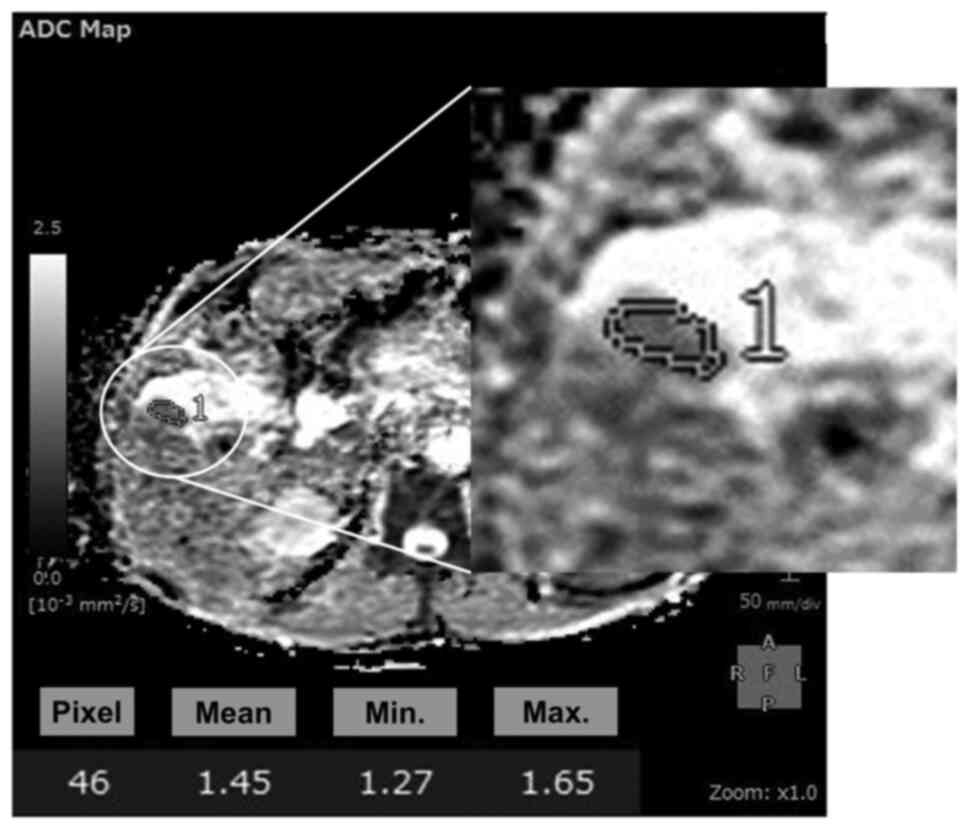
regions of interest with manual tracing (12). Synapse Vincent can obtain the
maximum, minimum and mean values in free-form green lines
automatically (Fig. 1). In the
present study, mean ADC values were used as previously reported
(15), and cases of gallbladder
cancer were divided into non-advanced gallbladder cancer, whereby
the tumor depth was the mucosa or muscular layer, and advanced
gallbladder cancer, in which the tumor depth was deeper than the
muscular layer. In advanced gallbladder cancer (n=25), patients
were divided into 2 groups using the ADC value: An
ADCHigh group (n=15) and an ADCLow group
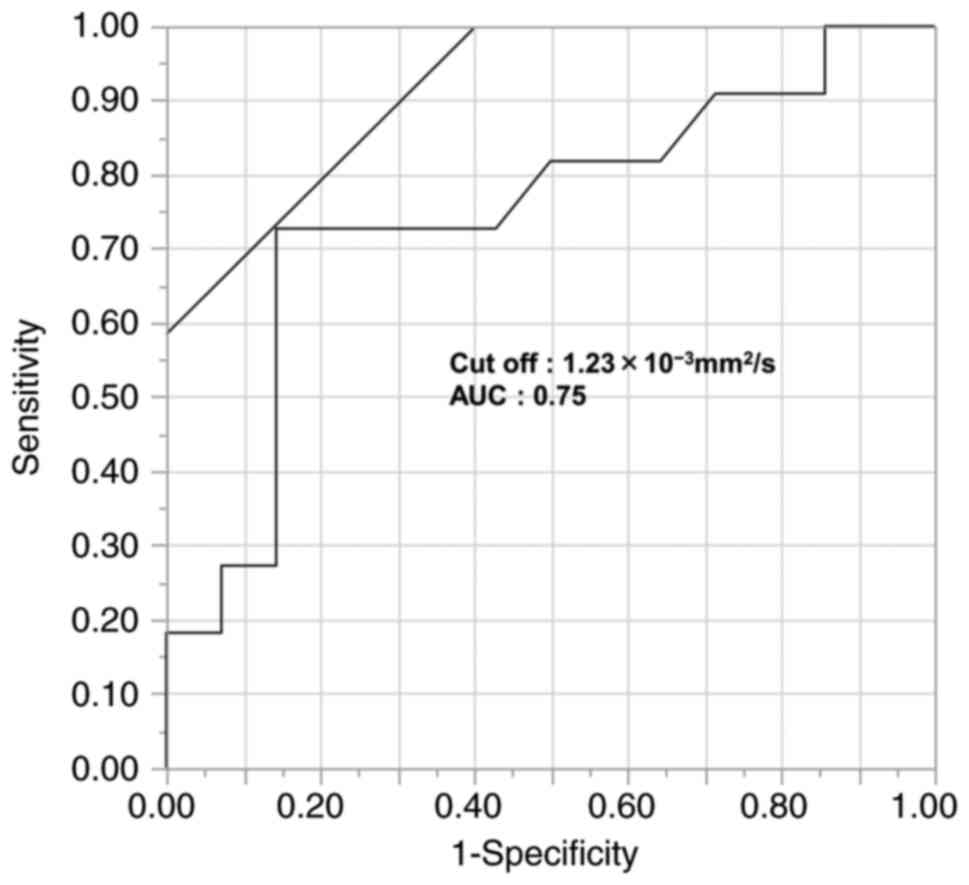
(n=10). Using receiver operating characteristic analysis, of which
the endpoint is cancer death, the cut-off value of
1.2×10−3 mm2/sec was defined (area under the
curve: 0.75, sensitivity: 72.7%, specificity: 85.7%, Fig. 2). The present study was approved by
the Institutional Review Board of the Tokushima University Hospital
(approval no. 3977-2; Tokushima, Japan). The requirement for
written consent was waived for the present study by the ethical
approval committee.
Statistical analysis
The Fisher's exact test was used to compare the
patients' backgrounds of the two groups. For multiple comparisons,
the Kruskal-Wallis test and Steel-Dwass post hoc test were used.
Disease-free survival (DFS) and OS curves were generated using the
Kaplan-Meier method and differences were evaluated by the log-rank
test. Cox proportional hazards model was used for multivariate
survival analysis. P<0.05 was considered to indicate a
statistically significant difference, and all statistical analyses
were performed by JMP v.8.0.1 (SAS Institute Inc.).
Results
ADC value of gallbladder tumors
In the present study, there were 33 benign tumor
cases, 5 non-advanced gallbladder cancers, and 25 advanced
gallbladder cases. Patients' characteristics in benign tumors and
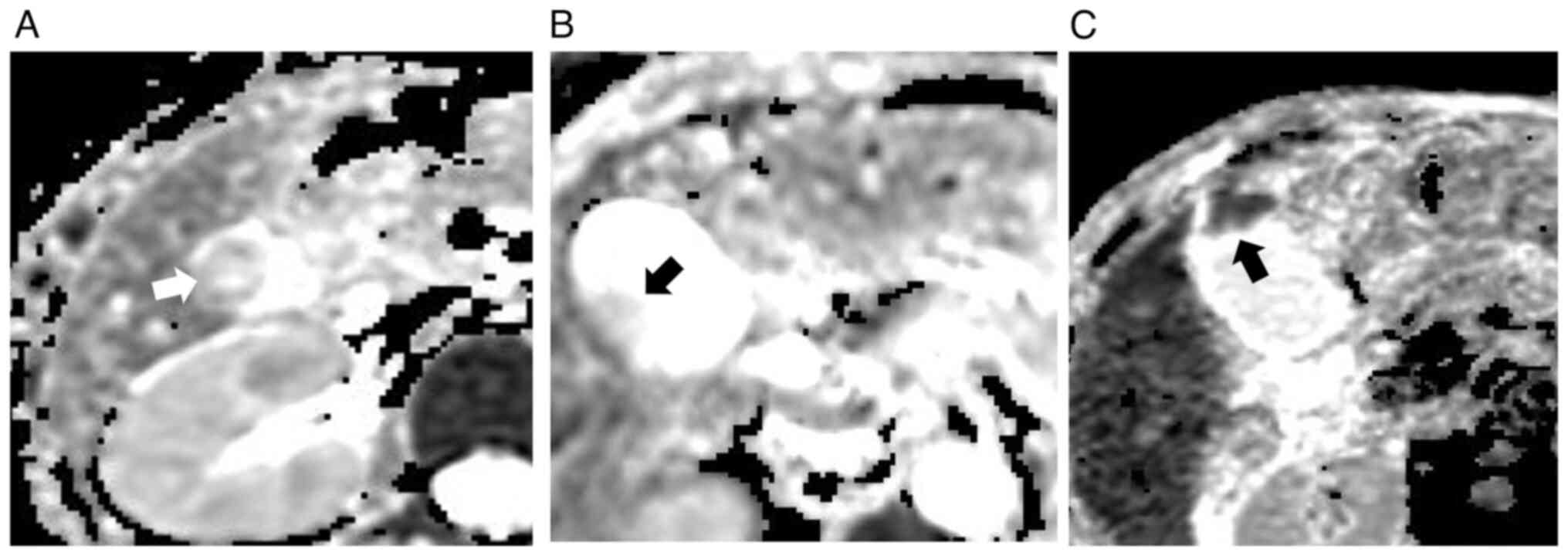
gallbladder cancers are presented in Tables I and II. Images of representative cases of
tumor are revealed in Fig. 3. ADC
values of benign tumors and gallbladder cancer are included in
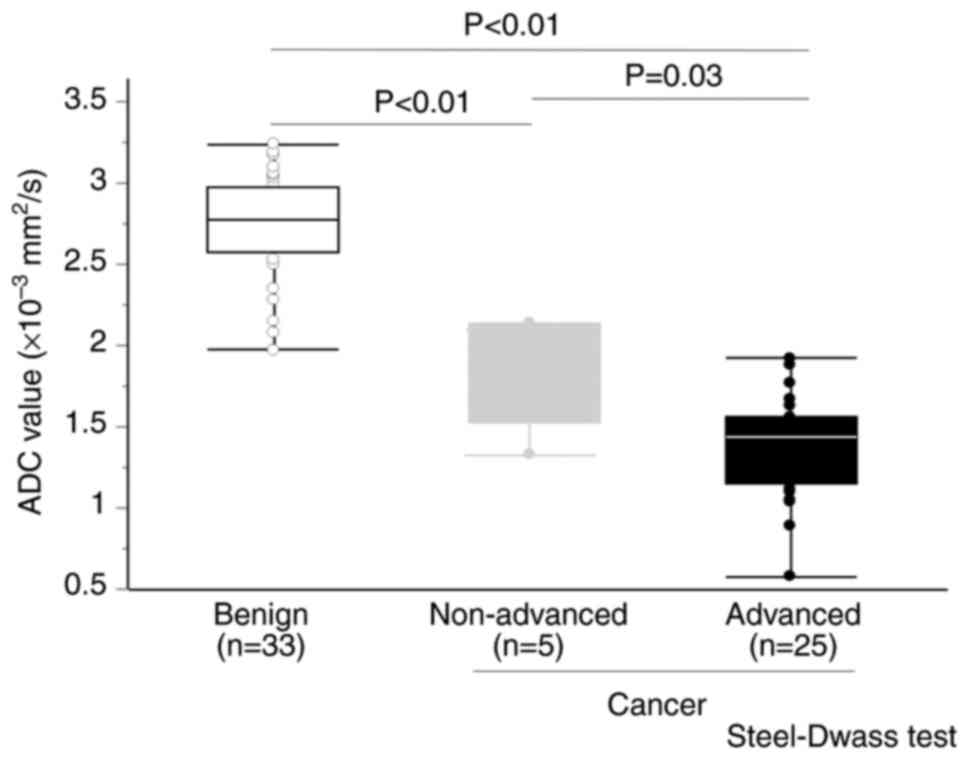
Tables I and II. The mean ADC value was
2.72×10−3 mm2/sec in benign tumors,
1.88×10−3 mm2/sec in non-advanced gallbladder
cancer and 1.36×10−3 mm2/sec in advanced
gallbladder cancer. ADC values in the three groups are demonstrated
in Fig. 4. ADC values in advanced
gallbladder cancer were significantly lower compared with benign
tumors and non-advanced gallbladder cancer (P<0.05).
Furthermore, ADC values in early gallbladder cancer were also
significantly lower compared with benign tumors (P<0.05).
 | Table I.Patients' characteristics in benign
gallbladder tumors. |
Table I.
Patients' characteristics in benign
gallbladder tumors.
| Variable | n=33 |
|---|
| Age, years | 52±13 |
| Sex
(male/female) | 10/23 |
| Diagnosis
(adenoma/hyperplastic polyp/cholesterol polyp) | 8/9/16 |
| Tumor size
(mm) | 10.5±3.9 |
| Tumor number
(single/multiple) | 14/19 |
| ADC value
(×10−3 mm2/sec) | 2.72±0.35 |
 | Table II.Patients' characteristics in
gallbladder carcinoma. |
Table II.
Patients' characteristics in
gallbladder carcinoma.
| Case | Age/Sex | Tumor
progression | Lymph node
metastasis |
Differentiation | Adjuvant
chemotherapy | Vessel
invasion | Recurrence | ADC value
(×10−3mm2/sec) |
|---|
| 1 | 59/F | Advanced | - | Por | + | + | + | 1.22 |
| 2 | 60/F | Advanced | + | Tub | + | - | + | 1.17 |
| 3 | 67/F | Advanced | + | Tub | + | + | - | 1.44 |
| 4 | 72/F | Advanced | - | Tub | + | + | + | 1.04 |
| 5 | 58/F | Advanced | - | Tub | - | + | + | 0.89 |
| 6 | 61/F | Advanced | + | Tub | + | + | + | 1.3 |
| 7 | 79/F | Advanced | + | Tub | - | + | + | 1.56 |
| 8 | 76/F | Advanced | + | Tub | + | + | + | 1.05 |
| 9 | 74/F | Advanced | - | Tub | - | - | - | 1.63 |
| 10 | 68/M | Advanced | + | Tub | + | + | + | 1.17 |
| 11 | 69/M | Advanced | + | Tub | + | + | + | 1.77 |
| 12 | 59/M | Advanced | - | Tub | + | + | - | 1.1 |
| 13 | 67/F | Advanced | - | Pap | - | - | - | 1.92 |
| 14 | 90/M | Advanced | - | Pap | - | + | - | 1.51 |
| 15 | 88/F | Advanced | + | Pap | - | + | - | 1.56 |
| 16 | 88/F | Advanced | + | Tub | - | + | + | 1.46 |
| 17 | 70/F | Advanced | + | Por | - | + | + | 1.12 |
| 18 | 72/M | Advanced | + | Por | + | + | + | 0.58 |
| 19 | 58/M | Advanced | - | Tub | - | + | - | 1.38 |
| 20 | 91/M | Advanced | - | Pap | - | + | + | 1.88 |
| 21 | 70/M | Advanced | - | Tub | - | + | - | 1.45 |
| 22 | 76/F | Advanced | - | Pap | - | + | - | 1.67 |
| 23 | 67/M | Advanced | - | Por | + | + | + | 1.23 |
| 24 | 63/F | Advanced | - | Tub | - | - | - | 1.49 |
| 25 | 62/F | Advanced | - | Tub | - | + | - | 1.46 |
| 26 | 68/F | Non-advanced | - | Pap | - | - | - | 1.72 |
| 27 | 84/F | Non-advanced | - | Tub | - | - | - | 1.33 |
| 28 | 71/F | Non-advanced | - | Pap | - | - | - | 2.08 |
| 29 | 68/F | Non-advanced | - | Tub | - | - | - | 2.14 |
| 30 | 70/F | Non-advanced | - | Pap | - | - | - | 2.13 |
Evaluation of malignancy with ADC in
advanced gallbladder cancer
The backgrounds of patients with advanced
gallbladder cancer are revealed in Table III. The ADCLow group
tended to have a higher rate of advanced stage disease
(P=0.21), and a significantly higher rate of adjuvant
chemotherapy compared with the ADCHigh group
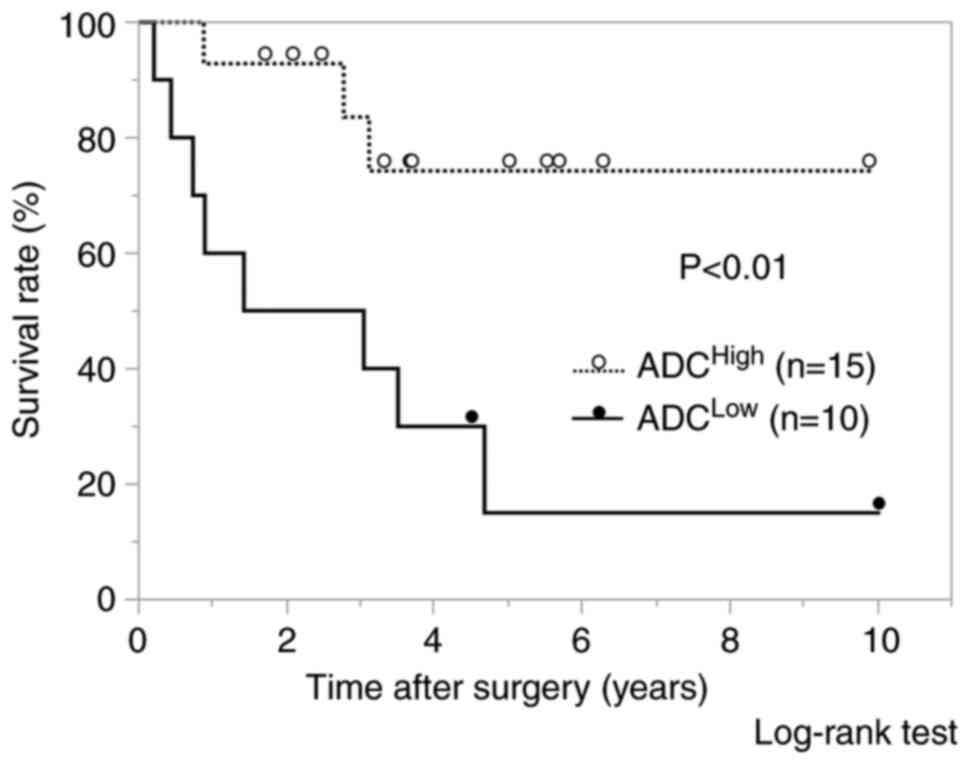
(P<0.05). Regarding OS, significantly worse prognosis was shown
in the ADCLow group compared with the ADCHigh
group (P<0.01) (Fig. 5). The
univariate and multivariate analyses of OS are shown in Table IV. Poor differentiation and low ADC
value were identified as independent prognostic factors. Regarding
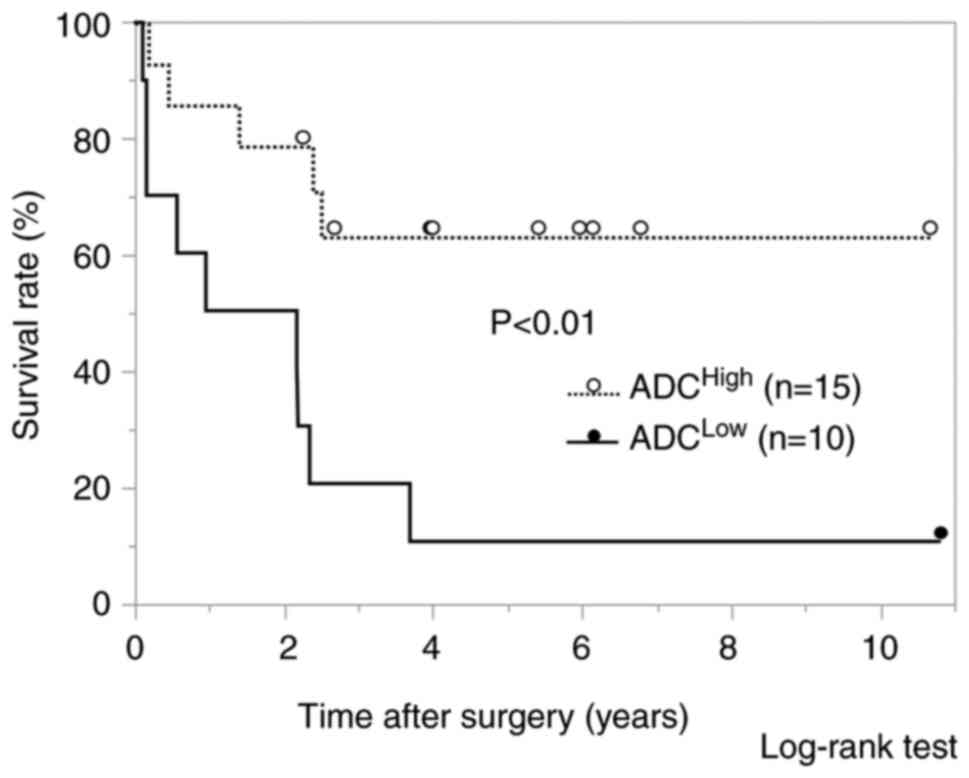
DFS, the ADCLow group also showed a significantly worse
prognosis than the ADCHigh group (P<0.01) (Fig. 6). The univariate and multivariate
analyses of DFS are demonstrated in Table V. Low ADC value was identified as an
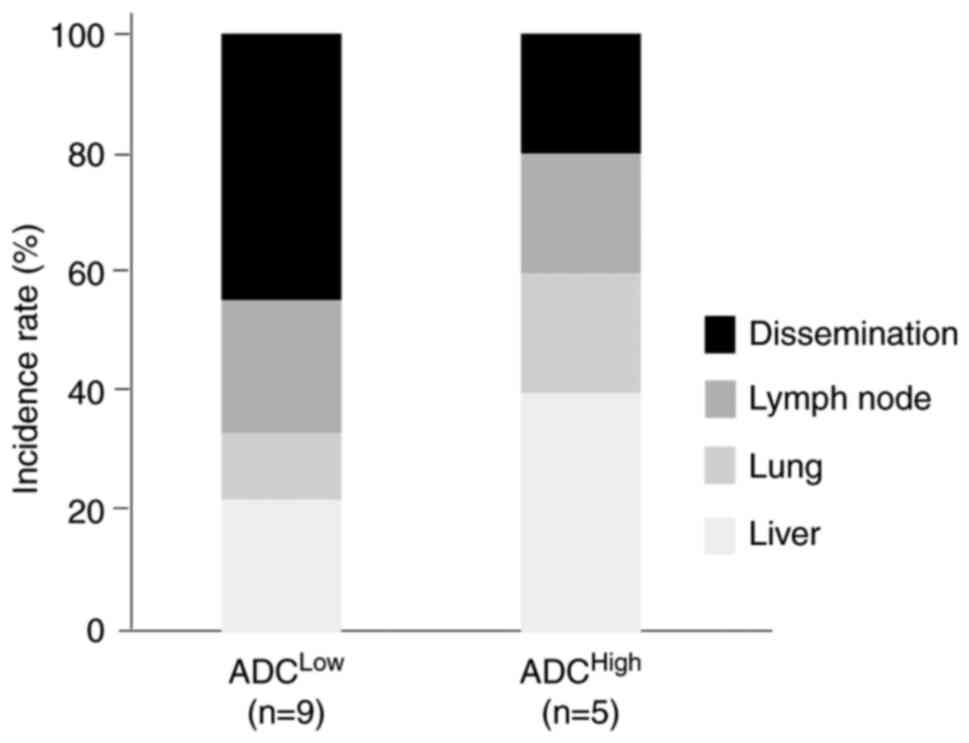
independent prognostic factor. Furthermore, the ADCLow
group revealed a higher rate of extra-hepatic recurrence compared
with ADCHigh group (Fig.
7).
 | Table III.Patients' characteristics in the ADC
low and high groups. |
Table III.
Patients' characteristics in the ADC
low and high groups.
|
| Expression level of
ADC |
|
|---|
|
|
|
|
|---|
| Variable | Low (n=10) | High (n=15) | P-value |
|---|
| Age (<70 /
>70 years) | 5/5 | 8/7 | >0.99 |
| Sex
(male/female) | 7/3 | 9/6 | 0.69 |
| CEA (<5 / >5
ng/ml) | 7/3 | 12/3 | 0.65 |
| CA19-9 (<37 /
>37 U/ml) | 3/7 | 7/8 | 0.68 |
| LN metastasis
(−/+) | 4/6 | 10/5 | 0.24 |
| Stage
(I/II/III) | 0/2/8 | 0/8/7 | 0.21 |
| Differentiation
(tub/others) | 7/3 | 14/1 | 0.27 |
| Vessel invasion
(−/+) | 1/9 | 3/12 | 0.63 |
| Adjuvant
chemotherapy (−/+) | 3/7 | 11/4 | 0.049 |
 | Table IV.Multivariate analysis for overall
survival. |
Table IV.
Multivariate analysis for overall
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value |
|---|
| Age [≥70 (Ref:
<70 years)] | 0.70 | 0.66
(0.05–7.98) | 0.75 |
| Sex [male (Ref:
female)] | 0.93 | 0.71
(0.09–5.44) | 0.74 |
| CEA [≥5 (Ref: <5
ng/ml)] | 0.10 | 5.16
(0.46–57.5) | 0.18 |
| CA19-9 [≥37 (Ref:
<37 U/ml)] | 0.53 | 1.30
(0.13–13.5) | 0.82 |
| LN metastasis [+
(Ref: -)] | 0.04 | 3.91
(0.34–44.3) | 0.27 |
| Differentiation
[others (Ref: tub)] | <0.01 | 19.3
(1.40–266.9) | 0.03 |
| Vessel invasion [+
(Ref: -)] | 0.28 | 4.73
(0.29–78.0) | 0.28 |
| Adjuvant
chemotherapy [+ (Ref: -)] | 0.32 | 0.09
(0.004–1.60) | 0.10 |
| ADC [low (Ref:
high)] | <0.01 | 9.51
(1.38–65.4) | 0.02 |
 | Table V.Multivariate analysis for
disease-free survival. |
Table V.
Multivariate analysis for
disease-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | P-value | HR (95% CI) | P-value |
|---|
| Age [≥70 (Ref:
<70 years)] | 0.70 | 0.76
(0.17–3.65) | 0.78 |
| Sex [male (Ref:
female)] | 0.74 | 0.95
(0.18–4.99) | 0.95 |
| CEA [≥5 (Ref: <5
ng/ml)] | 0.18 | 2.84
(0.39–20.7) | 0.30 |
| CA19-9 [≥37 (Ref:
<37 U/ml)] | 0.47 | 1.91
(0.23–15.9) | 0.55 |
| LN metastasis [+
(Ref: -)] | 0.04 | 2.41
(0.51–11.4) | 0.27 |
| Differentiation
[others (Ref: tub)] | <0.01 | 3.62
(0.63–20.9) | 0.15 |
| Vessel invasion [+
(Ref: -)] | 0.18 | 2.99
(0.32–28.2) | 0.34 |
| Adjuvant
chemotherapy [+ (Ref: -)] | 0.11 | 0.25
(0.03–2.00) | 0.19 |
| ADC [low (Ref:
high)] | <0.01 | 9.06
(1.44–57.1) | 0.02 |
Discussion
In the present study, the usefulness of ADC in the
estimation of malignancy and in prognostic prediction was
demonstrated. First, ADC values in malignant and benign gallbladder
tumors were investigated. ADC values in advanced gallbladder cancer
were significantly lower than those in benign tumors and
non-advanced gallbladder cancer, and ADC values in non-advanced
gallbladder cancer were significantly lower than those in benign
tumors. Differentiation of early malignant and benign gallbladder
tumors using conventional CT and MRI has been reported to be
difficult and challenging (16–18).
It is hard to diagnose gallbladder carcinoma when it reveals wall
thickness because it is more common than the wall thickness of
inflamed gallbladder. Furthermore, although polypoid tumors >1
cm in diameter have the potential to be cancer, benign gallbladder
tumors >1 cm are frequent (19,20).
The present study confirmed that non-advanced gallbladder cancer
could be distinguished from benign tumors using ADC values.
Therefore, when polypoid lesions show relatively low ADC values and
are suspicious for early gallbladder cancer, laparoscopic radical
cholecystectomy may be considered (21).
A small number of studies (7,22–29)
have used ADC values to differentiate gallbladder carcinoma from
benign tumors (Table VI). These
studies stated that ADC values are useful for distinguishing
gallbladder carcinoma and benign tumors. Lee et al (27) demonstrated sensitivity, specificity,
positive predictive value and negative predictive value of 97.2,
92.2, 83.3 and 98.8%, respectively. However, these cut-off points
vary between studies, which limits their clinical validity.
Kitazume et al (7) revealed
that the lesion to spinal cord ratio was more accurate than ADC.
Sulieman et al (29) showed
that the P-value between gallbladder carcinoma and benign tumor was
0.07, although the P-value of b800/b0 ratio was <0.01. This
correction of ADC value with other lesions using the b-value ratio
may reduce this validity.
 | Table VI.Summary of studies using the ADC
values in diagnosing gallbladder tumors. |
Table VI.
Summary of studies using the ADC
values in diagnosing gallbladder tumors.
| First author,
year | ADC in malignant
tumor | ADC in benign
tumor | P-value, cut-off
value | (Refs.) |
|---|
| Sugita et
al, 2009 | 1.28±0.41 | 1.92±0.21 | <0.01 | (22) |
| Irie et al,
2011 | 1.34±0.50 | 2.26±0.44 | 0.00016 | (23) |
| Ogawa et al,
2012 | 1.83±0.69 | 2.60±0.54 | 0.001 | (24) |
| Solak et al,
2012 | 0.98±0.13 | 1.96±0.26 | <0.01, 0.86 | (25) |
| Kim et al,
2013 | 1.46±0.45 | 2.16±0.56 | <0.0001 | (26) |
| Lee et al,
2014 | 1.04±0.38 | 2.20±0.72 | <0.001 | (27) |
| Kyung et al,
2016 | 1.041 | 2.039 | <0.001 | (28) |
| Kitazume et
al, 2016 | 1.06±0.37 | 1.85±0.32 | <0.001 | (7) |
| Sulieman et
al, 2021 | 1.27 | 1.62 | 0.07 | (29) |
Next, malignancy in advanced gallbladder cancer
using ADC values was estimated. A previous study reported that
tumor differentiation was inversely related to the ADC value
(13). It was previously reported
that low ADC value is associated with aggressive tumor types and
high HIF-1α expression, which accelerates tumor malignancy in
intrahepatic cholangiocarcinoma (12). Furthermore, Min et al
(15) showed that the low ADC value
in gallbladder cancer was associated with poor differentiation, T
stage, lymph node metastasis and progression stage. In the present
study, the ADCLow group tended to have a higher rate of
advanced stage, which represented tumor aggressiveness. Min et
al (15) also confirmed that
low ADC values could estimate long-term DFS. The current study
revealed a significant difference in OS, not only in DFS, and the
ADCLow group showed more extrahepatic distant recurrence
compared with the ADCHigh group, indicating an
aggressive recurrence pattern.
The present study has some limitations. First,
selection bias could exist because only patients with gallbladder
tumors who underwent MRI were retrospectively analyzed. Second,
patients in the present study underwent treatment at a single
center, and the number of patients was small. Statistical size
calculations were not conducted, and sample size of the present
study gave post hoc powers of 22%. Therefore, further analysis in a
larger, prospectively collected population is necessary to confirm
these results.
In conclusion, ADC values from DWI-MRI may estimate
the malignancy of gallbladder tumors and predict the prognosis of
patients with advanced gallbladder cancer.
Acknowledgements
The authors would like to thank Dr H. Nikki March
for editing a draft of this manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM, MS and SY designed the study. CN, SY, HT, YW,
YS, TI and TN contributed to collection of the data. SY and CN
wrote the main manuscript text, confirmed the authenticity of all
the raw data and prepared all figures. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tokushima University Hospital (approval no. 3977-2;
Tokushima, Japan). An information disclosure statement was
presented in the homepage of the institute website for opt-out, and
the requirement for informed consent was waived.
Patient consent for publication
An information disclosure statement was shown in the
homepage of Tokushima University Hospital website for opt-out; The
manuscript and images are published and freely available.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hickman L and Contreras C: Gallbladder
cancer: Diagnosis, surgical management, and adjuvant therapies.
Surg Clin North Am. 99:337–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cubertafond P, Gainant A and Cucchiaro G:
Surgical treatment of 724 carcinomas of the gallbladder. Results of
the French Surgical Association Survey. Ann Surg. 219:275–280.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim H, Seo DW, Park DH, Lee SS, Lee SK,
Kim MH and Hwang S: Prognostic factors in patients with gallbladder
cancer after surgical resection: Analysis of 279 operated patients.
J Clin Gastroenterol. 47:443–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alizadeh AA, Aranda V, Bardelli A,
Blanpain C, Bock C, Borowski C, Caldas C, Califano A, Doherty M,
Elsner M, et al: Toward understanding and exploiting tumor
heterogeneity. Nat Med. 21:846–853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scara S, Bottoni P and Scatena R: CA 19-9:
Biochemical and clinical aspects. Adv Exp Med Biol. 867:247–260.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ratanaprasatporn L, Uyeda JW, Wortman JR,
Richardson I and Sodickson AD: Multimodality imaging, including
dual-energy CT, in the evaluation of gallbladder disease.
Radiographics. 38:75–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitazume Y, Taura S, Nakaminato S, Noguchi
O, Masaki Y, Kasahara I, Kishino M and Tateishi U:
Diffusion-weighted magnetic resonance imaging to differentiate
malignant from benign gallbladder disorders. Eur J Radiol.
85:864–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parikh T, Drew SJ, Lee VS, Wong S, Hecht
EM, Babb JS and Taouli B: Focal liver lesion detection and
characterization with diffusion-weighted MR imaging: Comparison
with standard breath-hold T2-weighted imaging. Radiology.
246:812–822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang T, Xu JH, Zou Y, Chen R, Peng LR,
Zhou ZD and Yang M: Diffusion-weighted imaging (DWI) of
hepatocellular carcinomas: A retrospective analysis of the
correlation between qualitative and quantitative DWI and tumour
grade. Clin Radiol. 72:465–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurosawa J, Tawada K, Mikata R, Ishihara
T, Tsuyuguchi T, Saito M, Shimofusa R, Yoshitomi H, Ohtsuka M,
Miyazaki M and Yokosuka O: Prognostic relevance of apparent
diffusion coefficient obtained by Diffusion-Weighted MRI in
pancreatic cancer. J Magn Reson Imaging. 42:1532–1537. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parsian S, Giannakopoulos NV, Rahbar H,
Rendi MH, Chai X and Partridge SC: Diffusion-weighted imaging
reflects variable cellularity and stromal density present in breast
fibroadenomas. Clin Imaging. 40:1047–1054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada S..Morine Y, Imura S, Ikemoto T,
Arakawa Y, Saito Y, Yoshikawa M, Miyazaki K and Shimada M:
Prognostic prediction of apparent diffusion coefficient obtained by
diffusion-weighted MRI in mass-forming intrahepatic
cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 27:388–395. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee NK, Kim S, Moon JI, Shin N, Kim DU,
Seo HI, Kim HS, Han GJ, Kim JY and Lee JW: Diffusion-weighted
magnetic resonance imaging of gallbladder adenocarcinoma: Analysis
with emphasis on histologic grade. Clin Imaging. 40:345–351. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Japanese Society of Biliary Surgery, .
Classification of biliary tract carcinoma, second English edition.
Tokyo: Kanehara & Co., Ltd.; 2004
|
|
15
|
Min JH, Kang TW, Cha DI, Kim SH, Shin KS,
Lee JE, Jang KT and Ahn SH: Apparent diffusion coefficient as a
potential marker for tumour differentiation, staging and long-term
clinical outcomes in gallbladder cancer. Eur Radiol. 29:411–421.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun EJ, Cho SG, Park SW, Kim WH, Kim HJ
and Suh CH: Gallbladder carcinoma and chronic cholecystitis:
Differentiation with two-phase spiral CT. Abdom Imaging.
29:102–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimitsu K, Honda H, Kaneko K, Kuroiwa
T, Irie H, Ueki T, Chijiwa K, Takenaka K and Masuda K: Dynamic MRI
of the gallbladder lesions: Differentiation of benign from
malignant. J Magn Reson Imaging. 7:696–701. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Demachi H, Matsui O, Hoshiba K, Kimura M,
Miyata S, Kuroda Y, Konishi K, Tsuji M and Miwa A: Dynamic MRI
using a surface coil in chronic cholecystitis and gallbladder
carcinoma: Radiologic and histopathologic correlation. J Comput
Assist Tomogr. 21:643–651. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder carcinoma: Radiologicepathologic correlation.
RadioGraphics. 21:295–314, questionnaire, 549–555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levy AD, Murakata LA, Abbott RM and
Rohrmann CA Jr: From the archives of the AFIP. Benign tumors and
tumorlike lesions of the gallbladder and extrahepatic bile ducts:
Radiologic-pathologic correlation. Armed Forces Institute of
Pathology. Radiographics. 22:387–413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piccolo G and Piozzi GN: Laparoscopic
radical cholecystectomy for primary or incidental early gallbladder
cancer: The new rules governing the treatment of gallbladder
cancer. Gastroenterol Res Pract. 2017:85705022017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugita R, Yamazaki T, Furuta A, Itoh K,
Fujita N and Takahashi S: High b-value diffusion-weighted MRI for
detecting gallbladder carcinoma: Preliminary study and results. Eur
Radiol. 19:17942009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Irie H, Kamochi N, Nojiri J, Egashira Y,
Sasaguri K and Kudo S: High b-value diffusion-weighted MRI in
differentiation between benign and malignant polypoid gallbladder
lesions. Acta Radiol. 52:236–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogawa T, Horaguchi J, Fujita N, Noda Y,
Kobayashi G, Ito K, Koshita S, Kanno Y, Masu K and Sugita R: High
b-value diffusion-weighted magnetic resonance imaging for
gallbladder lesions: Differentiation between benignity and
malignancy. J Gastroenterol. 47:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solak A, Solak I, Genc¸ B and Sahin N: The
role of diffusion-weighted examination in non-polyploid gallbladder
malignancies: A preliminary study. Turk J Gastroenterol.
24:148–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Lee JM, Kim H, Yoon JH, Han JK and
Choi BI: Role of diffusion-weighted magnetic resonance imaging in
the diagnosis of gallbladder cancer. J Magn Reson Imaging.
38:127–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee NK, Kim S, Kim TU, Kim DU, Seo HI and
Jeon TY: Diffusion-weighted MRI for differentiation of benign from
malignant lesions in the gallbladder. Clin Radiol. 69:e78–e85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kyung N, Kim S, Moon JI, Shin N, Kim DU,
Seo HI, Kim HS, Han GJ, Kim JY and Lee JW: Diffusion-weighted
magnetic resonance imaging of gallbladder adenocarcinoma: Analysis
with emphasis on histologic grade. Clin Imaging. 40:345–351. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sulieman I, Mohamed S, Elmoghazy W,
Alaboudy A, Khalaf H and Elaffandi A: The value of
diffusion-weighted imaging in diagnosing gallbladder malignancy:
Performance of a new parameter. Clin Radiol. 76:709.e7–709.e12.
2021. View Article : Google Scholar : PubMed/NCBI
|