|
1
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Tian Q, Wang BY and Yang J, Zhao
SD and Yang J: The prognostic significance of TILs as a biomarker
in triple-negative breast cancer: What is the role of TILs in TME
of TNBC? Eur Rev Med Pharmacol Sci. 25:2885–2897. 2021.PubMed/NCBI
|
|
4
|
Luo C, Wang P, He S, Zhu J, Shi Y and Wang
J: Progress and prospect of immunotherapy for triple-negative
breast cancer. Front Oncol. 12:9190722022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Feng L, Huang Y, Wu Y and Xie N:
Mechanisms and strategies to overcome PD-1/PD-L1 blockade
resistance in triple-negative breast cancer. Cancers (Basel).
15:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He R, Yuan X, Chen Z and Zheng Y: Combined
immunotherapy for metastatic triple-negative breast cancer based on
PD-1/PD-L1 immune checkpoint blocking. Int Immunopharmacol.
113:1094442022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
So JY, Ohm J, Lipkowitz S and Yang L:
Triple negative breast cancer (TNBC): Non-genetic tumor
heterogeneity and immune microenvironment: Emerging treatment
options. Pharmacol Ther. 237:1082532022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Obidiro O, Battogtokh G and Akala EO:
Triple negative breast cancer treatment options and limitations:
Future outlook. Pharmaceutics. 15:17962023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solin LJ, Hwang WT and Vapiwala N: Outcome
after breast conservation treatment with radiation for women with
triple-negative early-stage invasive breast carcinoma. Clin Breast
Cancer. 9:96–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panoff JE, Hurley J, Takita C, Reis IM,
Zhao W, Sujoy V, Gomez CR, Jorda M, Koniaris L and Wright JL: Risk
of locoregional recurrence by receptor status in breast cancer
patients receiving modern systemic therapy and post-mastectomy
radiation. Breast Cancer Res Treat. 128:899–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan XB, Qu S, Jiang YM and Zhu XD: Triple
negative breast cancer versus non-triple negative breast cancer
treated with breast conservation surgery followed by radiotherapy:
A systematic review and meta-analysis. Breast Care (Basel).
10:413–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapdelaine AG and Sun G: Challenges and
opportunities in developing targeted therapies for triple negative
breast cancer. Biomolecules. 13:12072023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z,
Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y and
Chen-Yan Z: Recent treatment progress of triple negative breast
cancer. Prog Biophys Mol Biol. 151:40–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maqbool M, Bekele F and Fekadu G:
Treatment strategies against triple-negative breast cancer: An
updated review. Breast Cancer (Dove Med Press). 14:15–24.
2022.PubMed/NCBI
|
|
16
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powles T, Park SH, Voog E, Caserta C,
Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén
A, et al: Avelumab maintenance therapy for advanced or metastatic
urothelial carcinoma. N Engl J Med. 383:1218–1230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abad NM, Calabuig-Fariñas S, de Mena ML,
Torres-Martínez S, González CG, García JÁ, González-Cruz VI and
Herrero CC: Programmed death-ligand 1 (PD-L1) as immunotherapy
biomarker in breast cancer. Cancers (Basel). 14:3072022. View Article : Google Scholar
|
|
20
|
Qureshi S, Chan N, George M, Ganesan S,
Toppmeyer D and Omene C: Immune checkpoint inhibitors in triple
negative breast cancer: The search for the optimal biomarker.
Biomark Insights. 17:117727192210787742022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Zhu X, Tang C, Guan X and Zhang W:
Progress and challenges of immunotherapy in triple-negative breast
cancer. Biochim Biophys Acta Rev Cancer. 1876:1885932021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhang H, Merkher Y, Chen L, Liu N,
Leonov S and Chen Y: Recent advances in therapeutic strategies for
triple-negative breast cancer. J Hematol Oncol. 15:1212022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang F and Zheng P: Tumor cells versus
host immune cells: Whose PD-L1 contributes to PD-1/PD-L1 blockade
mediated cancer immunotherapy? Cell Biosci. 8:342018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Liang Y, Anders RA, Taube JM, Qiu
X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, et al: PD-L1
on host cells is essential for PD-L1 blockade-mediated tumor
regression. J Clin Invest. 128:580–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan M, Du K, Ai M, Wang B, Lin J, Ren A,
Chen C, Huang Z, Qiu W, Yuan Y and Tian Y: PD-L1 expression as
biomarker of efficacy of PD-1/PD-L1 checkpoint inhibitors in
metastatic triple negative breast cancer: A systematic review and
meta-analysis. Front Immunol. 14:10603082023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Institutes of Health, . National
Cancer Institute, U.S. Department of Health and Human Services.,
Common Terminology Criteria for Adverse Events (CTCAE) Version
4.0.
|
|
27
|
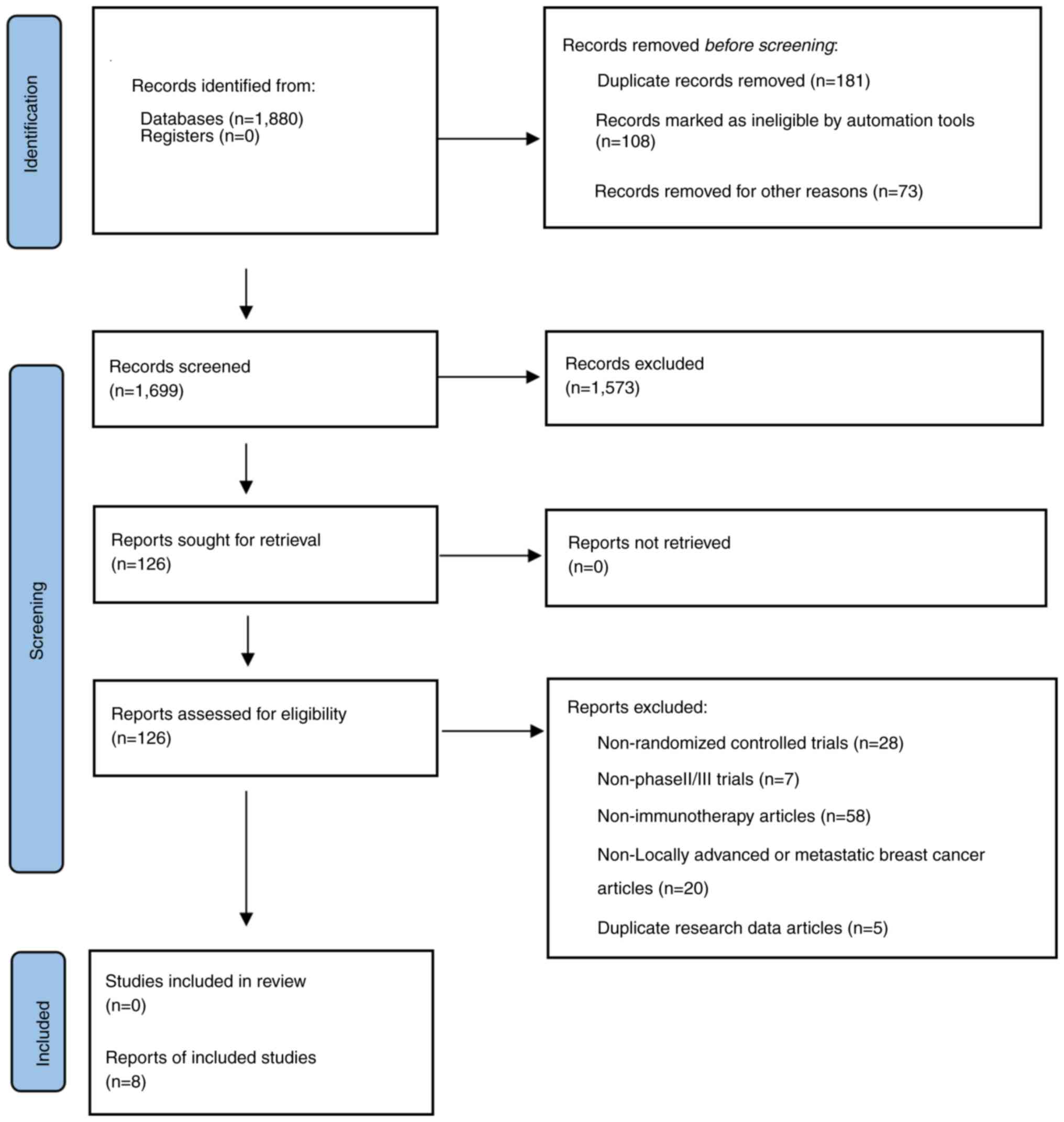
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
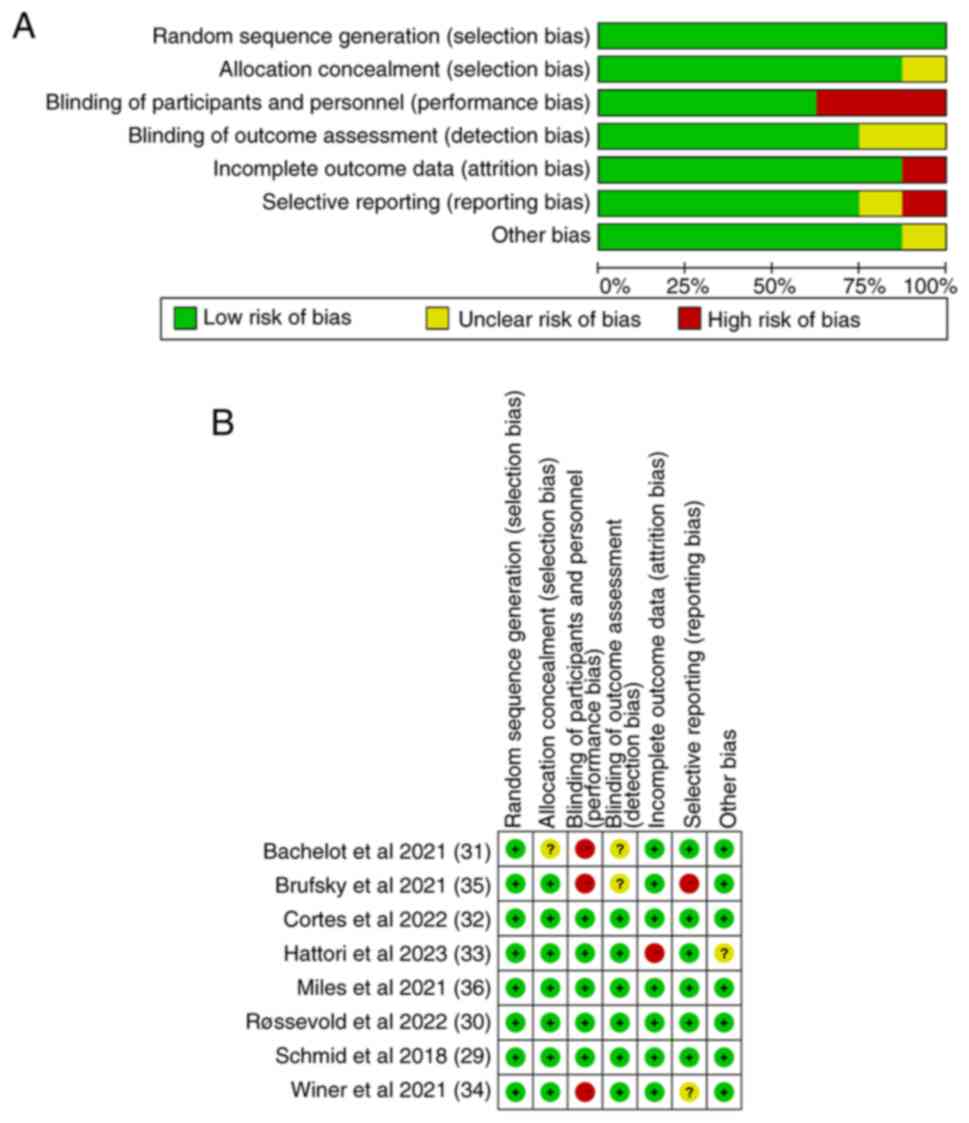
Sterne JA, Sutton AJ, Ioannidis JP, Terrin
N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH,
et al: Recommendations for examining and interpreting funnel plot
asymmetry in meta-analyses of randomised controlled trials. BMJ.
343:d40022011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
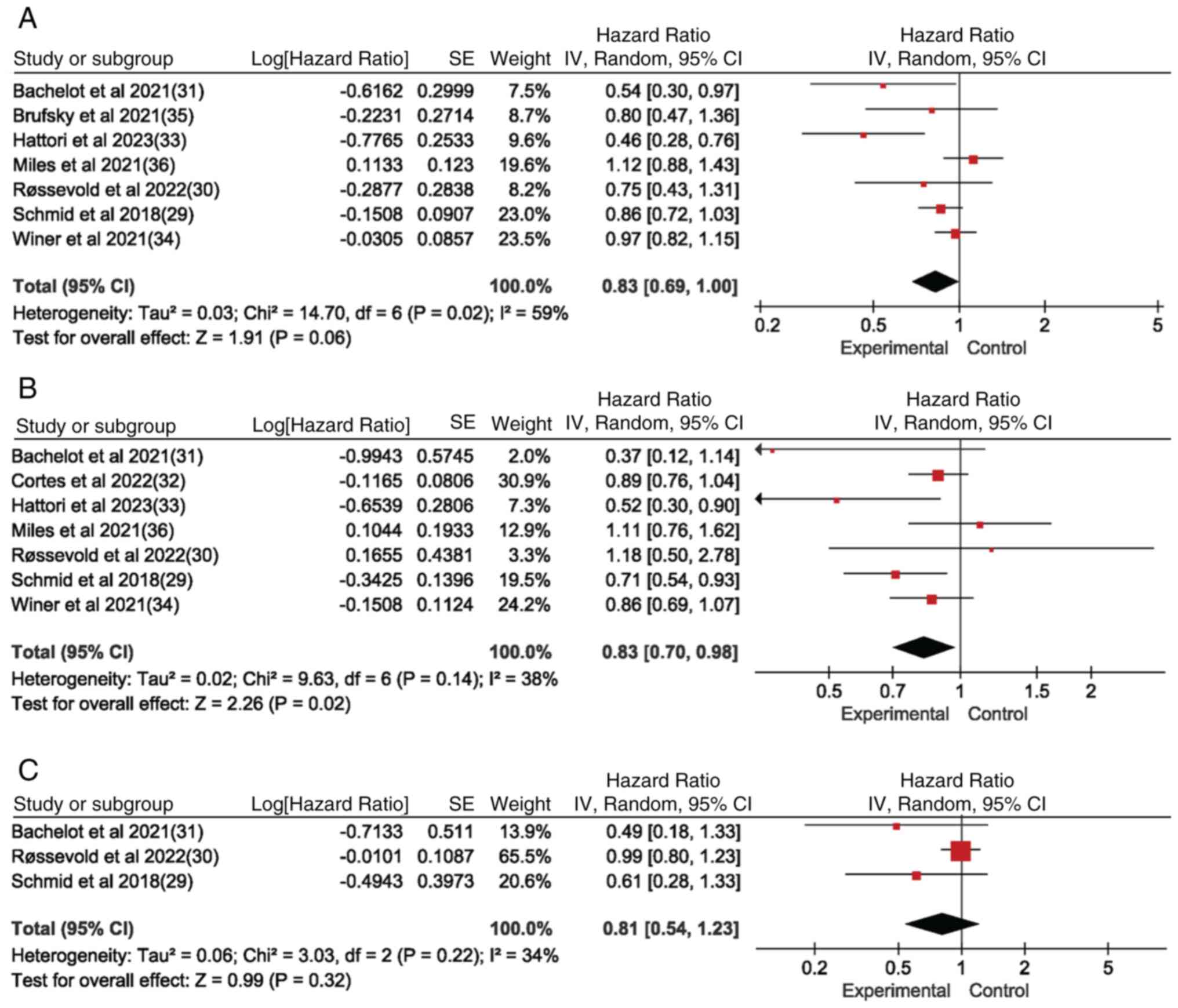
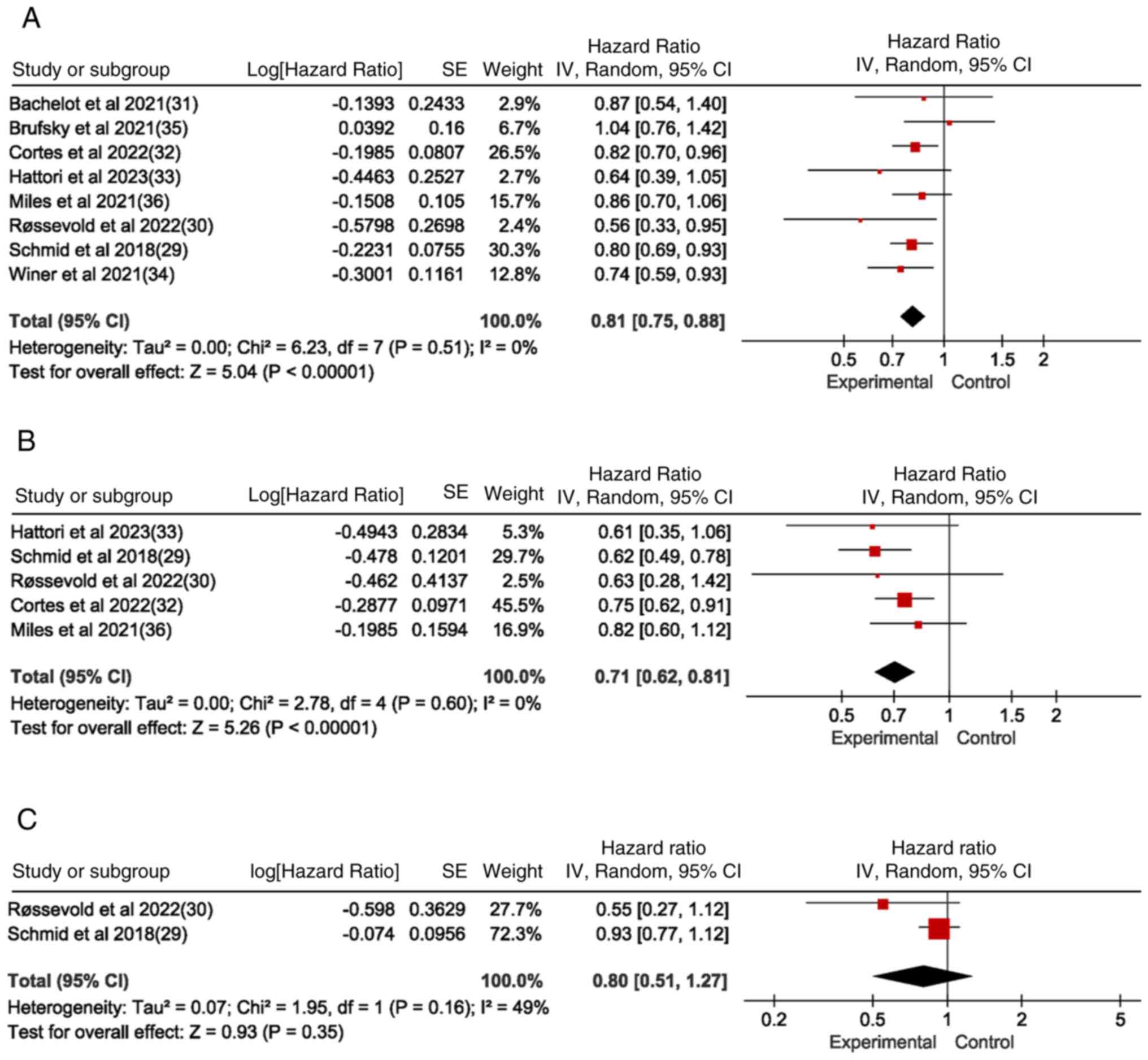
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Wright GS, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Røssevold AH, Andresen NK, Bjerre CA,
Gilje B, Jakobsen EH, Raj SX, Falk RS, Russnes HG, Jahr T,
Mathiesen RR, et al: Atezolizumab plus anthracycline-based
chemotherapy in metastatic triple-negative breast cancer: The
randomized, double-blind phase 2b ALICE trial. Nat Med.
28:2573–2583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bachelot T, Filleron T, Bieche I, Arnedos
M, Campone M, Dalenc F, Coussy F, Sablin MP, Debled M,
Lefeuvre-Plesse C, et al: Durvalumab compared to maintenance
chemotherapy in metastatic breast cancer: The randomized phase II
SAFIR02-BREAST IMMUNO trial. Nat Med. 27:250–255. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
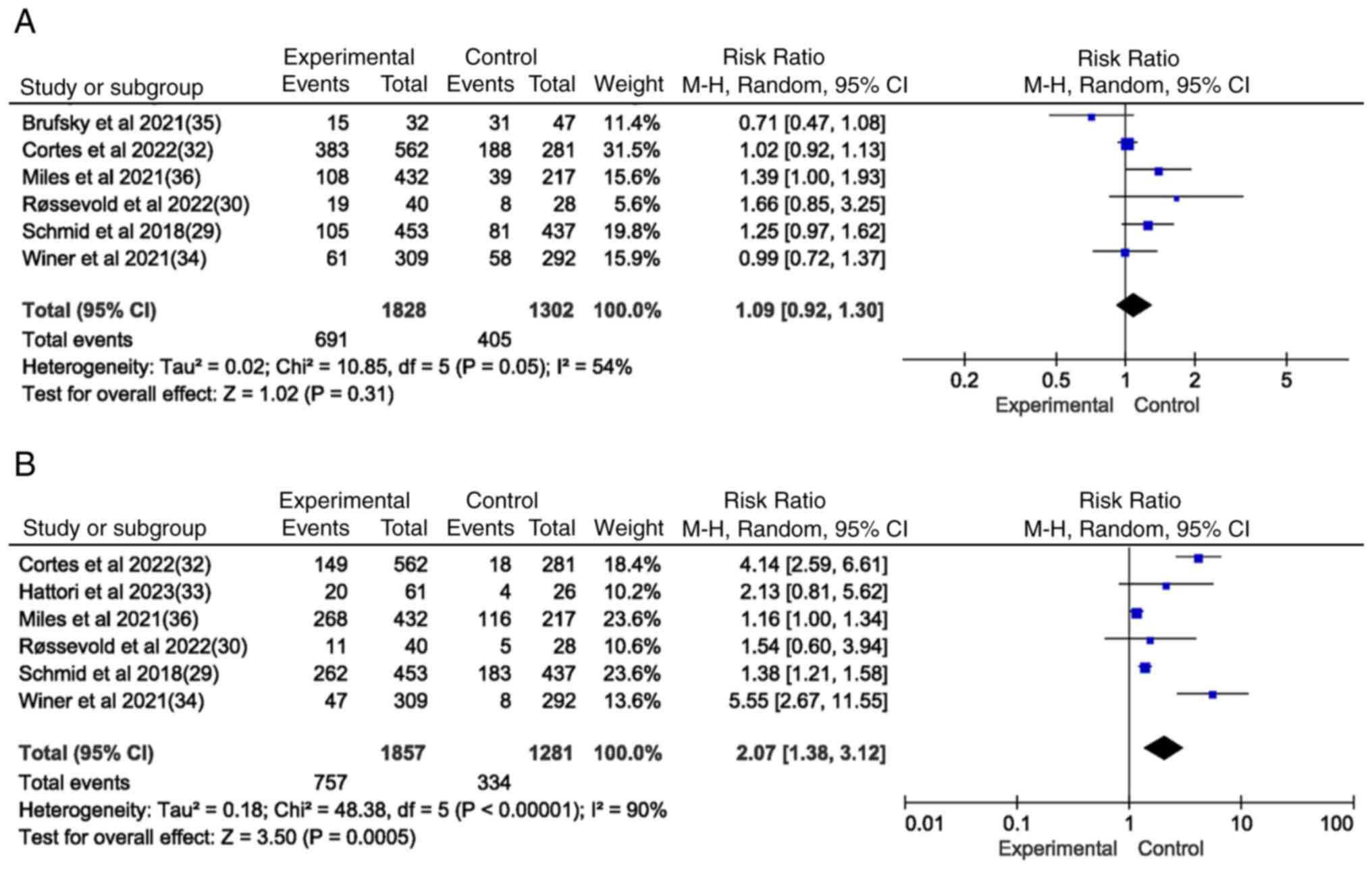
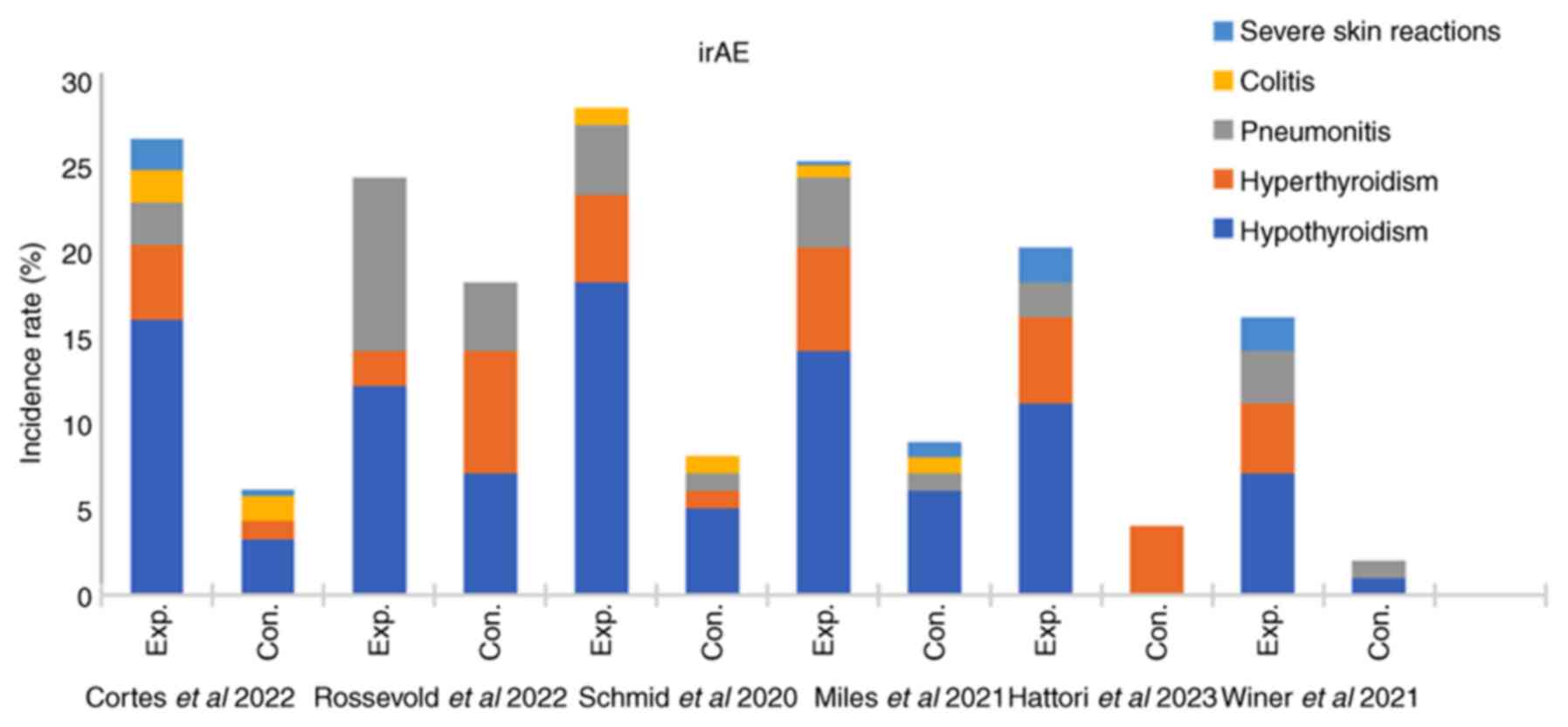
Cortes J, Rugo HS, Cescon DW, Im SA, Yusof
MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et
al: Pembrolizumab plus chemotherapy in advanced triple-negative
breast cancer. N Engl J Med. 387:217–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hattori M, Masuda N, Takano T, Tsugawa K,
Inoue K, Matsumoto K, Ishikawa T, Itoh M, Yasojima H, Tanabe Y, et
al: Pembrolizumab plus chemotherapy in Japanese patients with
triple-negative breast cancer: Results from KEYNOTE-355. Cancer
Med. 12:10280–10293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winer EP, Lipatov O, Im SA, Goncalves A,
Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, et
al: Pembrolizumab versus investigator-choice chemotherapy for
metastatic triple-negative breast cancer (KEYNOTE-119): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:499–511.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brufsky A, Kim SB, Zvirbule Ž, Eniu A,
Mebis J, Sohn JH, Wongchenko M, Chohan S, Amin R, Yan Y, et al: A
phase II randomized trial of cobimetinib plus chemotherapy, with or
without atezolizumab, as first-line treatment for patients with
locally advanced or metastatic triple-negative breast cancer
(COLET): Primary analysis. Ann Oncol. 32:652–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miles D, Gligorov J, André F, Cameron D,
Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, et
al: Primary results from IMpassion131, a double-blind,
placebo-controlled, randomised phase III trial of first-line
paclitaxel with or without atezolizumab for unresectable locally
advanced/metastatic triple-negative breast cancer. Ann Oncol.
32:994–1004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robert C: A decade of immune-checkpoint
inhibitors in cancer therapy. Nat Commun. 11:38012020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ledford H, Else H and Warren M: Cancer
immunologists scoop medicine nobel prize. Nature. 562:20–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Derakhshan F and Reis-Filho JS:
Pathogenesis of triple-negative breast cancer. Annu Rev Pathol.
17:181–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao BY, Lin GH, Zhao YX and Wang BC: The
efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: A
systematic review and meta-analysis. Transl Cancer Res.
9:3804–3818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Wang J, Hu T, Wang H, Long M and
Liang B: Adverse events of PD-1 or PD-L1 inhibitors in
triple-negative breast cancer: A systematic review and
meta-analysis. Life (Basel). 12:19902022.PubMed/NCBI
|
|
44
|
Baraibar I, Melero I, Ponz-Sarvise M and
Castanon E: Safety and tolerability of immune checkpoint inhibitors
(PD-1 and PD-L1) in cancer. Drug Saf. 42:281–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patel AB and Pacha O: Skin reactions to
immune checkpoint inhibitors. Adv Exp Med Biol. 995:175–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nguyen LT and Ohashi PS: Clinical blockade
of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol.
15:45–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang W, He Y, Tang Y, Dai W, Si Y, Mao F,
Xu J, Yu C and Sun X: A meta-analysis of application of PD-1/PD-L1
inhibitor-based immunotherapy in unresectable locally advanced
triple-negative breast cancer. Immunotherapy. 15:1073–1088. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang C: A meta-analysis of efficacy and
safety of PD-1/PD-L1 inhibitors in triple-negative breast cancer. J
Oncol. 2022:24072112022.PubMed/NCBI
|
|
49
|
Yu Y, Jin X, Zhu X, Xu Y, Si W and Zhao J:
PD-1/PD-L1 immune checkpoint inhibitors in metastatic
triple-negative breast cancer: A systematic review and
meta-analysis. Front Immunol. 14:12066892023. View Article : Google Scholar : PubMed/NCBI
|