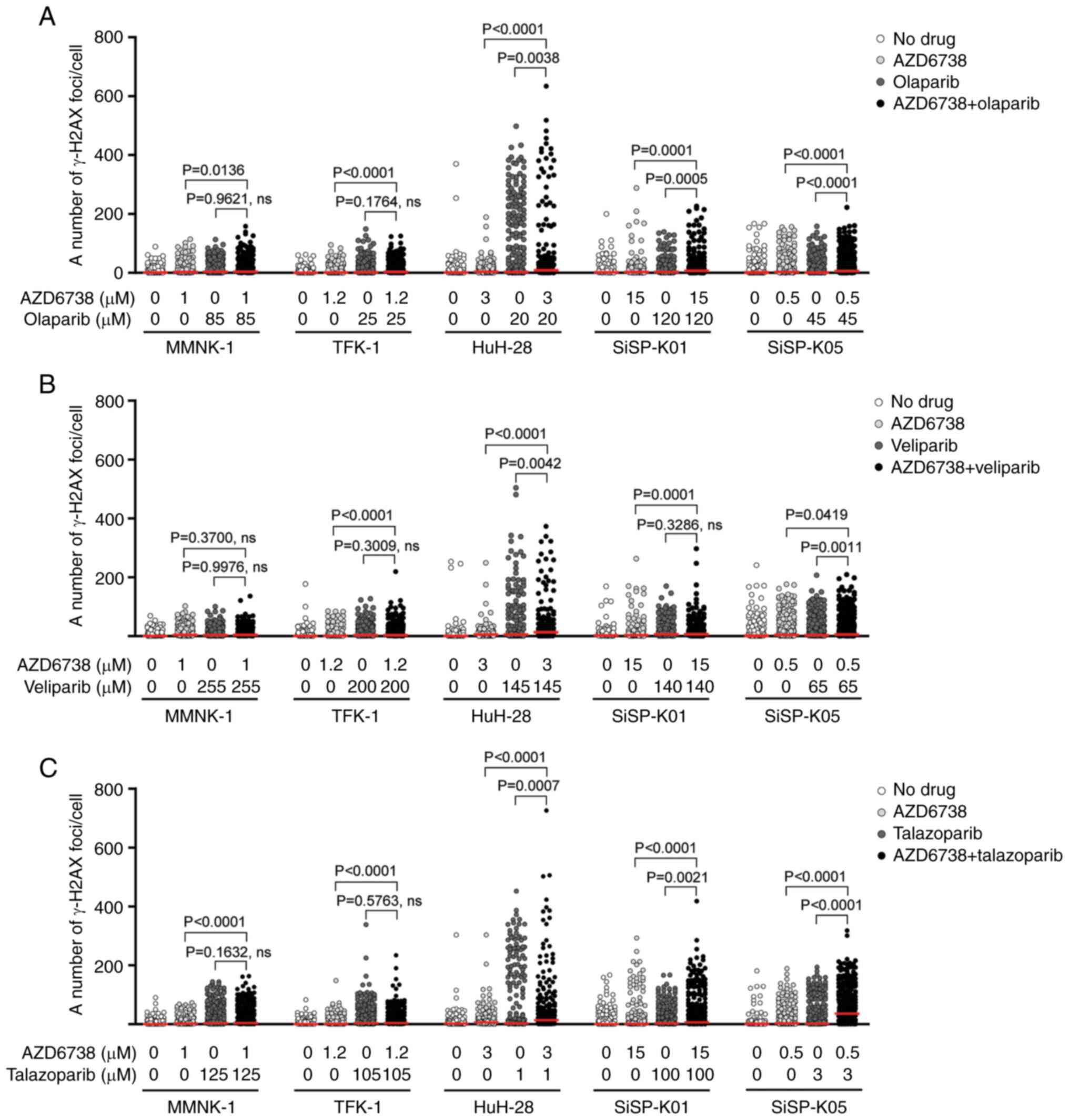
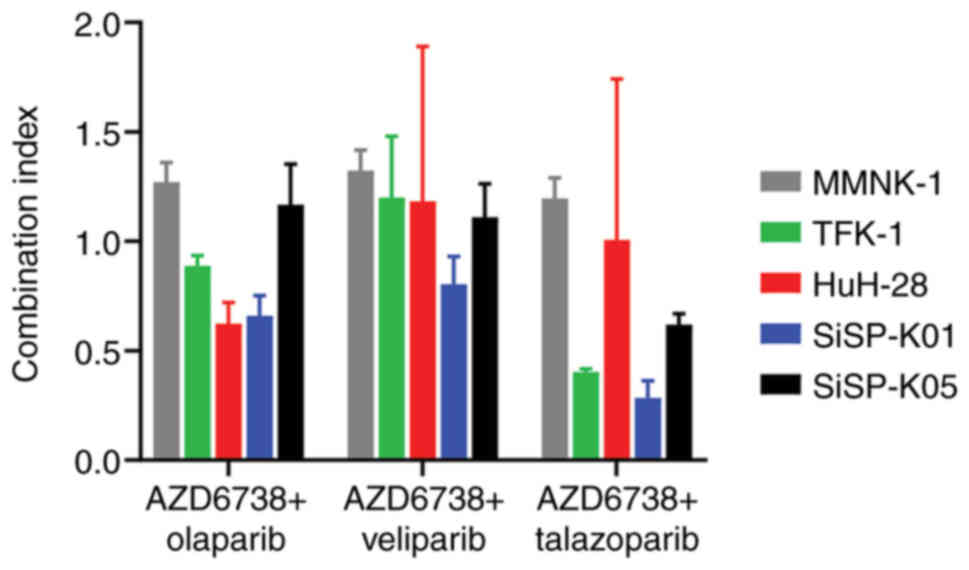
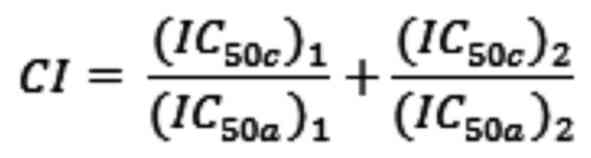|
1
|
Brindley PJ, Bachini M, Ilyas SI, Khan SA,
Loukas A, Sirica AE, The BT, Wongkham S and Gores GJ:
Cholangiocarcinoma. Nat Rev Dis Primers. 7:652021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohaegbulam KC, Koethe Y, Fung A, Mayo SC,
Grossberg AJ, Chen EY, Sharzehi K, Kardosh A, Farsad K, Rocha FG,
et al: The multidisciplinary management of cholangiocarcinoma.
Cancer. 129:184–214. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Squadroni M, Tondulli L, Gatta G, Mosconi
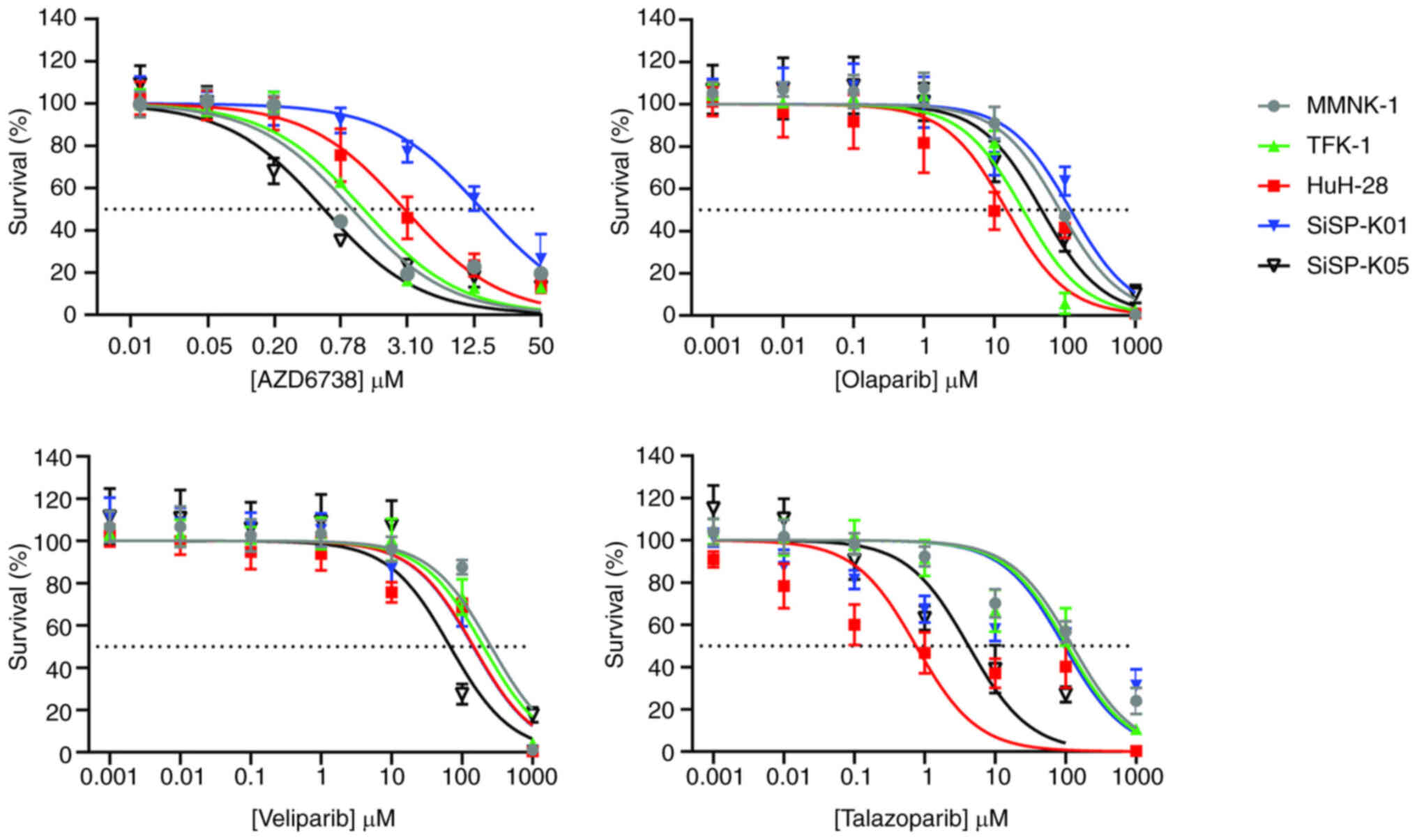
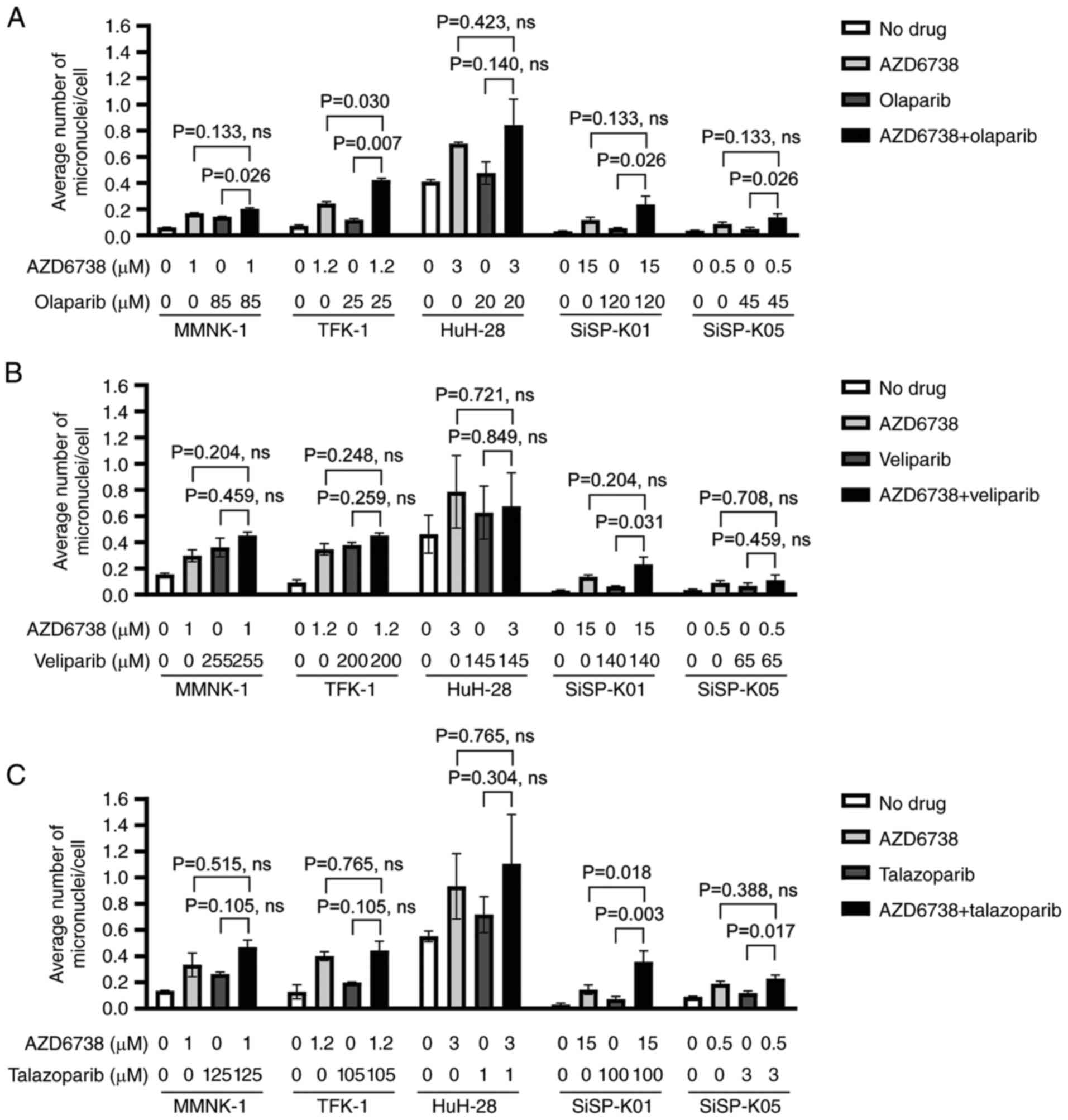
S, Beretta G and Labianca R: Cholangiocarcinoma. Crit Rev Oncol
Hematol. 116:11–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
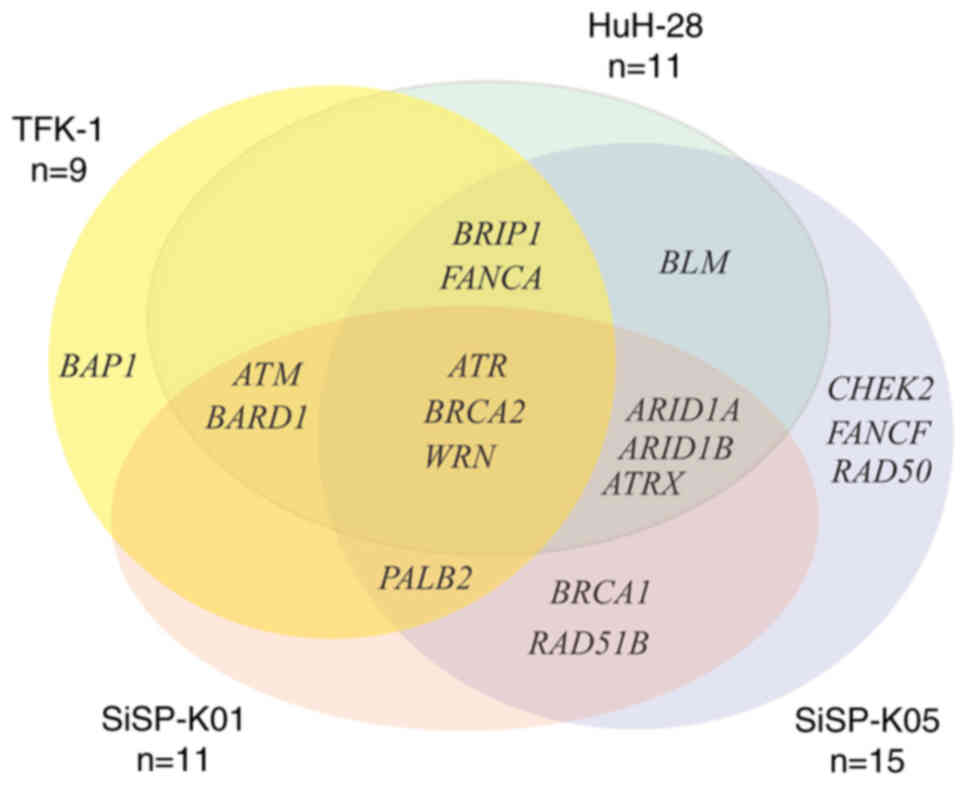
Gönül Geyik Ö, Anichini G, Ulukaya E,
Marra F and Raggi C: DNA damage response inhibitors in
cholangiocarcinoma: Current progress and perspectives. Cells.
11:14632022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chae H, Kim D, Yoo C, Kim KP, Jeong JH,
Chang HM, Lee SS, Park DH, Song TJ, Hwang S, et al: Therapeutic
relevance of targeted sequencing in management of patients with
advanced biliary tract cancer: DNA damage repair gene mutations as
a predictive biomarker. Eur J Cancer. 120:31–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn DH and Bekaii-Saab T: Biliary tract
cancer and genomic alterations in homologous recombinant
deficiency: Exploiting synthetic lethality with PARP inhibitors.
Chin Clin Oncol. 9:62020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beijersbergen RL, Wessels LFA and Bernards
R: Synthetic lethality in cancer therapeutics. Annu Rev Cancer
Biol. 1:141–161. 2017. View Article : Google Scholar
|
|
9
|
Pilié PG, Tang C, Mills GB and Yap TA:
State-of-the-art strategies for targeting the DNA damage response
in cancer. Nat Rev Clin Oncol. 16:81–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Andrea AD: Mechanisms of PARP inhibitor
sensitivity and resistance. DNA Repair (Amst). 71:172–176. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balasubramaniam S, Beaver JA, Horton S,
Fernandes LL, Tang S, Horne HN, Liu J, Liu C, Schrieber SJ, Yu J,
et al: FDA approval summary: rucaparib for the treatment of
patients with deleterious BRCA mutation-associated advanced ovarian
cancer. Clin Cancer Res. 23:7165–7170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoy SM: Talazoparib: First global
approval. Drugs. 78:1939–1946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li N, Bu H, Liu J, Zhu J, Zhou Q, Wang L,
Yin R, Wu X, Yao S, Gu K, et al: An open-label, multicenter,
single-arm, phase II study of fluzoparib in patients with germline
BRCA1/2 mutation and platinum-sensitive recurrent ovarian cancer.
Clin Cancer Res. 27:2452–2458. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Yin Y, Dong M, Song Y, Li W, Huang
X, Wang T, He J, Mu X, Li L, et al: Pamiparib dose escalation in
Chinese patients with non-mucinous high-grade ovarian cancer or
advanced triple-negative breast cancer. Cancer Med. 10:109–118.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
da Costa AABA, Chowdhury D, Shapiro GI,
D'Andrea AD and Konstantinopoulos PA: Targeting replication stress
in cancer therapy. Nat Rev Drug Discov. 22:38–58. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith G, Alholm Z, Coleman RL and Monk BJ:
DNA damage repair inhibitors-combination therapies. Cancer J.
27:501–505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mullard A: DNA damage response drugs for
cancer yield continued synthetic lethality learnings. Nat Rev Drug
Discov. 21:403–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shah PD, Wethington SL, Pagan C, Latif N,
Tanyi J, Martin LP, Morgan M, Burger RA, Haggerty A, Zarrin H, et
al: Combination ATR and PARP inhibitor (CAPRI): A phase 2 study of
ceralasertib plus olaparib in patients with recurrent,
platinum-resistant epithelial ovarian cancer. Gynecol Oncol.
163:246–253. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clinical Trials: NCT03330847: To assess
safety and efficacy of agents targeting DNA damage repair with
olaparib versus olaparib monotherapy. https://clinicaltrials.gov/show/NCT03330847June
4–2023
|
|
22
|
Clinical Trials: NCT03787680: Targeting
resistant prostate cancer with ATR and PARP inhibition (TRAP
Trial). https://clinicaltrials.gov/show/NCT03787680June
4–2023
|
|
23
|
Clinical Trials: NCT03878095: Testing
olaparib and AZD6738 in IDH1 and IDH2 mutant tumors. https://clinicaltrials.gov/show/NCT03878095June
4–2023
|
|
24
|
Clinical Trials: NCT04298021: DDR-umbrella
study of DDR targeting agents in advanced biliary tract cancer.
https://clinicaltrials.gov/study/NCT04298021November
13–2023
|
|
25
|
Serra-Camprubí Q, Verdaguer H, Oliveros W,
Lupión-Garcia N, Llop-Guevara A, Molina C, Vila-Casadesús M, Turpin
A, Neuzillet C, Frigola J, et al: Human metastatic
cholangiocarcinoma patient-derived xenografts and tumoroids for
preclinical drug evaluation. Clin Cancer Res. 29:432–445. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bezrookove V, Patino JM, Nosrati M,
Desprez PY, McAllister S, Soroceanu L, Baron A, Osorio R,
Kashani-Sabet M and Dar AA: Niraparib suppresses cholangiocarcinoma
tumor growth by inducing oxidative and replication stress. Cancers
(Basel). 13:44052021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moolmuang B and Ruchirawat M: The
antiproliferative effects of ataxia-telangiectasia mutated and ATM-
and Rad3-related inhibitions and their enhancements with the
cytotoxicity of DNA damaging agents in cholangiocarcinoma cells. J
Pharm Pharmacol. 73:40–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nam AR, Yoon J, Jin MH, Bang JH, Oh KS,
Seo HR, Kim JM, Kim TY and Oh DY: ATR inhibition amplifies
antitumor effects of olaparib in biliary tract cancer. Cancer Lett.
516:38–47. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maruyama M, Kobayashi N, Westerman KA,
Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A,
Stolz DB, et al: Establishment of a highly differentiated
immortalized human cholangiocyte cell line with SV40T and hTERT.
Transplantation. 77:446–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kusaka Y, Tokiwa T and Sato J:
Establishment and characterization of a cell line from a human
cholangiocellular carcinoma. Res Exp Med (Berl). 188:367–375. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saijyo S, Kudo T, Suzuki M, Katayose Y,
Shinoda M, Muto T, Fukuhara K, Suzuki T and Matsuno S:
Establishment of a new extrahepatic bile duct carcinoma cell line,
TFK-1. Tohoku J Exp Med. 177:61–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suntiparpluacha M, Chanwat R,
Limsrichamrern S, More-krong P, Srifa S, Kongsri K, Aroonpruksakul
S, Sathirareuangchai S, Sampatavanich S, Okada S and Jirawatnotai
S: Establishment of cholangiocarcinoma organoids from long-term
frozen tissues. J Basic Appl Pharmacol. 2:O11–O25. 2022.
|
|
33
|
Jamnongsong S, Kueanjinda P, Buraphat P,
Sakornsakolpat P, Vaeteewoottacharn K, Okada S, Jirawatnotai S and
Sampattavanich S: Comprehensive drug response profiling and
pan-omic analysis identified therapeutic candidates and prognostic
biomarkers for Asian cholangiocarcinoma. iScience. 25:1051822022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li MM, Datto M, Duncavage EJ, Kulkarni S,
Lindeman NI, Roy S, Tsimberidou AM, Vnencak-Jones CL, Wolff DJ,
Younes A and Nikiforova MN: Standards and guidelines for the
interpretation and reporting of sequence variants in cancer: A
joint consensus recommendation of the association for molecular
pathology, American society of clinical oncology, and college of
american pathologists. J Mol Diagn. 19:4–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jang SM, Redon CE, Fu H, Indig FE and
Aladjem MI: RepID-deficient cancer cells are sensitized to a drug
targeting p97/VCP segregase. Mol Cell Toxicol. 17:141–149. 2021.
View Article : Google Scholar
|
|
39
|
Wikiniyadhanee R, Lerksuthirat T,
Stitchantrakul W, Chitphuk S, Sura T and Dejsuphong D: TRIM29 is
required for efficient recruitment of 53BP1 in response to DNA
double-strand breaks in vertebrate cells. FEBS Open Bio.
10:2055–2071. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lerksuthirat T, Wikiniyadhanee R, Chitphuk
S, Stitchantrakul W, Sampattavanich S, Jirawatnotai S, Jumpathong J
and Dejsuphong D: DNA Repair Biosensor-Identified DNA Damage
Activities of Endophyte Extracts from Garcinia cowa.
Biomolecules. 10:16802020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Audeh MW, Carmichael J, Penson RT,
Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN,
Oaknin A, Loman N, et al: Oral poly(ADP-ribose) polymerase
inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and
recurrent ovarian cancer: A proof-of-concept trial. Lancet.
376:245–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martorana F, Da Silva LA, Sessa C and
Colombo I: Everything comes with a price: The toxicity profile of
DNA-damage response targeting agents. Cancers (Basel). 14:9532022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bradbury A, Hall S, Curtin N and Drew Y:
Targeting ATR as cancer therapy: A new era for synthetic lethality
and synergistic combinations? Pharmacol Ther. 207:1074502020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clinical Trials: NCT03462342: Combination
ATR and PARP inhibitor (CAPRI) trial with AZD6738 and olaparib in
recurrent ovarian cancer (CAPRI). https://clinicaltrials.gov/study/NCT03462342November
11–2023
|
|
45
|
Clinical Trials: NCT05269316: Study to
evaluate IMP9064 as a monotherapy or in combination in patients
with advanced solid tumors. https://www.clinicaltrials.gov/study/NCT05269316November
11–2023
|
|
46
|
Lecona E and Fernandez-Capetillo O:
Targeting ATR in cancer. Nat Rev Cancer. 18:586–595. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nam AR, Jin MH, Park JE, Bang JH, Oh DY
and Bang YJ: Therapeutic targeting of the DNA damage response using
an ATR inhibitor in biliary tract cancer. Cancer Res Treat.
51:1167–1179. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dorado Garcia H, Pusch F, Bei Y, von
Stebut J, Ibáñez G, Guillan K, Imami K, Gürgen D, Rolff J,
Helmsauer K, et al: Therapeutic targeting of ATR in alveolar
rhabdomyosarcoma. Nat Commun. 13:42972022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
King D, Southgate HED, Roetschke S,
Gravells P, Fields L, Watson JB, Chen L, Chapman D, Harrison D,
Yeomanson D, et al: Increased replication stress determines ATR
inhibitor sensitivity in neuroblastoma cells. Cancers (Basel).
13:62152021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhamidipati D, Haro-Silerio JI, Yap TA and
Ngoi N: PARP inhibitors: Enhancing efficacy through rational
combinations. Br J Cancer. 129:904–916. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yano K and Shiotani B: Emerging strategies
for cancer therapy by ATR inhibitors. Cancer Sci. 114:2709–2721.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sadeghi F, Asgari M, Matloubi M, Ranjbar
M, Karkhaneh Yousefi N, Azari T and Zaki-Dizaji M: Molecular
contribution of BRCA1 and BRCA2 to genome instability in breast
cancer patients: Review of radiosensitivity assays. Biol Proced
Online. 22:232020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sule A, Van Doorn J, Sundaram RK, Ganesa
S, Vasquez Juan C and Bindra Ranjit S: Targeting IDH1/2 mutant
cancers with combinations of ATR and PARP inhibitors. NAR Cancer.
3:zcab0182021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lloyd RL, Wijnhoven PWG, Ramos-Montoya A,
Wilson Z, Illuzzi G, Falenta K, Jones GN, James N, Chabbert CD,
Stott J, et al: Combined PARP and ATR inhibition potentiates genome
instability and cell death in ATM-deficient cancer cells. Oncogene.
39:4869–4883. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee EK and Matulonis UA: PARP inhibitor
resistance mechanisms and implications for post-progression
combination therapies. Cancers (Basel). 12:20542020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Onji H and Murai J: Reconsidering the
mechanisms of action of PARP inhibitors based on clinical outcomes.
Cancer Sci. 113:2943–2951. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Murai J, Huang SYN, Renaud A, Zhang Y, Ji
J, Takeda S, Morris J, Teicher B, Doroshow JH and Pommier Y:
Stereospecific PARP trapping by BMN 673 and comparison with
olaparib and rucaparib. Mol Cancer Ther. 13:433–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mosler T, Baymaz HI, Gräf JF, Mikicic I,
Blattner G, Bartlett E, Ostermaier M, Piccinno R, Yang J, Voigt A,
et al: PARP1 proximity proteomics reveals interaction partners at
stressed replication forks. Nucleic Acids Res. 50:11600–11618.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zamalloa LG, Pruitt MM, Hermance NM, Gali
H, Flynn RL and Manning AL: RB loss sensitizes cells to
replication-associated DNA damage after PARP inhibition by
trapping. Life Sci Alliance. 6:e2023020672023. View Article : Google Scholar : PubMed/NCBI
|