Introduction
Nivolumab, a human immunoglobulin G4 monoclonal
antibody against the immune checkpoint molecule programmed death-1
receptor, has demonstrated efficacy and safety in the treatment of
a variety of cancer types (1–4). In
2017, the ATTRACTION-2 trial showed that nivolumab treatment
significantly improved overall survival (OS) as a third-line or
later therapy for unresectable or recurrent gastric cancer (GC)
(5). In 2021, the combination of
nivolumab and chemotherapy as a first-line therapy for
HER2-negative unresectable or recurrent GC showed significantly
improved progression-free survival (PFS) and OS in the CheckMate649
trial (6) and significantly
improved PFS in the ATTRACTION-4 trial (7). Hence, nivolumab plus chemotherapy is
one of the recommended first-line therapies; moreover, nivolumab
monotherapy remains the recommended third-line or later therapy for
HER2-negative unresectable or recurrent GC (8–10).
Evaluation of tumor activity during systemic
chemotherapy is mainly conducted with tumor markers and computed
tomography (CT) examination. Increased tumor markers correlate with
increased tumor burden; however, depending on the histologic type,
tumor markers may be negative or not elevated until the late phase
of the disease course (11). CT
imaging evaluation is performed according to the Response
Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and
is based on the objective assessment of changes in tumor size in
solid tumors (12). Conversely, in
unresectable or recurrent GC, nonmeasurable lesion, such as
peritoneal dissemination, is often observed. This made it important
to comprehensively evaluate the clinical findings, hematologic
data, and other imaging examinations, such as
esophagogastroduodenoscopy, to evaluate the response of the primary
lesion (13). Newer indicators that
could be evaluated easily, less invasively, and reproductively
could help in the evaluation of tumor progression and activity.
The neutrophil-to-lymphocyte ratio (NLR), a marker
of systemic inflammation, reportedly reflects the systemic
inflammatory response associated with cancer progression, invasion
into surrounding tissues, and metastasis to distant organs
(14). Higher pre-treatment NLR or
increased NLR during treatment has been reported to worsen OS and
PFS in patients with unresectable or recurrent GC on nivolumab
monotherapy (14–17). Ogata et al (17). reported that advanced gastric cancer
patients with NLR <5 had significantly longer median OS and PFS
than did those with NLR >5 2 weeks after the first dose of
nivolumab. Furthermore, Ota et al (14). reported that an increase of ΔNLR60,
the NLR at 60 days after the first dose of nivolumab minus the NLR
value prior to treatment initiation, of ≥2 was associated with
significantly decreased OS. In previous reports, prognosis was
predicted by the NLR value at a certain point in time. In other
words, it is difficult to predict future clinical courses based on
the NLR value at the time of examination and the amount of change.
Therefore, whether the NLR can be a predictor of the timing of
disease progression is a clinical question, and there are no
reports on this subject. Moreover, there are few data on the
correlation between NLR and immune checkpoint inhibitor (ICI). This
study aimed to examine the relationship between dynamic changes in
NLR during treatment and disease progression in patients with
unresectable or recurrent GC treated with nivolumab
monotherapy.
Materials and methods
This retrospective observational study was approved
by the Institutional Review Board of Gifu University Hospital
(approval numbers:2021-B185) and was conducted in compliance with
the Declaration of Helsinki and Japanese Good Clinical Practice
guidelines. Informed consent was obtained in the form of opt-out on
the web-site. Those who did not provide consent were excluded.
The medical records of patients treated at Gifu
University Hospital were obtained and retrospectively analyzed.
Patients with unresectable or recurrent GC who received nivolumab
monotherapy in third-line or later between April 2017 and December
2021 were identified from the database. The patients received
standard doses of nivolumab 3 mg/kg or 240 mg/body intravenously
over 30 min every 2 weeks until disease progression, unacceptable
toxicity, or the patient refused to continue treatment. There was
no concomitant use of antiemetic drugs or steroids as part of the
regimen.
To evaluate the association between NLR changes and
disease condition during treatment with nivolumab, tumor response
was evaluated using RECIST v1.1 for CT examination. In patients
whose disease progression was not diagnosed with CT examination but
with clinical symptoms and blood examinations, including tumor
markers and non-CT examinations such as endoscopy, their events of
disease progression were defined as clinical progressive disease
(PD). These clinical and objective PDs diagnosed through CT
examinations were set as events of disease progression, and their
correlations with NLR, which was calculated based on data from
routine blood examinations, were analyzed. Hematological data were
analyzed every 2 weeks. Tumor markers, such as carcinoembryonic
antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were
evaluated once a month. CT examination was performed at 6–8 weeks.
NLR was defined as the number of neutrophils divided by the number
of lymphocytes. NLR was analyzed at the initiation of nivolumab
monotherapy and every 2 weeks during treatment.
Patient characteristics such as age, sex, Eastern
Cooperative Oncology Group performance status, histology, HER2
status, history of gastric resection, site of metastasis, treatment
regimen, and blood cell count were collected. Tumor histology was
classified according to Lauren's classification into intestinal
(well-differentiated, moderately differentiated, papillary
adenocarcinoma) and diffuse (poorly differentiated, mucinous
adenocarcinoma, signet-ring cell carcinoma) types. In the safety
analysis of nivolumab therapy, adverse events were evaluated based
on the National Cancer Institute Common Terminology Criteria for
Adverse Events version 4.03.
The primary outcome is PFS, while the secondary
outcome is OS and the best overall response and immunorelated
adverse events (IrAEs). PFS was defined as the time from the
initiation of nivolumab monotherapy to the disease progression date
with objective PD or clinical PD, the date of death from any cause,
or the last date of contact. OS was defined as the time from the
initiation of nivolumab monotherapy to death from any cause or the
last date of contact.
Statistical analysis. Patient characteristics were
summarized using medians and interquartile ranges for continuous
variables and counts and proportions for categorical variables. The
PFS and OS rates after the initiation of nivolumab monotherapy were
estimated using the Kaplan-Meier method. The median survival month
was calculated, and 95% confidence interval was estimated using the
Brookmeyer and Crowley method.
In analyzing the association between NLR and disease
progression, blood examinations within 2 weeks from disease
progression were linked with progressive disease. NLR was used as a
time-dependent variable in the analysis because it was collected as
repeated data from the start of nivolumab treatment to the last
observation. The association between PFS and NLR was evaluated
using a time-dependent Cox proportional hazards model. Nonlinearity
was considered using restricted cubic spline (RCS) curves to
accurately estimate the association between NLR and disease
progression. The RCS curve can express nonlinear relationships more
strongly as the number of knots is increased, though overfitting is
more likely to occur. The number of knots in RCS was set to 3 to
avoid overfitting. Moreover, the hazard ratios (HRs) predicted from
the time-dependent Cox model were plotted using the median NLR as a
reference. Separate from the model in the primary analysis
described above, an interaction term between the NLR at each
measurement time point and the baseline NLR was included in the
model to assess the modifying effect of NLR at the start of
nivolumab monotherapy. A multivariable time-dependent Cox
proportional hazards regression model was used to simultaneously
assess the independent association between NLR, CEA, and CA19-9
levels with disease progression. A two-sided P-value <0.05 was
considered statistically significant. All analyses were performed
using the R software version 4.2.2 (www.r-project.org).
Results
Table I summarizes
the clinical characteristics of the patients who received nivolumab
monotherapy for unresectable or recurrent GC. The most frequent
metastatic site was the peritoneum in 23 patients (52.3%), followed
by the lymph node in 14 patients (31.8%), liver in 9 patients
(20.1%), bone in 6 patients (13.6%), and others in 6 patients
(13.6%). Pretreatment regimens included S-1 plus oxaliplatin
(n=19), capecitabine plus oxaliplatin (n=8), S-1 plus cisplatin
(n=5), S-1 plus docetaxel (n=5), fluorouracil plus oxaliplatin
(n=3), capecitabine plus cisplatin (n=1), and S-1 (n=3) as the
first-line regimen. The second-line regimen included ramucirumab
plus paclitaxel (n=19), ramucirumab plus nab-paclitaxel (n=19),
ramucirumab plus trastuzumab (n=2), paclitaxel (n=2), ramucirumab
plus irinotecan (n=1), and ramucirumab (n=1). The third-line
regimen included irinotecan (n=2). Table II shows the patients' clinical
responses to nivolumab therapy. The response and disease control
rates were 6.8 and 27.3%, respectively. The median number of cycles
administered per patient was 3 (range, 1–29). Only one patient
maintained partial response (PR), and the others developed PD. None
of the patients continued nivolumab monotherapy after disease
progression. The median PFS of all included patients was 1.84
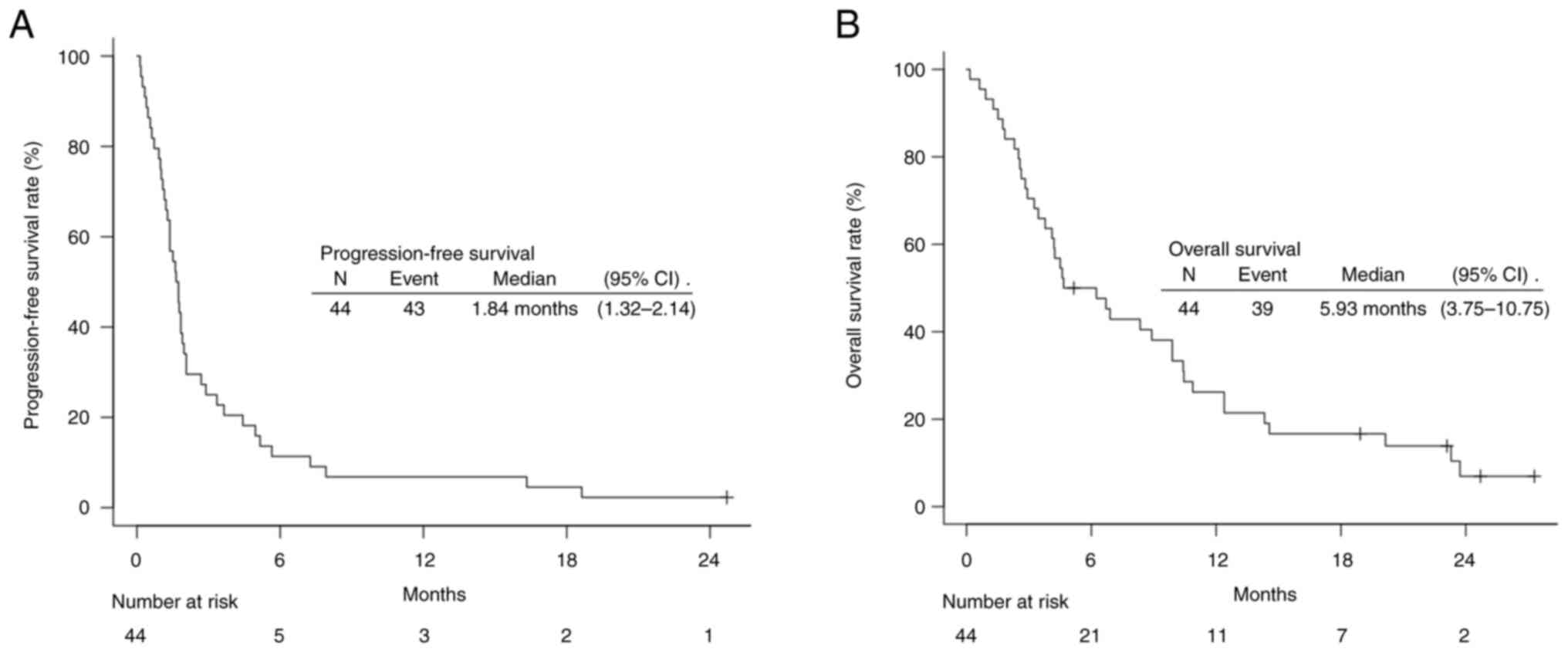
months [95% confidence interval (CI), 1.32–2.14], and the median OS
was 5.93 months (95% CI, 3.75–10.75) (Fig. 1). IrAEs of any degree were observed
in 36.4% of the patients (Table
III). Patients with grade ≤2 irAE were restarted on nivolumab
treatment after symptomatic improvement. Among patients with grade
3 irAEs, intestinal perforation occurred in one patient after four
cycles of treatment requiring surgery, and the other patient
presented with interstitial pneumonia after three cycles of
treatment requiring steroid therapy. Those two patients
subsequently survived after treatment for those adverse events. A
paired-sample t-test confirmed that there was no significant
difference in NLR values 2 or 4 weeks prior to the onset of irAEs
compared to NLR values at the onset of irAEs (P=0.200,
P=0.247).
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | N (%) |
|---|
| Sex |
|
| Male | 23 (52.3) |
|
Female | 21 (47.7) |
| Age, years | 70 (35–84) |
| ECOG performance
status |
|
| 0 | 21 (47.7) |
| 1 | 16 (36.4) |
| 2 | 1 (2.3) |
| 3 | 6 (13.6) |
| Histological type
(Lauren classification) |
|
|
Intestinal type | 13 (29.5) |
| Diffuse
type | 31 (70.5) |
| HER2 status |
|
|
Positive | 2 (4.5) |
|
Negative | 37 (84.1) |
|
Unknown | 5 (11.4) |
| Organs with
metastases |
|
|
<2 | 30 (68.2) |
| ≥2 | 14 (31.8) |
| Previous
gastrectomy |
|
| No | 13 (29.5) |
| Yes | 31 (70.5) |
| Previous treatment
regimens |
|
| 2 | 40 (90.9) |
| 3 | 4 (9.1) |
| Previous
therapies |
|
|
Pyrimidine analogues | 44 (100) |
|
Platinum | 36 (81.8) |
|
Taxane | 42 (95.5) |
|
Ramucirumab | 40 (90.9) |
|
Irinotecan | 3 (6.8) |
|
Trastuzumab | 2 (4.5) |
 | Table II.Clinical responses of patients to
nivolumab therapy. |
Table II.
Clinical responses of patients to
nivolumab therapy.
| Best overall
response | N (%) | Objective PD | Clinical PD |
|---|
| CR | 0 (0) |
|
|
| PR | 3 (6.8) | 2 |
|
| SD | 2 (4.5) | 2 |
|
| Non-CR/non-PD | 6 (13.6) | 6 |
|
| PD | 24 (54.5) | 24 |
|
| NE | 9 (20.5) |
| 9 |
 | Table III.Categorization of immune-related
adverse events. |
Table III.
Categorization of immune-related
adverse events.
|
| Grade, n (%) |
|---|
|
|
|
|---|
| Adverse event | 1/2 | 3≤ |
|---|
| Diarrhea | 5 (11.4) | 0 |
| Pruritus | 4 (9.1) | 0 |
| Fatigue | 4 (9.1) | 0 |
| Appetite loss | 3 (6.8) | 0 |
| Hypothyroidism | 3 (6.8) | 0 |
| Adrenal
insufficiency | 1 (2.3) | 0 |
| Colitis | 1 (2.3) | 0 |
| Liver
dysfunction | 1 (2.3) | 0 |
| Renal
dysfunction | 1 (2.3) | 0 |
| Colonic
perforation | 0 | 1 (2.3) |
| Interstitial
pneumonia | 0 | 1 (2.3) |
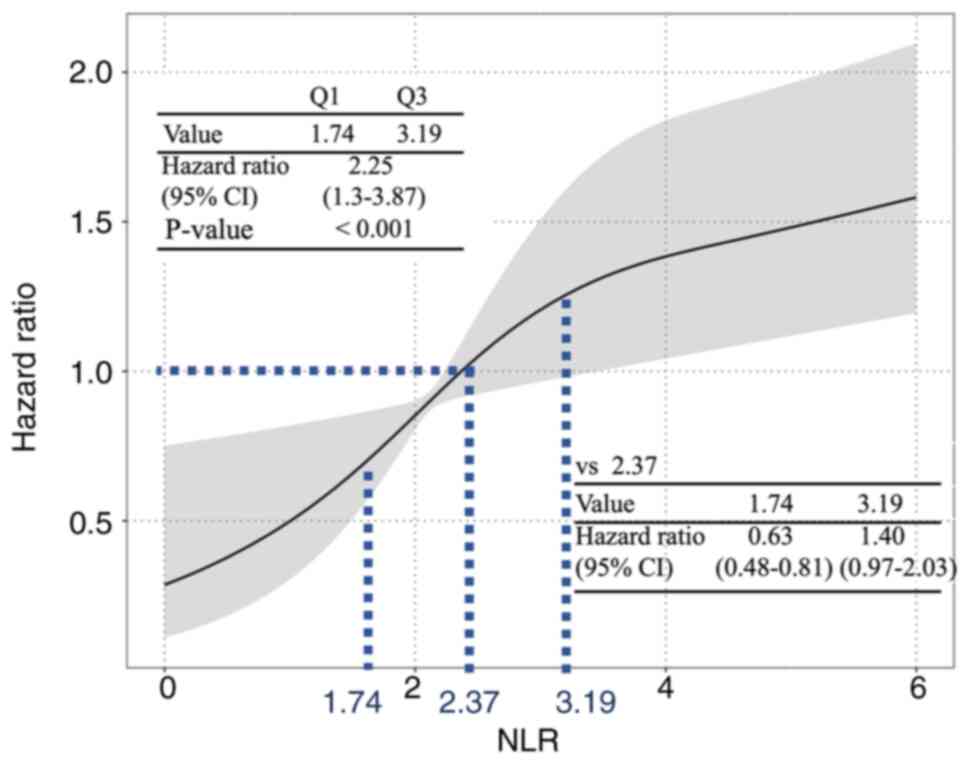
Fig. 2 shows the
results of the time-dependent Cox proportional hazards regression
analysis on the relationship between NLR and disease progression. A
median NLR of 2.37 was used as a reference to analyze the risk for
disease progression. The risk for disease progression was higher
when the NLR was above the reference value, and the risk was lower
when the NLR was below the reference value. The 25 and
75th percentile NLR values (1.74 and 3.19, respectively)
were used to calculate the representative HR for disease
progression, and the risk was increased with higher NLR (HR 2.25;
95% CI, 1.3–3.87; P<0.001).
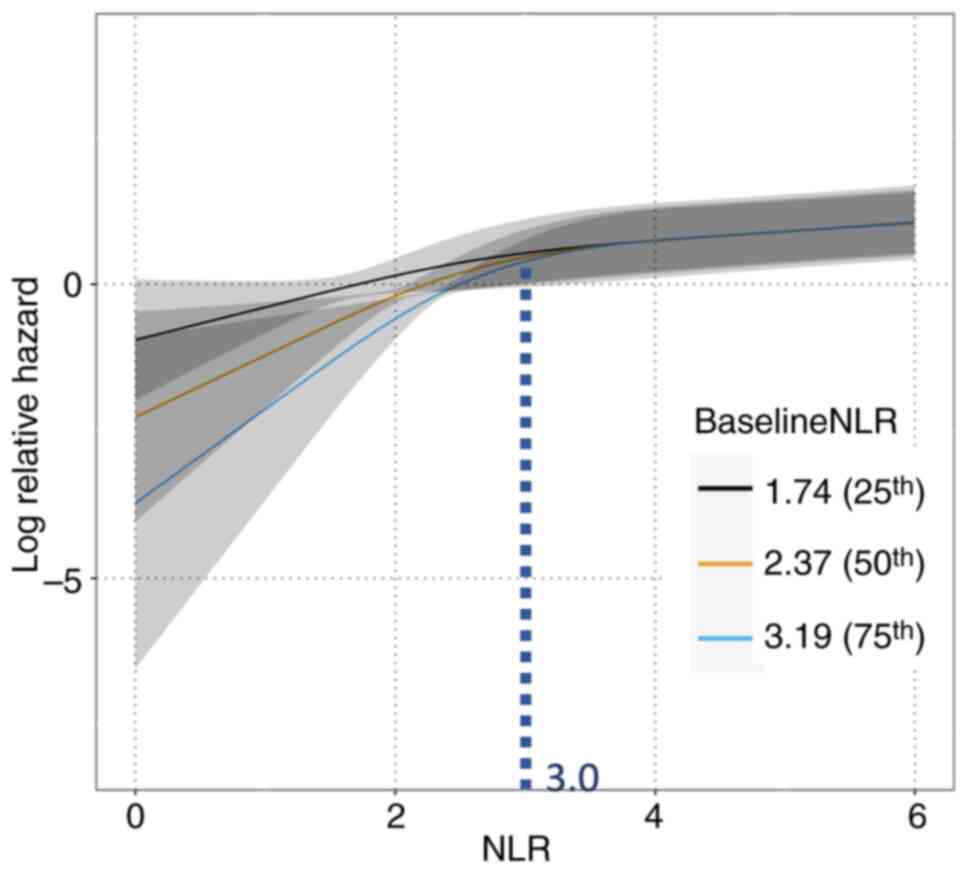
Fig. 3 shows the
three models of relationships between NLR and relative hazard for
disease progression, which were stratified based on the baseline
NLR values. Nonlinearities were considered using an RCS curve.
There was a significant difference in the interaction between the
NLR at each time point and the baseline NLR (P=0.009). Using the
NLR values (25% value: 1.74, 50% value: 2.37, and 75% value: 3.19)
as baseline NLR, the relative hazard of disease progression at each
NLR value during treatment was calculated. When the NLR value was
below 3.0, the risk of disease progression showed a more strongly
positive correlation to NLR in all three models. When the NLR value
increased compared to prior blood examination, the risk of disease
progression also increased. Furthermore, the baseline NLR values
stratified the relative risk each patient owed for their values of
NLR. The smaller the baseline NLR value, the larger the risk of
progressive disease, especially if the values and increments were
the same in all three models. Conversely, when the NLR value
exceeded 3.0, there were few differences in risk for subsequent
disease progression regardless of the NLR value and the baseline
NLR value.
Table IV shows the
analysis of correlations between NLR and tumor markers and disease
progression. The multivariable time-dependent Cox proportional
hazards regression analysis showed that NLR (HR 1.16; 95% CI,
1.04–1.29; P=0.006) and CEA (HR 1.25; 95% CI, 1.12–1.39;
P<0.001) correlated with disease progression.
 | Table IV.Multivariable time-dependent Cox
proportional hazards regression analysis. |
Table IV.
Multivariable time-dependent Cox
proportional hazards regression analysis.
| Variable | Q1 | Q3 | HR | 95% CI | P-value |
|---|
| NLR | 1.79 | 3.16 | 1.16 | 1.04, 1.29 | 0.006 |
| CEA | 2.1 | 7.25 | 1.25 | 1.12, 1.39 | <0.001 |
| CA19.9 | 9.05 | 203.85 | 0.99 | 0.98, 1 | 0.163 |
Discussion
In this study, the relationship between NLR and the
risk of disease progression was examined in patients with
unresectable or recurrent GC treated with nivolumab monotherapy as
a third-line or later regimen. As shown in Figures 2 and 3, NLR, which was calculated from daily
blood examinations, positively correlated with disease progression.
The higher the NLR, the higher the risk for disease progression,
especially if the NLR value is <3.0. If the NLR value is higher
compared to prior blood examination, the risk of disease
progression also increases. The multivariable time-dependent Cox
proportional hazards regression analysis showed that NLR was
correlated with disease progression. These findings suggested that
NLR, as a dynamic and convenient indicator of tumor condition,
could be better than tumor markers or imaging examinations in
predicting the risk of developing PD. We believe our results are
novel because previous literature has examined the NLR value prior
to treatment initiation or at some point in treatment for prognosis
but has not examined NLR dynamics and the risk of developing PD
(14–17).
Cancers are known to induce inflammatory responses
that affect the survival of patients with cancer (14–18).
Transcription factors, such as nuclear factor κB, in tumor cells
are activated to produce inflammatory mediators, such as cytokines
and chemokines (18). Cytokines
activate inflammatory cells, including neutrophils, to produce more
inflammatory mediators. This results in a cancer-associated
inflammatory microenvironment that increases cancer aggressiveness
in terms of cancer invasiveness, immune system resistance, enhanced
angiogenesis, and resistance to treatment (19,20).
Furthermore, cytokines and chemokines produced by neutrophils can
suppress lymphocyte immune activity, which plays an important role
in antitumor immunity which plays an important role in antitumor
immunity (21). Increased
infiltration of lymphocytes into tumors has been associated with
better response to cytotoxic therapy in *patients with cancer
(22). In this way, cancer
activates neutrophils, lymphocytes are suppressed, and the values
of NLR are elevated in case the disease progresses. In contrast, if
the cancers are controlled by systemic chemotherapy, the
inflammation is suppressed, and the values of NLR will decrease.
Ohashi reported that responders tend to have decreased neutrophils
and increased lymphocytes, while within 6 weeks after anti-PD-1
therapy for advanced malignant melanoma, the opposite trend is seen
in non-responders (23). The
balance between the values of neutrophils and lymphocytes may be an
important parameter in predicting disease conditions. In our study,
an increase in NLR value is associated with an increased risk of
disease progression (Fig. 2).
Fig. 3 shows the
risk of disease progression by stratification of baseline NLR.
Compared to previous reports (15,17,24,25),
the NLR interquartile range (1.74–3.19) in our study was in a
reasonable range. Previous reports (14–17)
have shown that patients with a high baseline NLR have poor PFS and
OS, while some patients who respond well to nivolumab therapy and
have decreased NLR may have long-term disease control. There were
16 cases with NLR >3, and 8 had a decrease in NLR after initial
nivolumab treatment in this study. One of them maintained PR until
the end of the observation period (752 days). It is important to
observe NLR trends while considering the baseline NLR to predict
the risk of disease progression.
As shown in Table
IV, NLR and CEA are significantly associated with developing
PD. NLR and tumor markers, including CEA and CA19-9, are biomarkers
that can be measured by blood tests. Tumor markers are measured
monthly, whereas neutrophils and lymphocytes are measured at each
treatment, which is more convenient. Elevated tumor markers
correlate with increased tumor burden and have been reported to be
useful for detecting recurrence or distant metastasis after radical
resection (11). CEA is
significantly associated with differentiated tumors and is an
independent predictor of liver metastases (26). CA19-9 has been associated with lymph
node metastasis (27). Conversely,
unresectable or recurrent GC often has nonmeasurable lesions,
including peritoneal dissemination. Peritoneal metastasis is often
observed in diffuse-type adenocarcinoma, and tumor marker elevation
is often not observed (13). In
some cases, despite the clinical benefits of chemotherapy, tumor
markers may show transient elevations after the initiation of
chemotherapy (11). It should be
noted that tumor markers may not accurately reflect tumor activity.
Therefore, not only tumor markers but also NLR should be considered
in order to understand tumor activity.
In clinical practice, the dynamics of the NLR can be
used to consider the treatment strategy. If the NLR remains above 3
during treatment, regardless of the baseline NLR, the tumor is
poorly controlled. Therefore, an early evaluation of treatment
efficacy, including CT imaging, is necessary, and a change in
treatment strategy should be considered. Conversely, if the NLR
remains below 3, a curve estimated by the Baseline NLR can be
generated, and treatment can be continued while keeping track of
the relative risk of PD.
Our study has several limitations. First, this was a
retrospective single-center study with a small sample size. Second,
infections, including pneumonia, may be a confounding factor. Cases
with infections were included. Neutrophils are elevated in
infection, and NLR is accordingly elevated. Patients who underwent
third- or later-line chemotherapy are generally frailer than those
in first-line chemotherapy, and infectious disease occurs more
frequently than the patients in first-line therapy. More accurate
results could be obtained if data with infectious conditions could
be excluded and still have larger sample sizes. Third, nivolumab is
currently used as a first-line therapy, and it is unclear whether
the results of a third-line or later therapy can be adapted for
first-line therapy. Therefore, further analysis is needed to
evaluate the correlation between NLR and tumor progression in
patients who underwent first-line chemotherapy in the future.
In conclusion, NLR during treatment could predict
the risk of developing PD and could be another new biomarker to
evaluate tumor activity other than tumor markers or imaging
examination in patients with unresectable or recurrent GC treated
with nivolumab monotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HH, IY, YS, SF, WC and NM conceptualized the study.
HH, ME, TH, RY, KMa, MK, YS, MF, RA, JYT, AM, SK, YT, KMu and TI
designed the methodology and analyzed the data. HH wrote the
original draft preparation. IY reviewed and edited the manuscript.
HH and NM confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective observational study was approved
by the Institutional Review Board of Gifu University Hospital
(approval no. 2021-B185) and was conducted in compliance with the
Declaration of Helsinki and Japanese Good Clinical Practice
guidelines. Informed consent was obtained from all
participants.
Patient consent for publication
Written informed consent was obtained from the
patient for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
GC
|
gastric cancer
|
|
RCS
|
restricted cubic spline
|
|
RECIST v1.1
|
Response Evaluation Criteria in Solid
Tumors version 1.1
|
|
ICI
|
immune checkpoint inhibitor
|
References
|
1
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar
|
|
3
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar
|
|
4
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar
|
|
5
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomized,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar
|
|
6
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomized, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar
|
|
7
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomized, multicentre, double-blind,
Placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar
|
|
8
|
Japanese Gastric Cancer Association, .
Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition).
Gastric Cancer. 26:1–25. 2023. View Article : Google Scholar
|
|
9
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN Clinical Practice Guidelines
in Oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar
|
|
10
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar
|
|
11
|
Iwasa S, Kudo T, Takahari D, Hara H, Kato
K and Satoh T: Practical guidance for the evaluation of disease
progression and the decision to change treatment in patients with
advanced gastric cancer receiving chemotherapy. Int J Clin Oncol.
25:1223–1232. 2020. View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
13
|
Hasegawa H, Fujitani K, Nakazuru S, Hirao
M, Yamamoto K, Mita E and Tsujinaka T: Optimal treatment change
criteria for advanced gastric cancer with Non-measurable peritoneal
metastasis: Symptom/tumor Marker-based versus CT-based. Anticancer
Res. 34:5169–5174. 2014.
|
|
14
|
Ota Y, Takahari D, Suzuki T, Osumi H,
Nakayama I, Oki A, Wakatsuki T, Ichimura T, Ogura M, Shinozaki E,
et al: Changes in the Neutrophil-to-lymphocyte ratio during
nivolumab monotherapy are associated with gastric cancer survival.
Cancer Chemother Pharmacol. 85:265–272. 2020. View Article : Google Scholar
|
|
15
|
Namikawa T, Yokota K, Tanioka N, Fukudome
I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M,
et al: Systemic inflammatory response and nutritional biomarkers as
predictors of nivolumab efficacy for gastric cancer. Surg Today.
50:1486–1495. 2020. View Article : Google Scholar
|
|
16
|
Fujita K, Haruki N, Kurehara H, Ochi N,
Yamakawa Y, Harata S, Tsumoto C, Tsuji T, Ito T, Izumi A, et al:
Neutrophil-lymphocyte ratio as a prognostic indicator in patients
treated with nivolumab for gastric cancer. Gan To Kagaku Ryoho.
47:923–926. 2020.(In Japanese).
|
|
17
|
Ogata T, Satake H, Ogata M, Hatachi Y,
Inoue K, Hamada M and Yasui H: Neutrophil-to-lymphocyte ratio as a
predictive or prognostic factor for gastric cancer treated with
nivolumab: A multicenter retrospective study. Oncotarget.
9:34520–34527. 2018. View Article : Google Scholar
|
|
18
|
Tsujimoto H, Ono S, Ichikura T, Matsumoto
Y, Yamamoto J and Hase K: Roles of inflammatory cytokines in the
progression of gastric cancer: Friends or foes? Gastric Cancer.
13:212–221. 2010. View Article : Google Scholar
|
|
19
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
20
|
Granja S, Tavares-Valente D, Queirós O and
Baltazar F: Value of pH regulators in the diagnosis, prognosis and
treatment of cancer. Semin Cancer Biol. 43:17–34. 2017. View Article : Google Scholar
|
|
21
|
Nakaya A, Kurata T, Yoshioka H, Takeyasu
Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T and Nomura S:
Neutrophil-to-lymphocyte ratio as an early marker of outcomes in
patients with advanced non-small-cell lung cancer treated with
nivolumab. Int J Clin Oncol. 23:634–640. 2018. View Article : Google Scholar
|
|
22
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar
|
|
23
|
Ohashi H, Takeuchi S, Miyagaki T and
Kadono T: Increase of lymphocytes and eosinophils, and decrease of
neutrophils at an early stage of anti-PD-1 antibody treatment is a
favorable sign for advanced malignant melanoma. Drug Discov Ther.
14:117–121. 2020. View Article : Google Scholar
|
|
24
|
Nakao M, Muramatsu H, Kagawa Y, Suzuki Y,
Sakai Y, Kurokawa R, Fujita K and Sato H: Immunological status may
predict response to nivolumab in non-small cell lung cancer without
driver mutations. Anticancer Res. 37:3781–3786. 2017.
|
|
25
|
Suzuki K, Terakawa T, Furukawa J, Harada
K, Hinata N, Nakano Y and Fujisawa M: C-reactive protein and the
neutrophil-to-lymphocyte ratio are prognostic biomarkers in
metastatic renal cell carcinoma patients treated with nivolumab.
Int J Clin Oncol. 25:135–144. 2020. View Article : Google Scholar
|
|
26
|
Maehara Y, Sugimachi K, Akagi M, Kakegawa
T, Shimazu H and Tomita M: Serum carcinoembryonic antigen level
increases correlate with tumor progression in patients with
differentiated gastric carcinoma following noncurative resection.
Cancer Res. 50:3952–3955. 1990.
|
|
27
|
Kodera Y, Yamamura Y, Torii A, Uesaka K,
Hirai T, Yasui K, Morimoto T, Kato T and Kito T: The prognostic
value of preoperative serum levels of CEA and CA19-9 in patients
with gastric cancer. Am J Gastroenterol. 91:49–53. 1996.
|