Introduction
Pulmonary artery intimal sarcoma (PAIS) is an
uncommon and extremely aggressive mesenchymal tumor originating
from the intimal layer of the pulmonary artery (1), with only a few hundred documented
cases in the medical literature to date since being initially
reported by Mandelstamm in 1923 (2). The early diagnosis of PAIS poses a
significant challenge, with the sarcoma often being misidentified
as a pulmonary thromboembolism (PTE) due to overlapping clinical
symptoms (such as dyspnea, chest pain and hemoptysis) and imaging
findings (such as pulmonary artery filling defect). This delayed
and inaccurate diagnosis impedes timely intervention for patients,
resulting in a poor prognosis and a marked shortening of overall
survival time. Surgical excision currently remains the primary
treatment strategy for PAIS. Early diagnosis and surgical resection
provide substantial benefits to patients, potentially prolonging
survival time (3). Furthermore,
postoperative adjuvant therapies have the potential to improve
clinical outcomes. Untreated PAIS results in a median survival time
of only 6 weeks (3); however,
advancements in surgical techniques and emerging treatment
approaches hold promise for extending median survival time.
Nevertheless, the overall long-term prognosis remains notably poor
(4). The present study aims to
deepen the clinical understanding of PAIS by presenting a unique
case report, thereby providing a valuable reference for the
diagnosis and treatment protocols of healthcare professionals and
ultimately optimizing patient outcomes.
Case report
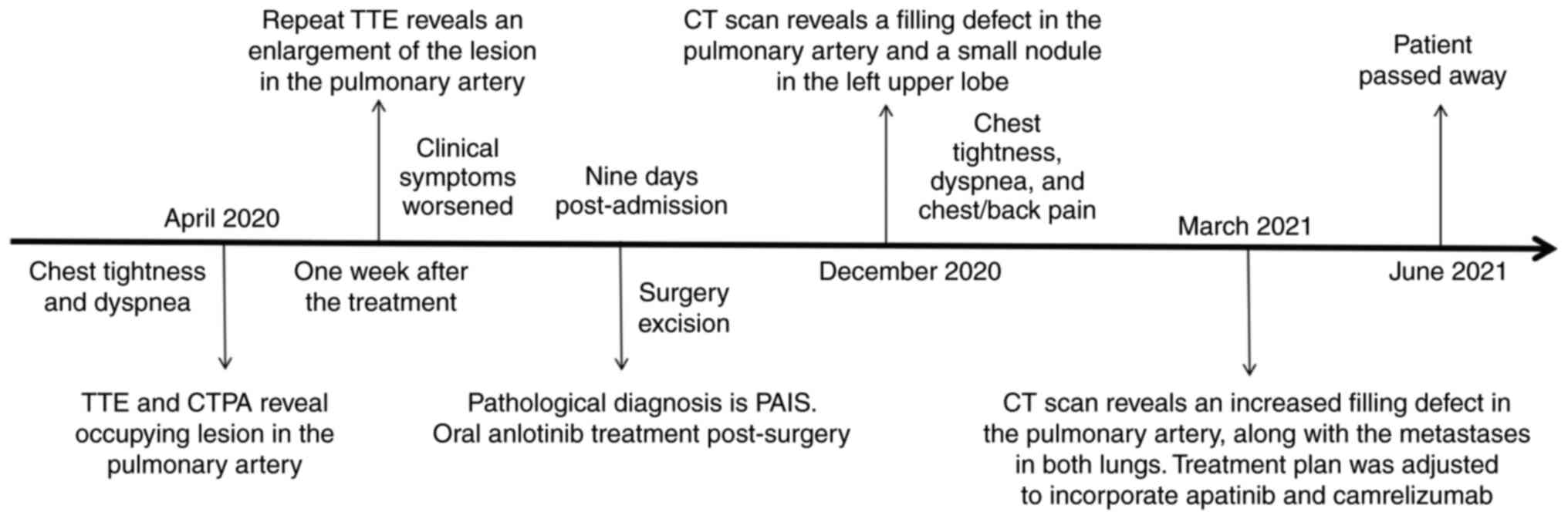
A 68-year-old female patient was admitted to The
Affiliated People's Hospital of Jiangsu University (Zhenjiang,
China) in April 2020, presenting with chief complaints of chest
tightness and dyspnea that had persisted for 4 days. The symptoms
worsened post-activity and did not completely alleviate after rest,
accompanied by palpitations, dizziness, fatigue and a poor
appetite. The patient had sustained a right wrist fracture along
with soft-tissue injuries to both lower extremities due to an
accident 6 days ago that had necessitated splinting of the right
wrist joint. Additionally, the patient had undergone a right-sided
mastectomy 10 years prior, but had no history of hypertension or
diabetes, nor any family history of hereditary diseases.
Upon the current admission, the patient presented
with a blood pressure of 113/81 mmHg (normal range, 90–139/60–89
mmHg), a heart rate of 104 beats per min (normal range, 60–100
beats per min) and a respiratory rate of 19 breaths per min (normal
range, 12–18 breaths per min). Oxygen saturation levels were
maintained at 98% (normal range, 95–100%) while breathing room air.
Physical examination revealed a grade 3 out of 6 sighing systolic
murmur over the pulmonary valve area along the left sternal border.
Laboratory tests showed elevated D-dimer levels at 4.7 mg/l
(reference range, 0.1–0.55 mg/l), and the level of CA125 was 32.1
U/ml (reference range, 0–35 U/ml), while other parameters were
within the normal ranges.
Venous ultrasound indicated no signs of thrombosis
in the limbs. The electrocardiogram displayed sinus tachycardia,
atrial premature contractions and ST-T wave abnormalities.
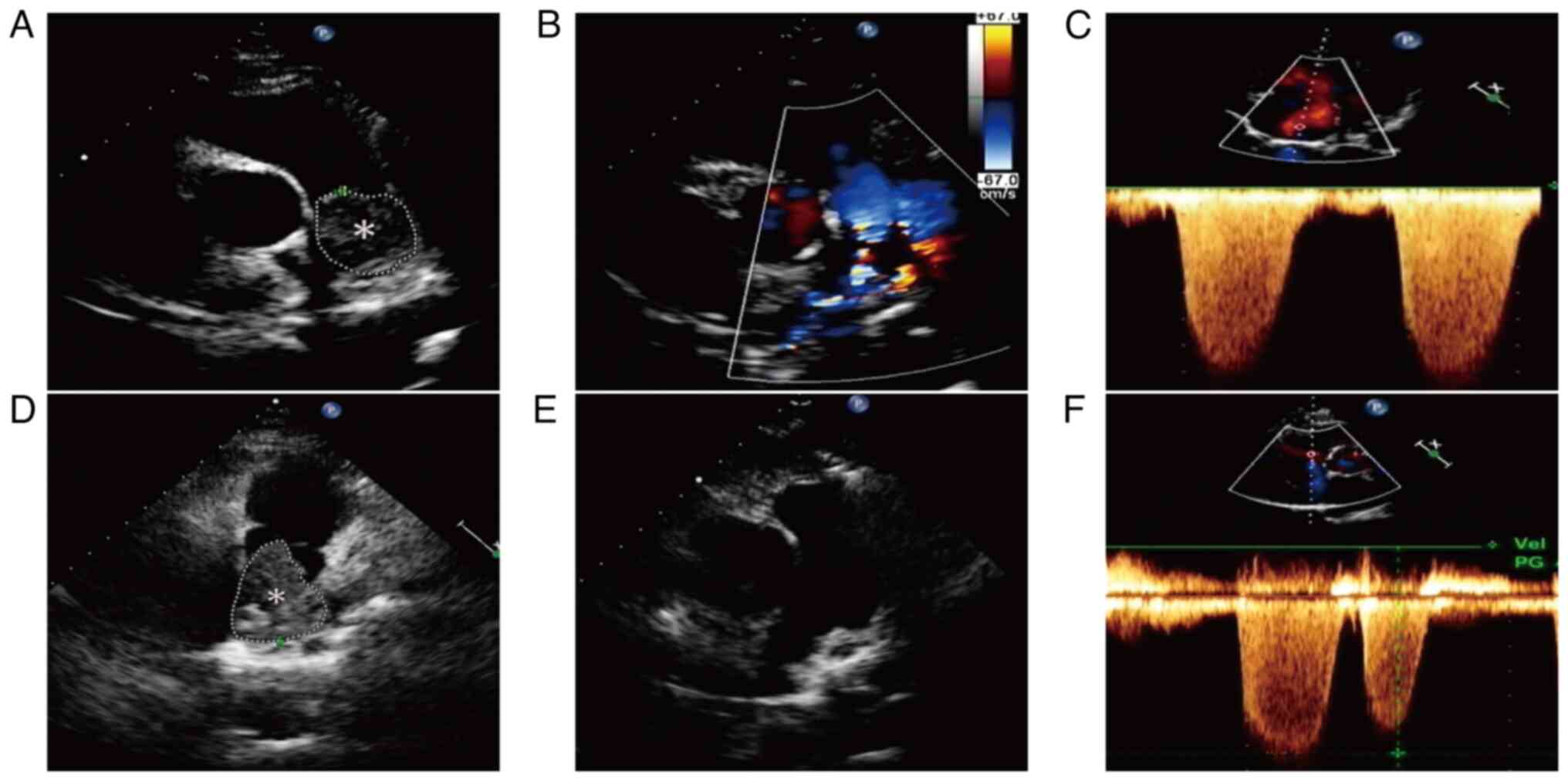
Transthoracic echocardiography (TTE) detected right ventricular
enlargement and an isoechoic mass measuring ~8.79 cm2
within the main pulmonary artery (Fig.
1A), which exhibited well-defined margins and a fixed position
without evidence of blood filling (Fig.
1B). Additionally, TTE imaging demonstrated severe pulmonary
hypertension (mean pulmonary artery pressure, 94 mmHg; normal
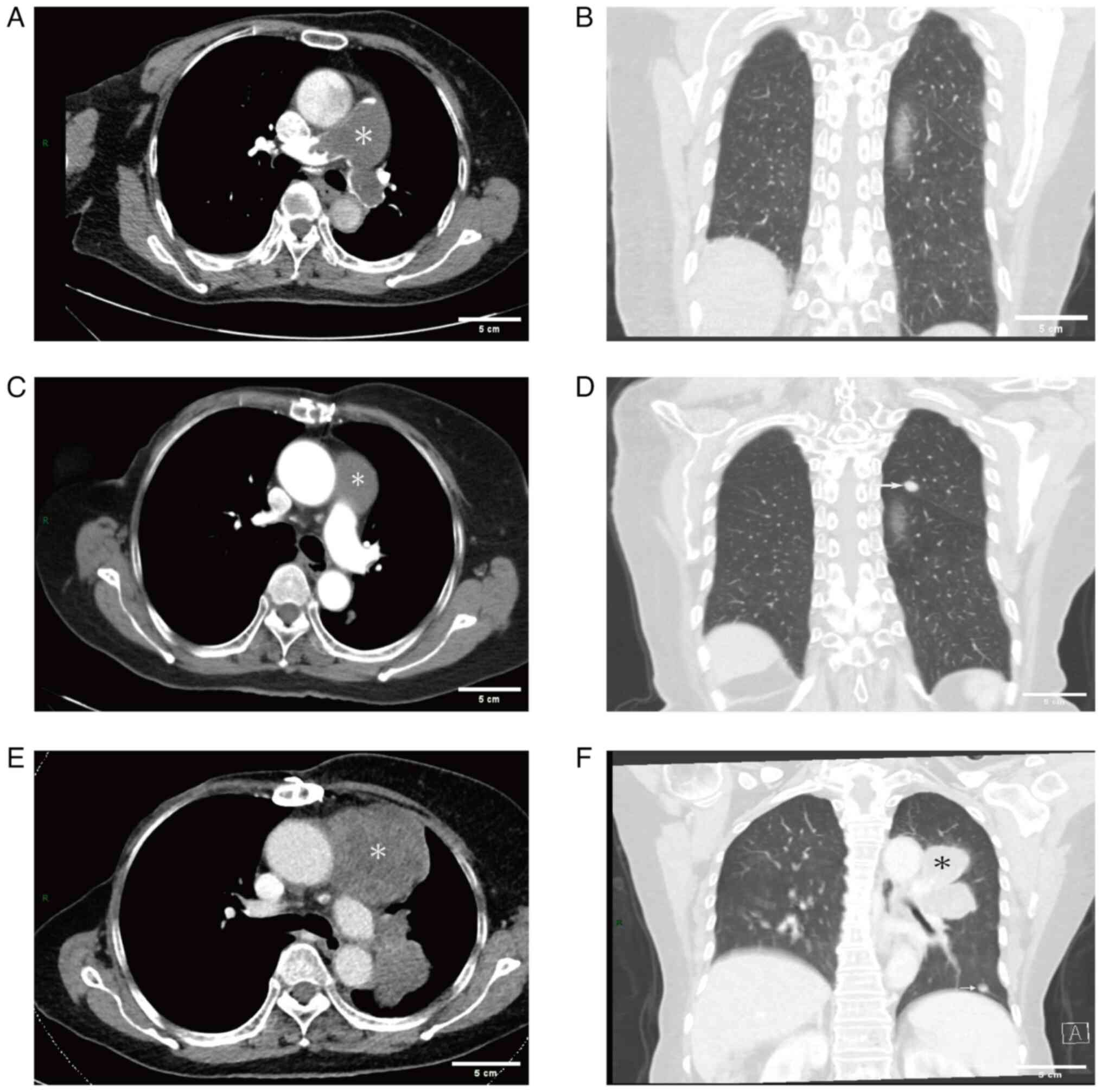
range, 18–25 mmHg) and marked tricuspid regurgitation (Fig. 1C). Computed tomography pulmonary
angiography (CTPA) was performed to further investigate the
suspicion of a PTE. The imaging revealed regions of decreased
density in the main pulmonary artery, the proximal segment of the
right pulmonary artery, the left pulmonary artery, the left upper
lung and branches of the left lower pulmonary artery (Fig. 2A), with no nodules in the lungs
(Fig. 2B). Furthermore, localized
enlargement measuring a diameter of ~37 mm was noted in the main
pulmonary artery. Minimal fluid accumulation was also identified in
both the pericardium and right pleural cavity. The initial
diagnosis was PTE, for which the patient received subcutaneous
injections of low molecular weight heparin (4,000 IU every 12 h)
and oral warfarin (2.5 mg per day) anticoagulation therapy.
However, despite a week of treatment, the patient's clinical
symptoms gradually worsened. Subsequent repeat TTE was performed to
assess the efficacy of anticoagulant therapy, revealing an increase
in the size of the isoechoic mass within the main pulmonary artery
(~11.4 cm2) (Fig. 1D).
The patient was promptly transferred to the Department of
Cardiothoracic Surgery for further intervention. Under general
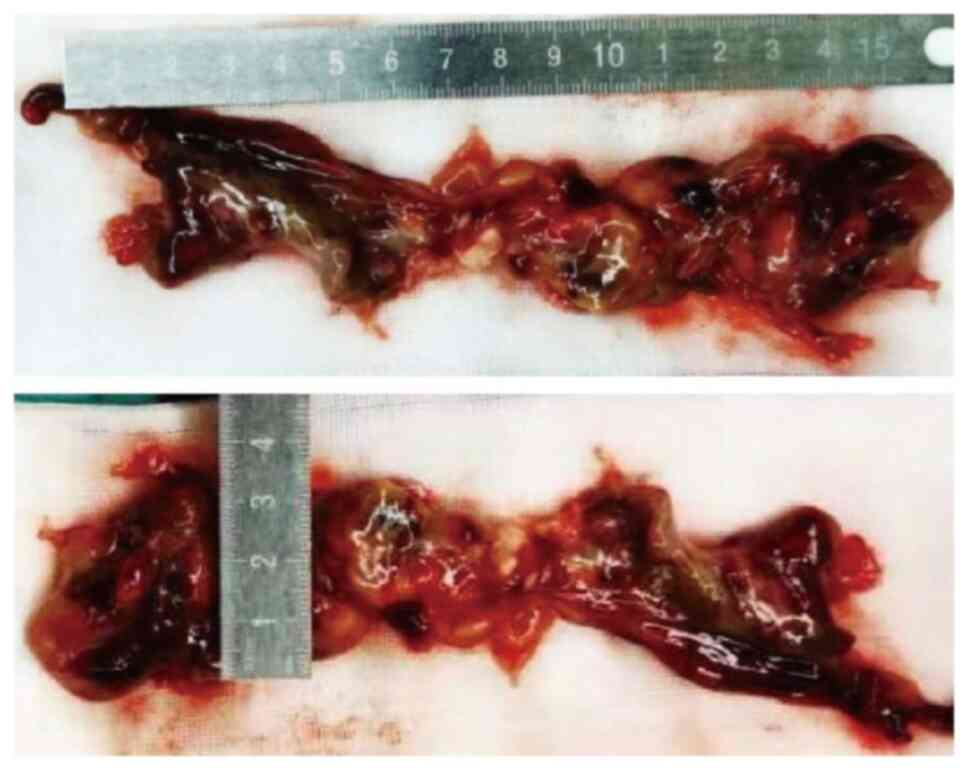
anesthesia and cardiopulmonary bypass, pulmonary endarterectomy was
conducted at 9 days post-admission to alleviate the deteriorating
clinical symptoms, revealing a 15×5-cm mass in the main pulmonary
artery (Fig. 3), closely adherent
to its intima and extending throughout both the left and right
pulmonary arteries. The mass was successfully excised, with
intraoperative transesophageal echocardiography confirming the
absence of residual masses in the main pulmonary artery and no
marked tricuspid valve regurgitation. The resected specimens were
fixed in 10% formalin at room temperature for 24 h for
histopathological examination. Histopathological staining and
immunohistochemistry were performed using 4-µm thick
paraffin-embedded sections, and the specific protocol was carried
out in accordance with the method described by Neuville et
al (5). The BOND-MAX fully
automatic IHC and ISH staining system (Leica Microsystems) was used
for immunohistochemical staining. Slides were inspected under a
light microscope (Olympus BX51TF; Olympus Corporation). Hematoxylin
and eosin staining revealed that the tumor cells exhibited an
epithelioid and short fusiform morphology, with marked
heterogeneity in cell density. Tumor cells were preferentially
aggregated around blood vessels, accompanied by desmoplastic
mucinous degeneration and localized hemorrhage. Notably, the tumor
cells displayed pronounced atypia, with identification of
pathological mitotic figures (Fig.
4A). Immunohistochemical examination revealed positive vimentin
(cat. no. RTU-VIM-V9-QH; AQ Medical Technology) and Ki-67 (40%)
(antibody dilution, 1:150; cat. no. NCL-L-Ki67-MIB1; AQ Medical
Technology) staining, but negative staining for anaplastic lymphoma
kinase (ALK; cat. no. GT226602; Gene Tech, Co., Ltd.), CD34 (cat.
no. RTU-END-QH; AQ Medical Technology), CD117 (cat. no.
RTU-CD117-QH; AQ Medical Technology), CD163 (cat. no. RTU-CD163-QH;
AQ Medical Technology), cytokeratin (CK; antibody dilution, 1:200;
cat. no. NCL-L-AE1/AE3-601; AQ Medical Technology),
erythroblastosis transformation specific-1 related gene (ERG-1;
cat. no. RTU-ERG-QH; AQ Medical Technology), p53 (antibody
dilution, 1:100; cat. no. NCL-L-P53-DO7; AQ Medical Technology),
S100 (cat. no. RTU-S100-QH; AQ Medical Technology) and smooth
muscle actin (SMA; cat. no. RTU-SMA-QH; AQ Medical Technology)
(Fig. 4B-L). The final diagnosis of
PAIS was confirmed based on the results of the pathological
examination and the specific tumor localization (6,7).
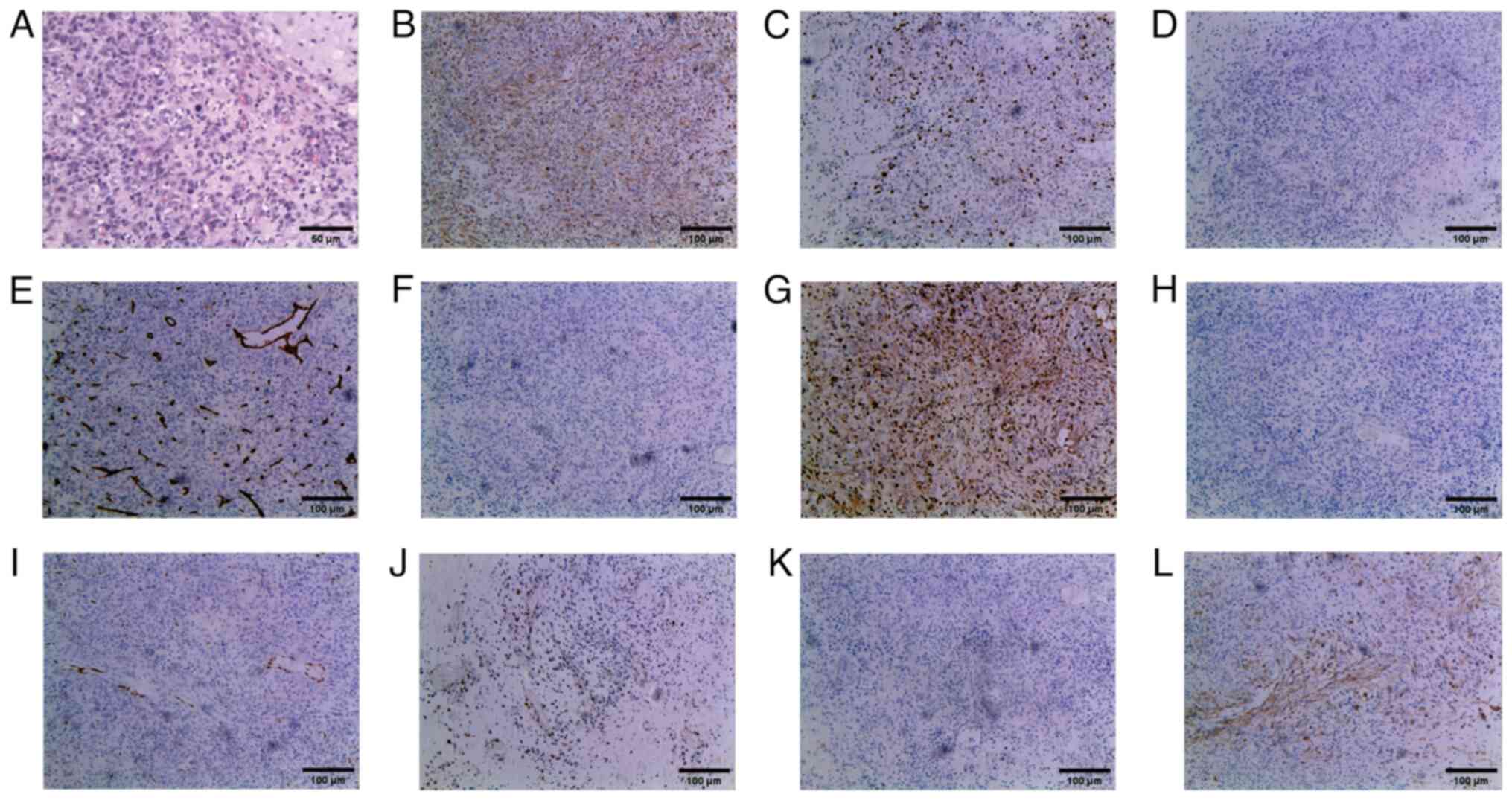 | Figure 4.Microscopy examination of the
pulmonary artery intimal sarcoma specimen. (A) Postoperative
hematoxylin and eosin staining revealed that the tumor cells
exhibited an epithelioid and short fusiform morphology, with
mucinous degeneration and local hemorrhage. These cells displayed
pronounced atypia, with identification of pathological mitotic
figures (original magnification, ×200). The immunohistochemical
staining of (B) vimentin (positive), (C) Ki-67 (40% positive), (D)
anaplastic lymphoma kinase (negative), (E) CD34 (negative), (F)
CD117 (negative), (G) CD163 (negative), (H) cytokeratin (negative),
(I) erythroblastosis transformation specific-1 related gene
(negative), (J) p53 (negative), (K) S100 (negative) and (L) smooth
muscle actin (negative) (magnification, ×100). |
Following surgery, the patient experienced a
satisfactory recovery with notable improvement in clinical
symptoms. A postoperative TTE revealed no abnormal mass in the
pulmonary artery and a marked decrease in pulmonary artery pressure
(34 mmHg) compared with preoperative levels (Fig. 1E and F). Despite the recommendation
for a positron emission tomography-CT (PET-CT) scan, the patient
declined and was discharged in May 2020. Subsequently, the patient
opted to undergo treatment with oral anlotinib (12 mg on days 1–14)
according to the 2019 Chinese Society of Clinical Oncology
treatment guidelines for soft-tissue sarcoma (8) and due to the demonstrated significant
efficacy of anlotinib in treating soft-tissue sarcoma in multiple
clinical trials (9). In December
2020, the patient reported chest tightness, dyspnea and chest/back
pain. An enhanced CT scan of the chest performed on 3 days later
revealed a low-density filling defect in the main pulmonary artery
along with a 10-mm nodule in the left upper lobe initially
suspected to be metastatic lesions (Fig. 2C and D). The patient opted to
continue oral anlotinib treatment while remaining hesitant to
modify the treatment regimen. A subsequent enhanced CT scan of
chest and abdomen performed in March 2021 showed substantial
enlargement of the low-density lesions within the main pulmonary
artery (~68×67 mm), as well as multiple fresh soft-tissue masses in
the left lung; however, no abnormalities were observed within the
abdominal region (Fig. 2E and F).
Concurrently, the levels of tumor marker CA125 were increased to
66.9 U/ml (reference value, 0–35 U/ml) and exhibited a progressive
upward trend as the disease progressed. By April 2021, the level of
this marker had peaked at 267 U/ml.
Despite receiving combined treatment with apatinib
(250 mg orally per day) and camrelizumab (200 mg intravenously,
twice), no marked improvement in the patient's condition was
observed. Subsequently, the patient's condition continued to
deteriorate, necessitating palliative care. The patient passed away
in June 2021. The procedures for specific diagnosis and treatment
are elaborated in Fig. 5.
Discussion
PAIS is a rare neoplasm characterized by
intraluminal growth resulting in vascular occlusion or embolization
to distant sites (6,10). PAIS originates from multipotent
mesenchymal stem cells within the intimal layer of the pulmonary
artery (7), which can exhibit
complete undifferentiated or contain heterologous components such
as rhabdomyosarcoma and chondrosarcoma (11). The reported occurrence rates range
from 0.001 to 0.03% (1,12). The age of onset for the initial
symptoms varies widely among individuals, spanning from infancy to
late adulthood, with a mean age of onset around 48 years old
(6). The prevalence of illness in
women is slightly higher than that in men, with a ratio of 1.3:1
(6).
The clinical presentation of PAIS lacks specificity
and is closely linked to the location and extent of tumor invasion.
In the early stages, patients may be asymptomatic; however, as the
tumor obstructs blood vessels, it can lead to a spectrum of
respiratory issues, including dyspnea, chest or back pain, and
hemoptysis. Additionally, non-specific symptoms, including
palpitations, fatigue, fever and weight loss, may also contribute
to the diagnosis of PAIS (13).
The clinical signs and imaging features of PAIS
closely resemble those of PTE, usually leading to the misdiagnosis
as PTE, which poses a significant challenge for early diagnosis and
prompt, effective intervention (14,15).
Radiological examination technologies, such as TTE, CTPA, magnetic
resonance imaging (MRI) and PET-CT, can considerably aid in
distinguishing between the two conditions (6).
TTE has been validated as a reliable modality for
identifying pulmonary artery lesions, enabling visualization of
their location, size, shape and activity. Additionally, it can
detect blood flow signals within the mass and aid in assessing the
impact of these lesions on pulmonary artery hemodynamics, as well
as cardiac structure and function (16). In echocardiographic images, PAIS
typically presents as areas of moderate to low echogenicity, with
irregularity and potential activity; it primarily affects the main
pulmonary artery, with rare involvement of the right ventricular
outflow tract and pulmonary valve (10). Color Doppler imaging can reveal
discernible blood flow signals within these abnormal areas, while
contrast-enhanced echocardiography can provide a more detailed
display of neovascularization within the mass (7). By contrast, thrombi generally appear
as fixed avascular homogeneous hypoechoic lesions, without
involving the right ventricular outflow tract or pulmonary valve
(16). In the present case, an
isoechoic mass was present in the main pulmonary artery, rather
than a hypoechoic mass, which indicated a sarcoma, which aligns
with the finding of Bai and Ruan (7).
CTPA is currently the predominant imaging modality
for diagnosing pulmonary artery diseases. Previous research has
indicated that CTPA can differentiate between PAIS and PTE based on
their location and morphological characteristics. PAIS usually
affects two or more pulmonary arteries, with the main pulmonary
artery trunk, and the left or right pulmonary artery being the most
common sites of involvement. In rare cases, it may also extend in a
retrograde manner to the right ventricle and pulmonary valve
(1). These lesions appear as
cauliflower-like polyps with bulging or lobulating contours,
showing heterogeneous attenuation on imaging, accompanied by the
‘wall eclipse sign’ and intratumoral vessels, along with localized
aneurysm dilatation of the affected vessels (17–19).
However, PTE primarily affects the right or both lower lungs and
rarely involves the main pulmonary artery. PTE presents as a
tubular-polypoid shape with straight proximal edges, homogeneous
attenuation on imaging, but without the ‘wall eclipse sign’, and
intratumoral vessels. In the present case, multiple filling defects
were observed in the main pulmonary artery and in both left and
right pulmonary arteries, along with dilatation of the affected
arteries, indicative of sarcoma, which is consistent with the
previous literature (18). However,
no heterogeneous attenuation or ‘wall eclipse sign’ characteristics
of the lesion were observed on the CTPA, which could potentially
result in a misdiagnosis of PTE.
MRI provides excellent soft-tissue contrast and can
effectively distinguish between tumors and thrombi due to its
unique signal characteristics. In MRI, PAIS demonstrates restricted
diffusion and heterogeneous enhancement, a condition not previously
observed in PTE (20).
Additionally, MRI can provide superior diagnostic information in
specific sequences (fat-suppressed T2-weighted imaging) without the
need for iodinated contrast agents, reducing potential risks such
as allergies and contrast-induced nephropathy for patients.
However, thoracic MRI is more time-consuming compared with CT, and
may be susceptible to respiratory and motion artifacts (21).
PET-CT is crucial for accurately distinguishing PAIS
from PTE and serves as an invaluable tool for assessing potential
systemic metastasis. PET-CT imaging reveals that a sarcoma exhibits
high positive uptake of fluorine-18-fluorodeoxyglucose (18F-FDG),
whereas a thrombus demonstrates no uptake (19). The study by Ren et al
(22) demonstrated that the maximum
standardized uptake value of PAIS (median, 8.0; range, 3.0–17.2)
was significantly elevated compared with that of PTE (median, 1.8;
range, 0.8–3.7; P<0.001). When a cut-off value of 2.9 was
applied (identified using the Youden index), the sensitivity and
specificity were recorded at 100.0 and 93.9%, respectively
(22). This finding aligns with
those from Ito et al (23)
and Xi et al (24),
indicating that the maximum standardized uptake value of PET-CT can
serve as a critical guiding parameter for differentiating PAIS from
PTE in clinical settings. However, it is essential to acknowledge
that a previous study has reported lower uptake of 18F-FDG in PAIS
cases (25). This may be related to
the presence of sarcoma-intermingled thrombi (25). Consequently, it must be recognized
in clinical practice that relying solely on low 18F-FDG uptake
should not be the only determining factor for excluding PAIS.
The final diagnosis of PAIS relies on the
pathological findings (6,7). Current primary biopsy methods include
percutaneous intravascular aspiration, intravascular
ultrasound-guided needle aspiration biopsy, percutaneous
intravascular biopsy and surgical biopsy. Percutaneous
intravascular aspiration has a certain failure rate due to its
inconsistent ability to obtain sufficient tumor tissue (26). Intrabronchial ultrasound-guided
needle aspiration biopsy enables real-time guidance and
visualization of pulmonary artery flow and artery stenosis;
however, the obtained tissue is also subject to limitations, such
as insufficient core tissue (27).
Percutaneous intravascular biopsy can be repeated to obtain
multiple tissue samples, making it a relatively ideal method for
biopsies. However, potential complications such as pulmonary artery
perforation, bleeding and tumor dissemination still exist (28).
The prognosis for patients diagnosed with PAIS is
typically fairly unfavorable. In one study, those who did not
undergo surgical intervention had a median survival duration of
only 6 weeks, attributable to the rapid progression of the
condition. By contrast, patients who underwent surgical resection
exhibited a survival spectrum ranging from 8 to 36 months, with a
median postoperative survival period of ~10 months (29). Surgically excising the tumor not
only relieves the clinical symptoms caused by vascular blockage,
but also markedly prolongs the overall survival of the patients.
Radical surgery remains the preferred therapeutic approach for PAIS
due to its exceptional efficacy. However, early detection is
challenging due to its subtle presentation and lack of obvious
symptoms or characteristic indicators (3,6). Some
patients diagnosed with metastasis may miss the opportunity for
surgical intervention. Therefore, accurate and timely
identification of patients with PAIS who are suitable for surgery
is crucial for improving their prognosis. Studies have indicated
that utilizing a combination of multiple treatment modalities, such
as surgery, radiotherapy and chemotherapy, may yield superior
survival outcomes compared with the use of a single treatment
modality, particularly in cases where the disease is inoperable or
relapse occurs after initial surgical intervention (3,30). In
recent years, the use of molecular targeted therapies has emerged
as a promising strategy for managing PAIS and has demonstrated
favorable clinical outcomes. Sanada et al (31) conducted experiments using cell lines
and xenograft models to investigate PAIS, and observed an
upregulation of specific tyrosine kinase receptor expression in
PAIS cells. The specific tyrosine kinase receptor inhibitor
pazopanib displayed marked effectiveness in inhibiting the growth
of PAIS cells and xenograft tumors, consistent with the findings by
Funatsu et al (32). While
Wu et al (33) suggested
potential therapeutic effects of anlotinib for PAIS with lung
metastasis, the present case study revealed that postoperative
application of anlotinib did not yield the anticipated results.
Therefore, further clinical trials are warranted to evaluate the
precise impact of molecular targeted drugs in treating PAIS.
In the current case, the patient presented with
symptoms of chest tightness and dyspnea, along with a history of
prior upper limb fracture fixation preceding the onset of the
condition. A slight elevation in D-dimer levels was observed, and
evidence of pulmonary artery obstruction was detected by both TTE
and CTPA, indicating a preliminary diagnosis of PTE. Despite
undergoing anticoagulation therapy, the therapeutic response was
unsatisfactory. Subsequently, surgical biopsy disclosed the
presence of PAIS. Upon re-evaluation of the case, it was noted that
the patient presented with non-specific symptoms, including low
tolerance and fatigue. The increment in D-dimer was not
substantial, and limb ultrasound examination did not reveal any
signs of thrombosis. TTE demonstrated moderate echogenicity within
the pulmonary artery rather than the expected low echogenicity. The
presence of multiple filling defects on CTPA, coupled with a lack
of imaging improvement despite anticoagulant therapy, raised the
suspicion of PAIS. However, due to the rarity of PAIS in clinical
practice and the non-specific nature of its hidden symptoms,
diagnosis frequently hinges on histopathological tissue biopsy,
resulting in a delayed diagnosis. The MRI examination could not be
completed due to the patient's dyspnea. An immediate surgical
resection was performed after the intraoperative diagnosis,
followed by adjunctive anlotinib targeted therapy. Despite these
interventions, the therapeutic response was unsatisfactory and led
to tumor recurrence and local metastasis, ultimately resulting in
the patient's death. It is essential to note that a gradual
increase in the patient's CA125 level was observed as the disease
progressed. Currently, there is a paucity of literature
investigating the efficacy of tumor markers in diagnosing PAIS.
Further comprehensive research is imperative to ascertain whether
CA125 can be employed as a potential biomarker for PAIS.
The present case report has some limitations.
Firstly, previous literature has reported that PAIS may exhibit
amplification of murine double minute 2 (MDM2) and platelet-derived
growth factor receptor α (PDGFRA) genes, implying potential
efficacy of targeted therapy against MDM2 or PDGFRA (34,35).
However, cytogenetic testing was not performed in the present case.
Secondly, the therapeutic effect and mechanism of action of
anlotinib in PAIS remain unclear and require further investigative
clinical trials, despite the substantial clinical efficacy of
anlotinib in the treatment of advanced soft-tissue sarcoma, as
supported by a number of clinical trials (9,36,37).
In conclusion, PAIS is a rare malignant neoplasm
that affects the pulmonary vascular intima, leading to
physiological changes and clinical symptoms similar to those
observed in PTE. Due to the relatively low prevalence of PAIS,
healthcare professionals may possess limited knowledge and
experience in managing this specific medical condition, increasing
the risk of misdiagnosis. In scenarios where clinical symptoms
resemble PTE and imaging shows a blockage in the pulmonary artery,
with normal or only slightly elevated D-dimer levels, but where
patients do not respond well to anticoagulant therapy, alternative
non-thrombotic diseases should be considered. A comprehensive
analysis of the patient's medical history and imaging data is
essential at this point, with particular attention given to
evaluating the chest CT pulmonary window. MRI and PET-CT can serve
as second-line imaging methods for differential diagnosis. If new
abnormalities are detected, a timely pathological biopsy should be
performed for a definitive diagnosis. This helps effectively reduce
the likelihood of misdiagnosis and facilitate prompt diagnostic
accuracy.
Acknowledgements
Not applicable.
Funding
This study was supported by funding from the the Research
Project of Jiangsu University Affiliated People's Hospital (grant
nos. YP2023005 and BJRH-2024-3) and the Project of Zhenjiang City
Social Development (grant no. SH2023046).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JG and RQ conceptualized the study. YH and YW
acquired and analyzed the data, and wrote reviewed and edited the
manuscript. BK, FZ, YD and BH obtained medical images (such as TTE
and CT scans) and analyzed patient data. BK, FZ, YD and BH confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated People's Hospital of Jiangsu University (Zhenjiang,
China; approval no. K-2022003-W). Written informed consent for
participation was obtained from the patient.
Patient consent for publication
Written informed consent for publication of this
case report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PAIS
|
pulmonary artery intimal sarcoma
|
|
PTE
|
pulmonary thromboembolism
|
|
TTE
|
transthoracic echocardiography
|
|
CTPA
|
computed tomography pulmonary
angiography
|
|
MRI
|
magnetic resonance imaging
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
18F-FDG
|
fluorine-18-fluorodeoxyglucose
|
References
|
1
|
Burke AP and Virmani R: Sarcomas of the
great vessels. A clinicopathologic study. Cancer. 71:1761–1773.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandelstamm M: On primary neoplasms of the
heart. Virchows Arch Pathol Anat. 245:43–54. 1923.(In German).
View Article : Google Scholar
|
|
3
|
Blackmon SH, Rice DC, Correa AM, Mehran R,
Putnam JB, Smythe WR, Walkes JC, Walsh GL, Moran C, Singh H, et al:
Management of primary pulmonary artery sarcomas. Ann Thorac Surg.
87:977–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Liu X, Li R and Zhang W: Primary
pulmonary artery tumors easily misdiagnosed as pulmonary embolism:
A review. Medicine (Baltimore). 102:e333372023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neuville A, Collin F, Bruneval P, Parrens
M, Thivolet F, Gomez-Brouchet A, Terrier P, de Montpreville VT, Le
Gall F, Hostein I, et al: Intimal sarcoma is the most frequent
primary cardiac sarcoma: Clinicopathologic and molecular
retrospective analysis of 100 primary cardiac sarcomas. Am J Surg
Pathol. 38:461–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Assi T, Kattan J, Rassy E, Moussa T,
Nassereddine H, Honore C, Adam J, Terrier P, Dumont S, Mir O and Le
Cesne A: A comprehensive review on the diagnosis and management of
intimal sarcoma of the pulmonary artery. Crit Rev Oncol Hematol.
147:1028892020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai X and Ruan L: A case report of primary
pulmonary artery intimal sarcoma. Eur J Med Res. 26:892021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niu X WJX, Yu S, Zhang X, Huang Z, Cai J,
Cai Z, Chen J and Cheng X: Chinese society of clinical oncology
(CSCO) guidelines for diagnosis and treatment of soft tissue
sarcoma. 1st edition. People's Medical Publishing House; Beijing:
pp. 1082019, (nN Chinese). PubMed/NCBI
|
|
9
|
Gao Y, Liu P and Shi R: Anlotinib as a
molecular targeted therapy for tumors. Oncol Lett. 20:1001–1014.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah DK, Joyce LD, Grogan M, Aubry MC,
Miller JA, Ding W and Haddock MG: Recurrent pulmonary intimal
sarcoma involving the right ventricular outflow tract. Ann Thorac
Surg. 91:e41–e42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Zhang Y, Liu M, Tao X, Xie W, Wan
J and Zhai Z: Radiological, histopathological findings, and
clinical outcome of pulmonary artery sarcoma. Pulm Circ.
11:20458940209405372021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mussot S, Ghigna MR, Mercier O, Fabre D,
Fadel E, Le Cesne A, Simonneau G and Dartevelle P: Retrospective
institutional study of 31 patients treated for pulmonary artery
sarcoma. Eur J Cardiothorac Surg. 43:787–793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Liu L, Song LX, Zhang YH, Liu Y, Gu
S, Wang JF, Huang Q, Ma ZH, Guo XJ, et al: Clinical features and
outcomes of pulmonary artery sarcoma. Heart Lung Circ. 31:230–238.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rijal R, Mridha AR, Arava SK and Behera C:
Primary intimal sarcoma of the pulmonary artery misdiagnosed as
pulmonary thromboembolism: A case confirmed at medicolegal autopsy.
J Forensic Sci. 66:403–406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srivali N, Yi ES and Ryu JH: Pulmonary
artery sarcoma mimicking pulmonary embolism: A case series. QJM.
110:283–286. 2017.PubMed/NCBI
|
|
16
|
Jiang W, Liu M, Guo X, Li J, Gong J, Yang
M, Liu Y, Gu S, Li Y, Yang Y and Lv X: Echocardiographic
characteristics of pulmonary artery intimal sarcoma: Comparison
with CTPA. Heart Lung Circ. 32:1080–1088. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pu X, Song M, Huang X, Zhu G, Chen D, Gan
H and Huang L: Clinical and radiological features of pulmonary
artery sarcoma: A report of nine cases. Clin Respir J.
12:1820–1829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo R, Yang H, Xi L, Liu A, Deng M, Liu H,
Gao Q, Xie W, Zhen Y, Huang Z and Liu M: Comparison of pulmonary
artery sarcoma and pulmonary thromboembolism according to clinical
and computed tomography pulmonary angiography and magnetic
resonance imaging characteristics: A single-center retrospective
study. Quant Imaging Med Surg. 14:1686–1698. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim C, Kim MY, Kang JW, Song JS, Lee KY
and Kim SS: Pulmonary artery intimal sarcoma versus pulmonary
artery thromboembolism: CT and clinical findings. Korean J Radiol.
19:792–802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Luo C, Wang Y, Guo X, Ma Z, Yang Y
and Zhang T: Multiparametric MRI in differentiating pulmonary
artery sarcoma and pulmonary thromboembolism: A preliminary
experience. Diagn Interv Radiol. 23:15–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar P, Singh A, Deshmukh A and Kumar S:
Cardiac MRI for the evaluation of cardiac neoplasms. Clin Radiol.
75:241–253. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren J, Li H, Zhang Q, Liu E, Zeng B, Huang
Y, Wang L and Jiang L: Clinical utility of 18F-FDG
PET/CT imaging in patients with pulmonary artery sarcoma. EJNMMI
Res. 12:182022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito K, Kubota K, Morooka M, Shida Y, Hasuo
K, Endo H and Matsuda H: Diagnostic usefulness of 18F-FDG PET/CT in
the differentiation of pulmonary artery sarcoma and pulmonary
embolism. Ann Nucl Med. 23:671–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi XY, Gao W, Gong JN, Guo XJ, Wu JY, Yang
YH and Yang MF: Value of 18F-FDG PET/CT in
differentiating malignancy of pulmonary artery from pulmonary
thromboembolism: A cohort study and literature review. Int J
Cardiovasc Imaging. 35:1395–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suto H, Suto M, Inui Y and Okamura A:
Difficulty in distinguishing pulmonary arterial intimal sarcoma
from pulmonary thromboembolism using FDG PET/CT. In Vivo.
36:1519–1522. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pomoni A, Sotiriadis C, Gay F, Jouannic AM
and Qanadli SD: Percutaneous endovascular biopsy of intravascular
masses: Efficacy and safety in establishing pre-therapy diagnosis.
Eur Radiol. 28:301–307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie WM, Zhai ZG, Wang LF, Wan J, Yang YH
and Wang C: Endovascular catheter-guided forceps biopsy for the
diagnosis of suspected pulmonary artery sarcoma: A preliminary
study of eight cases. Chin Med J (Engl). 129:2246–2249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong C, Lin JL, Chen HM, Guo WL, Li XY and
Wu XF: Percutaneous endovascular biopsy for the diagnosis of
pulmonary artery masses: A preliminary study of single-center. Pulm
Circ. 13:e122342023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto Y, Shintani Y, Funaki S, Taira M,
Ueno T, Kawamura T, Kanzaki R, Minami M, Sawa Y and Okumura M:
Aggressive surgical resection of pulmonary artery intimal sarcoma.
Ann Thorac Surg. 106:e197–e199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuft C, Maheepala K, Raguparan A, Naeem A,
Lodh S and Lindstrom S: Pulmonary artery sarcoma: An important
mimic of pulmonary embolism-case reports and literature review.
Respirol Case Rep. 10:e08972022. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanada TJ, Sakao S, Naito A,
Ishibashi-Ueda H, Suga M, Shoji H, Miwa H, Suda R, Iwasawa S, Tada
Y, et al: Characterization of pulmonary intimal sarcoma cells
isolated from a surgical specimen: In vitro and in vivo study. PLoS
One. 14:e02146542019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Funatsu Y, Hirayama M, Shiraishi J,
Asakura T, Wakaki M, Yamada E, Fujimoto K, Satomi R, Inaki S,
Murata Y and Oyamada Y: Intimal sarcoma of the pulmonary artery
treated with pazopanib. Intern Med. 55:2197–2202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Huang J, Wang Q, Zhang M, Luo Y,
Wang X, Zhu X and Liu H: Whole-exome sequencing insights into
pulmonary artery sarcoma mimicking pulmonary embolism: A case
report and review. Onco Targets Ther. 12:6227–6235. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roszik J, Khan A, Conley AP, Livingston
JA, Groisberg R, Ravi V, Carmagnani Pestana R, Sen S and Subbiah V:
Unique aberrations in intimal sarcoma identified by next-generation
sequencing as potential therapy targets. Cancers (Basel).
11:12832019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin J, Ng CS, He P, Lin X, Lin X and Hou
P: Pulmonary artery intimal sarcoma-A primeval or rediscovered
tumor? A report of 14 new cases with literature review. Pathol Res
Pract. 224:1535482021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang
G, Du F, Sun Y, Wu Q, Qu G, et al: Safety and efficacy of
anlotinib, a multikinase angiogenesis inhibitor, in patients with
refractory metastatic soft-tissue sarcoma. Clin Cancer Res.
24:5233–5238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li T, Dong Y, Wei Y, Wang S, Liu Y, Chen
J, Xiong W, Lin N, Huang X, Liu M, et al: First-line anlotinib
treatment for soft-tissue sarcoma in chemotherapy-ineligible
patients: An open-label, single-arm, phase 2 clinical trial. Clin
Cancer Res. 30:4310–4317. 2024. View Article : Google Scholar : PubMed/NCBI
|