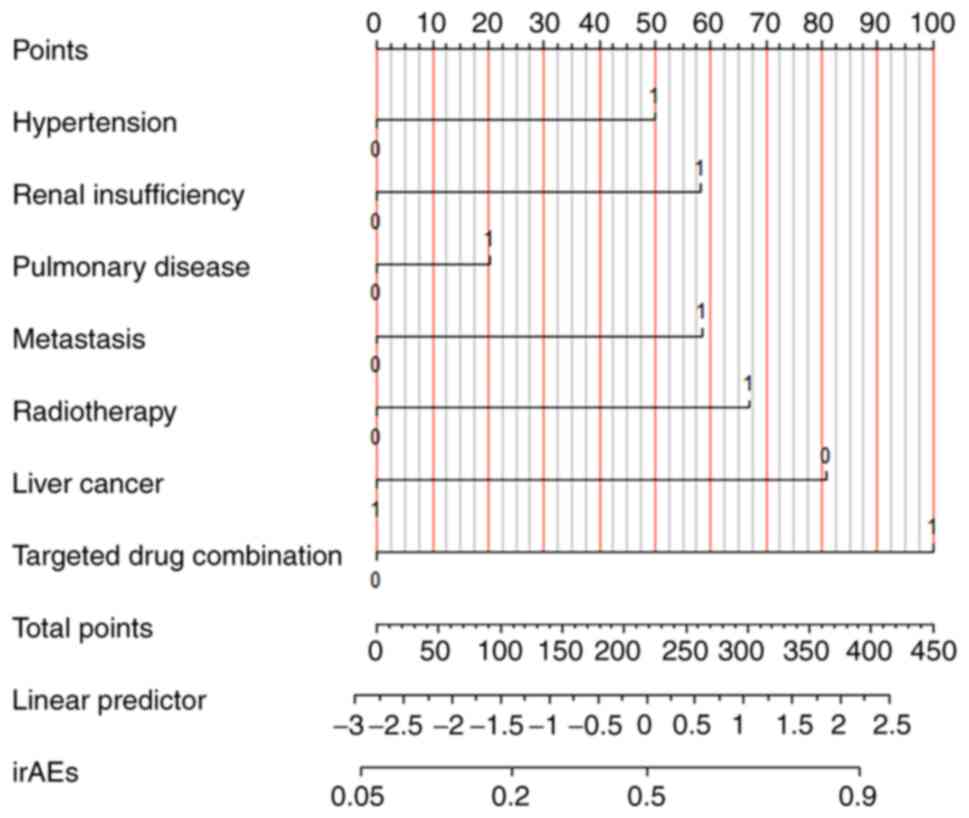
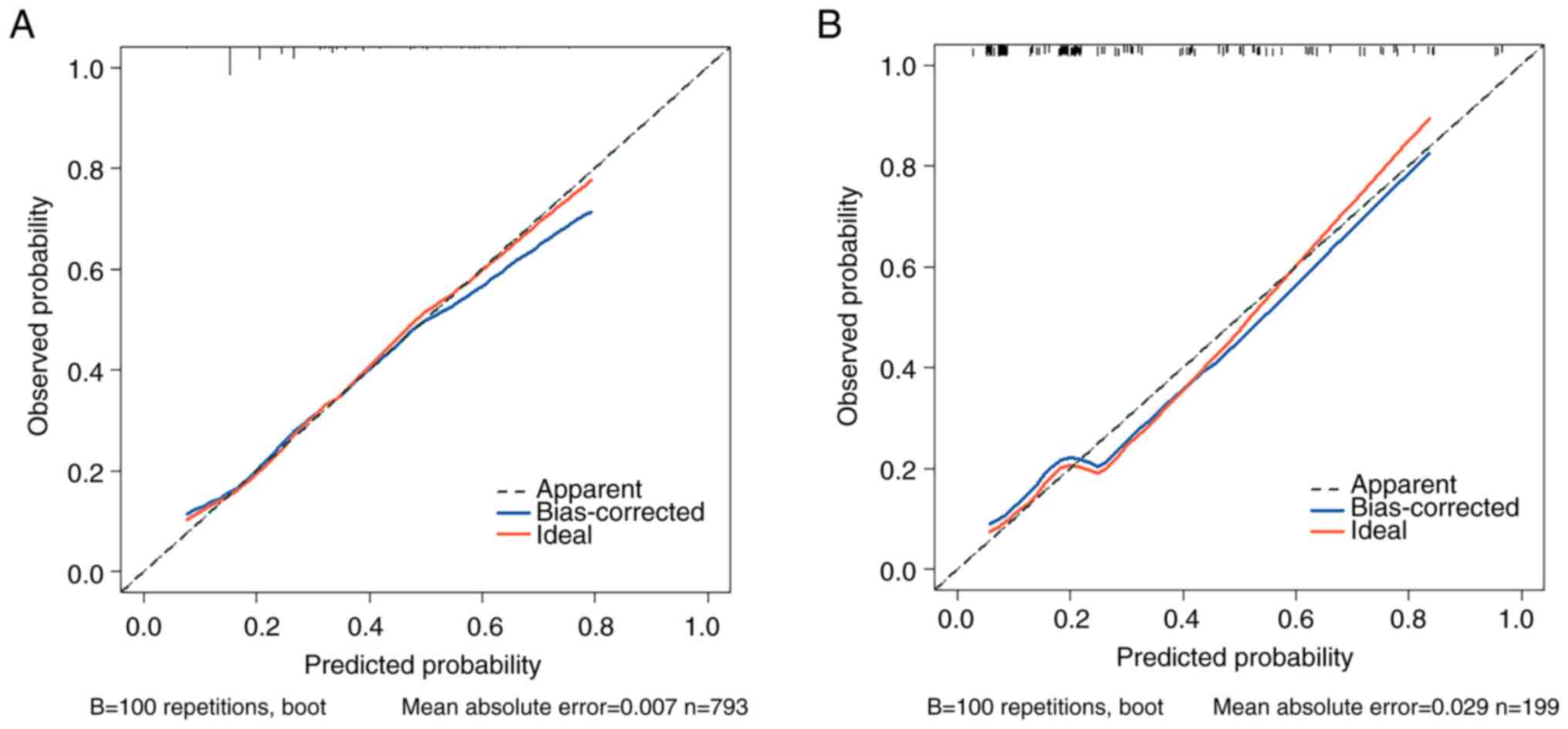
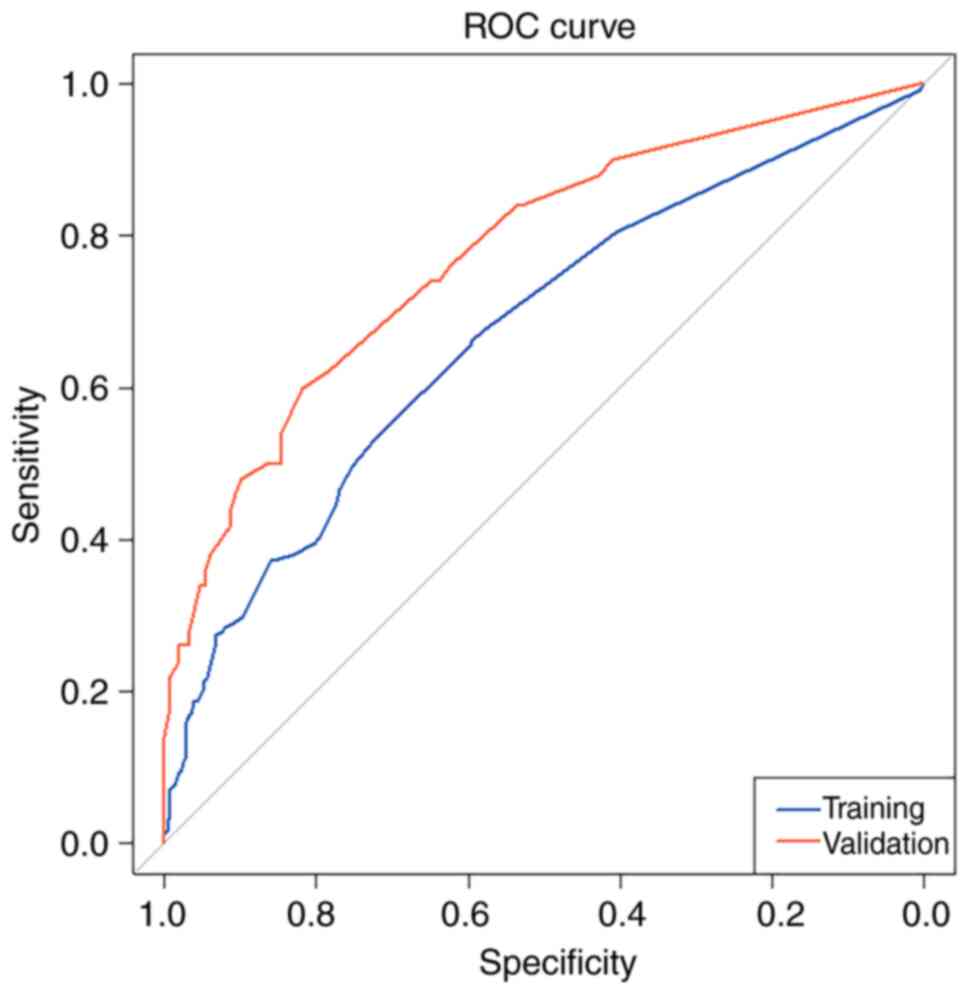
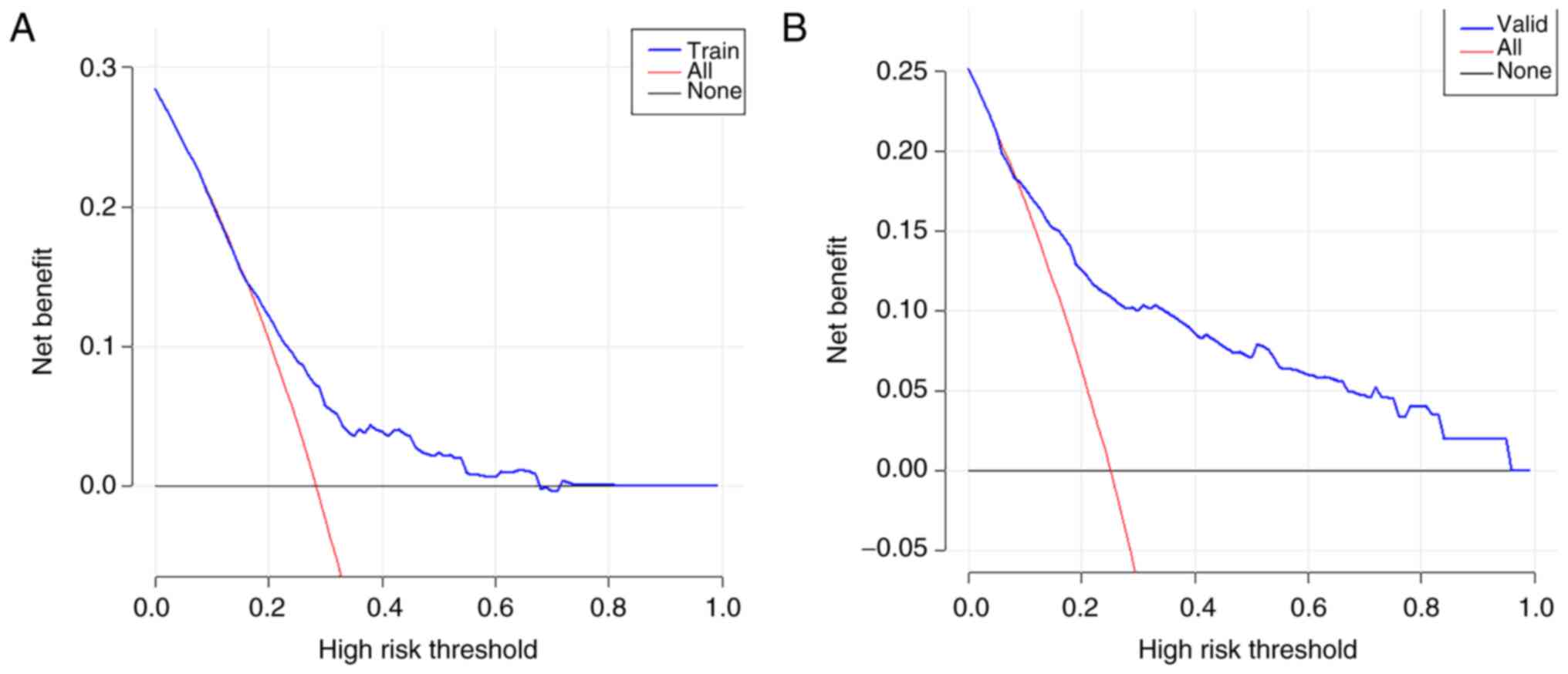
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv3242016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robert C: A decade of immune-checkpoint
inhibitors in cancer therapy. Nat Commun. 11:38012020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramos-Casals M and Siso-Almirall A:
Immune-Related adverse events of immune checkpoint inhibitors. Ann
Intern Med. 177:ITC17–ITC32. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin Q, Wu L, Han L, Zheng X, Tong R, Li L,
Bai L and Bian Y: Immune-related adverse events of immune
checkpoint inhibitors: A review. Front Immunol. 14:11679752023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Champiat S, Lambotte O, Barreau E, Belkhir
R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M,
Durrbach A, et al: Management of immune checkpoint blockade
dysimmune toxicities: A collaborative position paper. Ann Oncol.
27:559–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dougan M, Luoma AM, Dougan SK and
Wucherpfennig KW: Understanding and treating the inflammatory
adverse events of cancer immunotherapy. Cell. 184:1575–1588. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara Y, Horita N, Adib E, Zhou S,
Nassar AH, Asad ZUA, Cortellini A and Naqash AR: Treatment-related
adverse events, including fatal toxicities, in patients with solid
tumours receiving neoadjuvant and adjuvant immune checkpoint
blockade: A systematic review and meta-analysis of randomised
controlled trials. Lancet Oncol. 25:62–75. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang X, Li W, Du Y, Hu W and
Zhao J: Biomarkers and risk factors for the early prediction of
immune-related adverse events: A review. Hum Vaccin Immunother.
18:20188942022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCulloch JA, Davar D, Rodrigues RR,
Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM,
Fernandes MR, et al: Intestinal microbiota signatures of clinical
response and immune-related adverse events in melanoma patients
treated with anti-PD-1. Nat Med. 28:545–556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smithy JW, Faleck DM and Postow MA: Facts
and hopes in prediction, diagnosis, and treatment of immune-related
adverse events. Clin Cancer Res. 28:1250–1257. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponvilawan B, Khan AW, Subramanian J and
Bansal D: Non-invasive predictive biomarkers for immune-related
adverse events due to immune checkpoint inhibitors. Cancers
(Basel). 16:12252024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan L, Li Y, Chen JY, Zheng YF and Xu XM:
Immune checkpoint modulators in cancer immunotherapy: Recent
advances and combination rationales. Cancer Lett. 456:23–28. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makuku R, Khalili N, Razi S,
Keshavarz-Fathi M and Rezaei N: Current and future perspectives of
PD-1/PDL-1 blockade in cancer immunotherapy. J Immunol Res.
2021:66614062021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russano M, Cortellini A, Giusti R, Russo
A, Zoratto F, Rastelli F, Gelibter A, Chiari R, Nigro O, De Tursi
M, et al: Clinical outcomes of NSCLC patients experiencing early
immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors
leading to treatment discontinuation. Cancer Immunol Immunother.
71:865–874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Felip E, Altorki N, Zhou C, Csoszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M,
Lee HC, Yopp AC, Zhou J, Wang L, Wen X, et al: Atezolizumab plus
bevacizumab versus active surveillance in patients with resected or
ablated high-risk hepatocellular carcinoma (IMbrave050): A
randomised, open-label, multicentre, phase 3 trial. Lancet.
402:1835–1847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khoja L, Day D, Chen TWW, Siu LL and
Hansen AR: Tumour- and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elshafie O, Khalil AB, Salman B, Atabani A
and Al-Sayegh H: Immune checkpoint inhibitors-induced
endocrinopathies: Assessment, management and monitoring in a
comprehensive cancer centre. Endocrinol Diabetes Metab.
7:e005052024. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panagiotou E, Ntouraki S, Vathiotis IA,
Livanou ME, Trimis A, Evangelou G, Charpidou A, Syrigos K and Peppa
M: Endocrine immune-related adverse events are independent
predictors of survival in patients with lung cancer. Cancers
(Basel). 16:17642024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sears CR, Peikert T, Possick JD, Naidoo J,
Nishino M, Patel SP, Camus P, Gaga M, Garon EB, Gould MK, et al:
Knowledge gaps and research priorities in immune checkpoint
inhibitor-related pneumonitis. An official American thoracic
society research statement. Am J Respir Crit Care Med. 200:e31–e43.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bracamonte-Baran W and Kim ST: The current
and future of biomarkers of immune related adverse events. Rheum
Dis Clin North Am. 50:201–227. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu HR, Zhu PF, Deng YY, Chen ZL and Yang
L: Predictive value of NLR and PLR for immune-related adverse
events: A systematic review and meta-analysis. Clin Transl Oncol.
26:1106–1116. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chennamadhavuni A, Abushahin L, Jin N,
Presley CJ and Manne A: Risk factors and biomarkers for
immune-related adverse events: A practical guide to identifying
high-risk patients and rechallenging immune checkpoint inhibitors.
Front Immunol. 13:7796912022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh MV, Chapleau MW, Harwani SC and
Abboud FM: The immune system and hypertension. Immunol Res.
59:243–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao W, Liu W, Chang R, Yang M, Xin K, Liu
J, Wang Y, Ren M, Xie J and Yang Y: Safety and clinical efficacy of
immune checkpoint inhibitors in advanced gastric cancer in the real
world. J Cancer Res Clin Oncol. 150:1802024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Remolina-Bonilla YA, Jimenez-Franco B, Lam
ET and Bourlon MT: Immune-related adverse events involving multiple
organ sites in a patient treated with nivolumab plus ipilimumab.
Oncology (Williston Park). 34:171–174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Aren Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bardoscia L, Pasinetti N, Triggiani L,
Cozzi S and Sardaro A: Biological bases of immune-related adverse
events and potential crosslinks with immunogenic effects of
radiation. Front Pharmacol. 12:7468532021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaverdian N, Beattie J, Thor M, Offin M,
Shepherd AF, Gelblum DY, Wu AJ, Simone CB II, Hellmann MD, Chaft
JE, et al: Safety of thoracic radiotherapy in patients with prior
immune-related adverse events from immune checkpoint inhibitors.
Ann Oncol. 31:1719–1724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu X, Wang J, Zhang T, Zhou Z, Deng L,
Wang X, Wang W, Liu W, Tang W, Wang Z, et al: Comprehensive
pneumonitis profile of thoracic radiotherapy followed by immune
checkpoint inhibitor and risk factors for radiation recall
pneumonitis in lung cancer. Front Immunol. 13:9187872022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoos A: Development of immuno-oncology
drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug
Discov. 15:235–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reynolds KL, Arora S, Elayavilli RK, Louv
WC, Schaller TH, Khandelwal A, Rothenberg M, Khozin S, Guidon AC,
Dougan M, et al: Immune-related adverse events associated with
immune checkpoint inhibitors: A call to action for collecting and
sharing clinical trial and real-world data. J Immunother Cancer.
9:e0028962021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn MJ, Sun JM, Lee SH, Ahn JS and Park K:
EGFR TKI combination with immunotherapy in non-small cell lung
cancer. Expert Opin Drug Saf. 16:465–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spigel DR, Reynolds C, Waterhouse D, Garon
EB, Chandler J, Babu S, Thurmes P, Spira A, Jotte R, Zhu J, et al:
Phase 1/2 study of the safety and tolerability of nivolumab plus
crizotinib for the first-line treatment of anaplastic lymphoma
kinase translocation-positive advanced non-small cell lung cancer
(CheckMate 370). J Thorac Oncol. 13:682–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Lv F, Wang Y and Du Z: Establishment
and validation of nomogram for predicting immuno checkpoint
inhibitor related pneumonia. BMC Pulm Med. 22:3312022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong Z, Liu G, Tu L, Su X and Yu Y:
Establishment of a prediction model of postoperative infection
complications in patients with gastric cancer and its impact on
prognosis. J Gastrointest Oncol. 14:1250–1258. 2023. View Article : Google Scholar : PubMed/NCBI
|