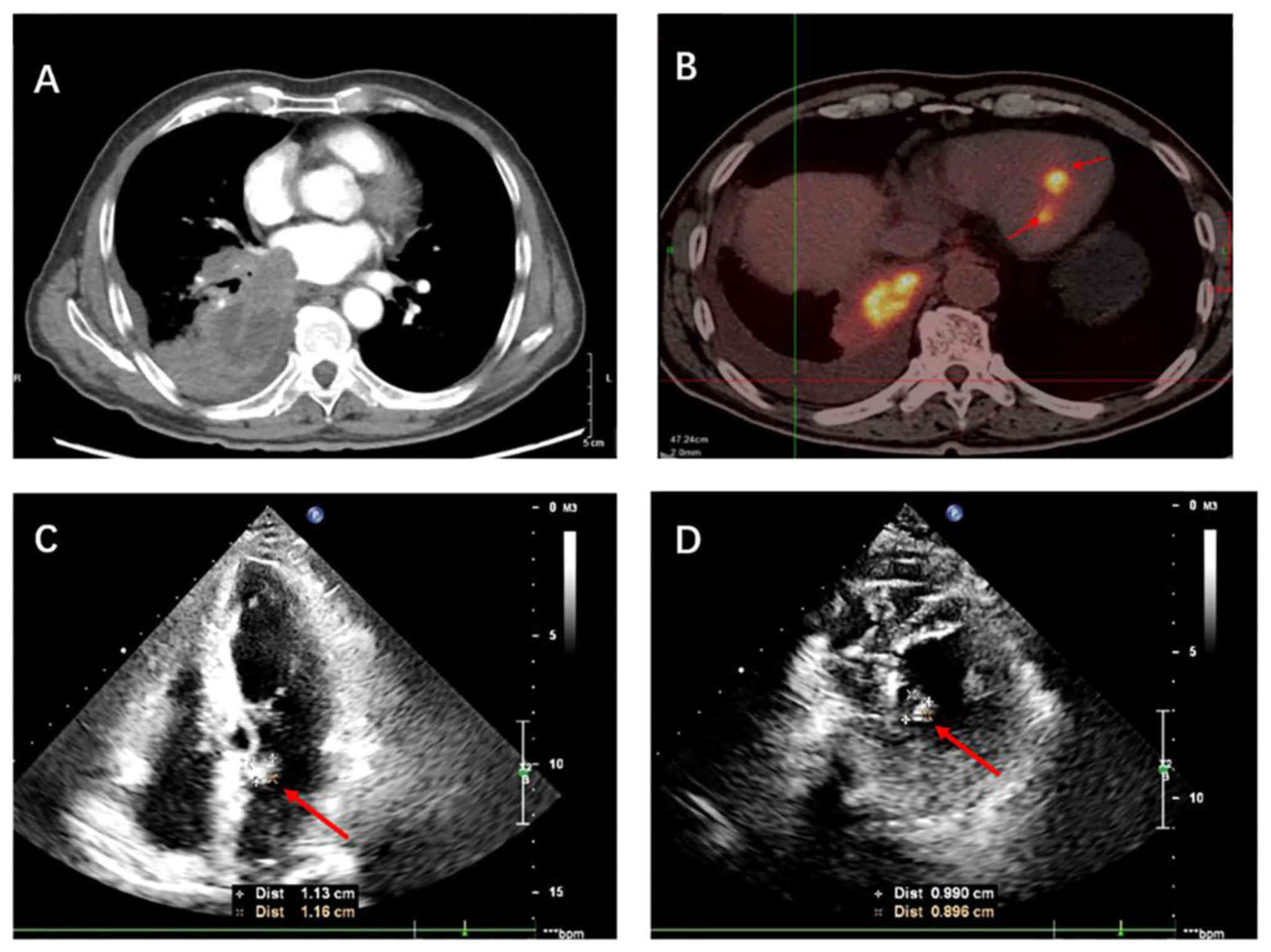
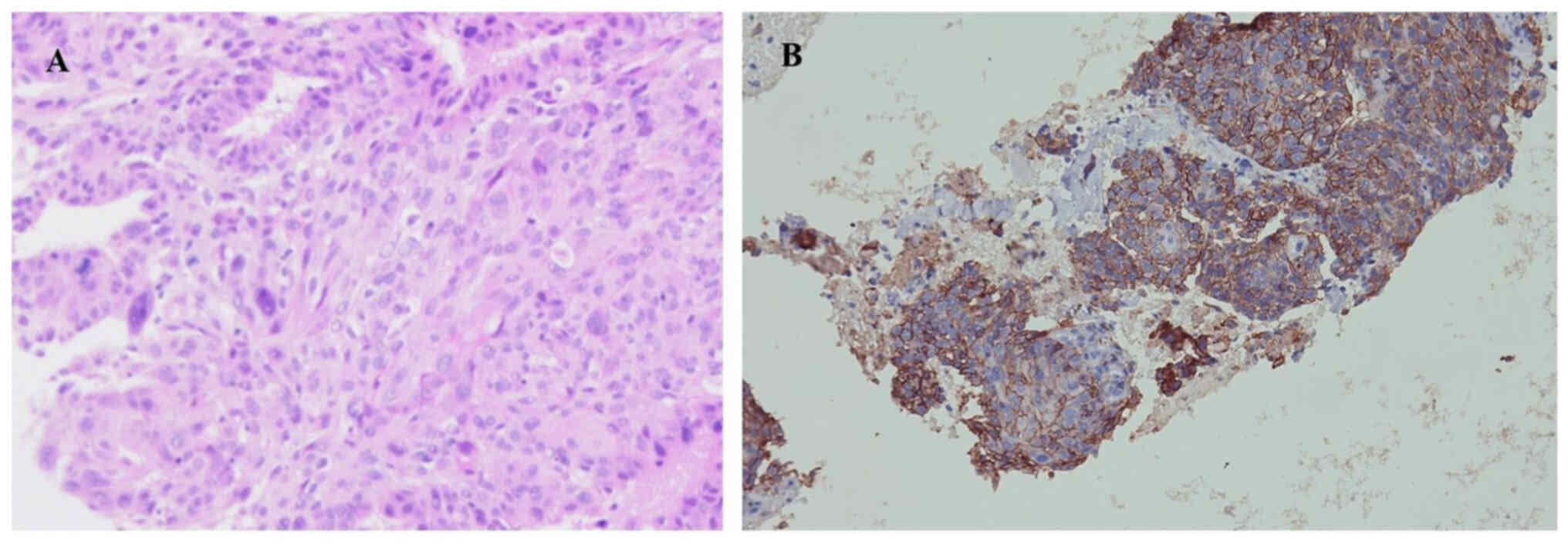
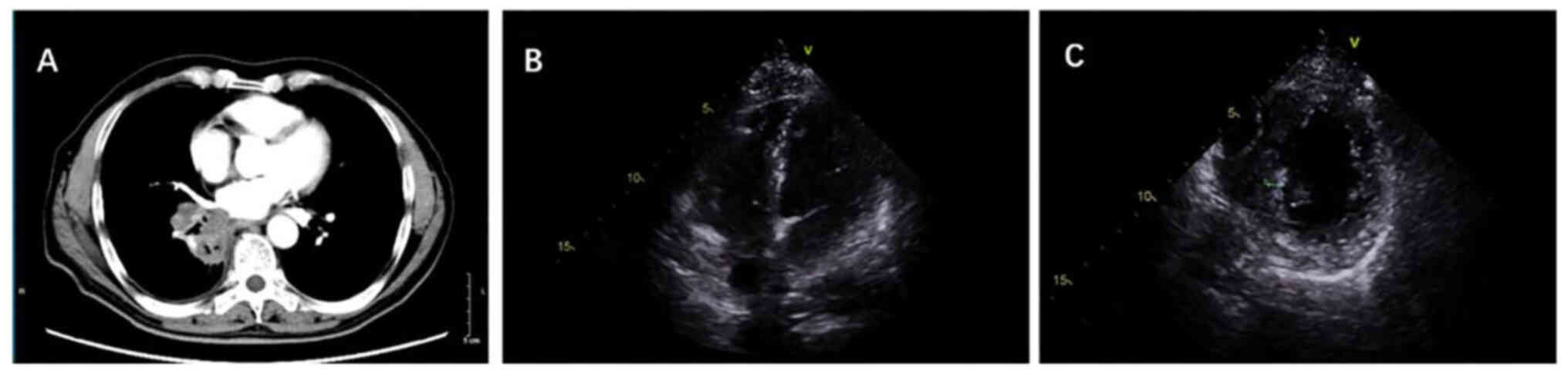
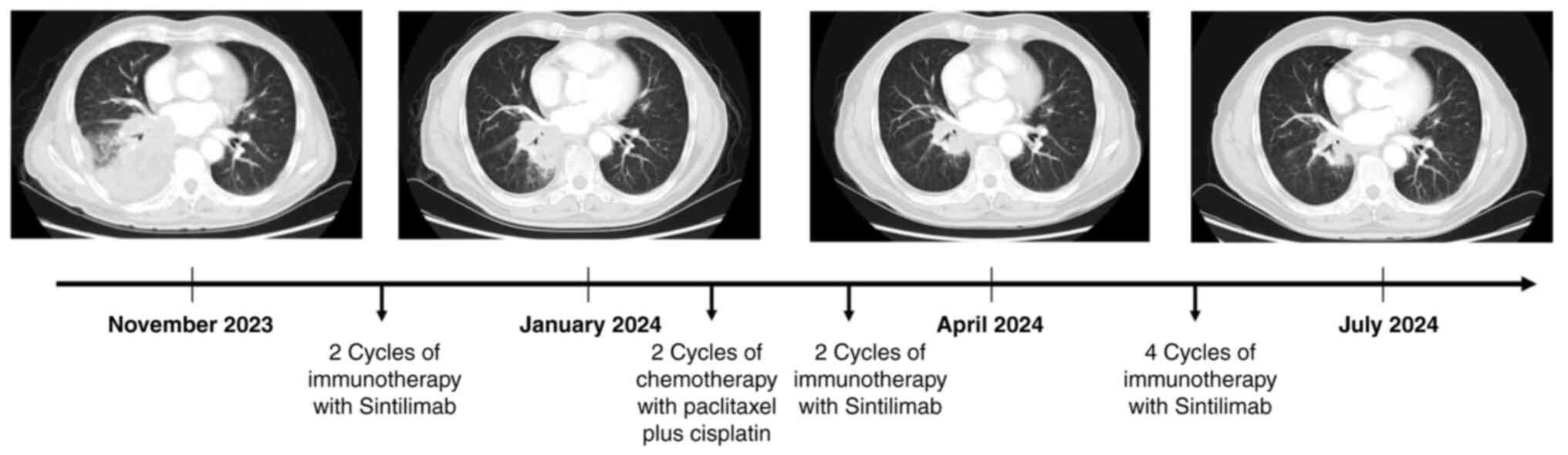
|
1
|
Nova-Camacho LM, Gomez-Dorronsoro M,
Guarch R, Cordoba A, Cevallos MI and Panizo-Santos A: Cardiac
metastasis from solid cancers: A 35-year single-center autopsy
study. Arch Pathol Lab Med. 147:177–184. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts WC: Primary and secondary
neoplasms of the heart. Am J Cardiol. 80:671–682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Vaccarella S, Morgan E, Li M,
Etxeberria J, Chokunonga E, Manraj SS, Kamate B, Omonisi A and Bray
F: Global variations in lung cancer incidence by histological
subtype in 2020: A population-based study. Lancet Oncol.
24:1206–1218. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waterhouse D, Lam J, Betts KA, Yin L, Gao
S, Yuan Y, Hartman J, Rao S, Lubinga S and Stenehjem D: Real-world
outcomes of immunotherapy-based regimens in first-line advanced
non-small cell lung cancer. Lung Cancer. 156:41–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung Cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
Non-Small-Cell Lung Cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park K, Vansteenkiste J, Lee KH,
Pentheroudakis G, Zhou C, Prabhash K, Seto T, Voon PJ, Tan DSW,
Yang JCH, et al: Pan-Asian adapted ESMO Clinical Practice
Guidelines for the management of patients with locally-advanced
unresectable non-small-cell lung cancer: A KSMO-ESMO initiative
endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol.
31:191–201. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaiyesimi IA, Leighl NB, Ismaila N, Alluri
K, Florez N, Gadgeel S, Masters G, Schenk EL, Schneider BJ, Sequist
L, et al: Therapy for stage IV non-small cell lung cancer without
driver alterations: ASCO living guideline. version 2023.3. Clin
Oncol. 42:e23–e43. 2024.PubMed/NCBI
|
|
13
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-Small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oncology Society of Chinese Medical
Association and Chinese Medical Association Publishing House, .
Chinese Medical Association guideline for clinical diagnosis and
treatment of lung cancer (2023 edition). Zhonghua Yi Xue Za Zhi.
103:2037–2074. 2023.(In Chinese). PubMed/NCBI
|
|
15
|
Maggie Liu SY, Huang J, Deng JY, Xu CR,
Yan HH, Yang MY, Li YS, Ke EE, Zheng MY, Wang Z, et al: PD-L1
expression guidance on sintilimab versus pembrolizumab with or
without platinum-doublet chemotherapy in untreated patients with
advanced non-small cell lung cancer (CTONG1901): A phase 2,
randomized, controlled trial. Sci Bull (Beijing). 69:535–543. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 5. US Department of Health and Human
Services, National Institutes of Health, National Cancer Institute,
. 2017.Available from. https://ctep.cancer.gov/search/search.asp?zoom_query=CTCAE&Action=Go%3EOctober
10–2024
|
|
17
|
Maleszewski JJ and Anavekar NS: Neoplastic
pericardial disease. Cardiol Clin. 35:589–600. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dragoescu EA and Liu L: Pericardial fluid
cytology: An analysis of 128 specim-ens over a 6-year period.
Cancer Cytopathol. 121:242–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Butany J, Leong SW, Carmichael K and
Komeda M: A 30-year analysis of cardiac neoplasms at autopsy. Can J
Cardiol. 21:675–680. 2005.PubMed/NCBI
|
|
20
|
Xie S, Wu Z, Qi Y, Wu B and Zhu X: The
metastasizing mechanisms of lung cancer: Recent advances and
therapeutic challenges. Biomed Pharmacother. 138:1114502021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu FY, Zhou Q, Yang JJ, Zhong WZ, Chen
ZH, Deng W, He YY, Chen HJ, Zeng Z, Ke EE, et al: Distribution and
prognosis of uncommon metastases from non-small cell lung cancer.
BMC Cancer. 16:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Ma W, Wu D, Lyu M, Zheng Q, Wang
T, Zhou J and Liu C: Prognostic relevance of immune-related adverse
events in lung cancer patients undergoing immune checkpoint
inhibitor therapy: A systematic review and meta-analysis. Transl
Lung Cancer Res. 13:1559–1584. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thompson JA, Schneider BJ, Brahmer J,
Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M,
Dunnington D, et al: NCCN Guidelines Insights: Management of
Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc
Netw. 18:230–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brahmer JR, Lacchetti C and Thompson JA:
Management of Immune-Related Adverse Events in Patients Treated
With Immune Checkpoint Inhibitor Therapy: American Society of
Clinical Oncology Clinical Practice Guideline Summary. J Oncol
Pract. 14:247–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K; ESMO Guidelines Committee, :
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl 4):iv264–iv266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and Efficacy of Re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018. View Article : Google Scholar : PubMed/NCBI
|