Introduction
Cancer, a prevalent malignant neoplasm in clinical
settings, can affect various organs, and is characterized by its
rapid progression and high fatality rate, posing a threat to human
health. The World Health Organization estimates a 60% rise in
global cancer incidence within the next 2 decades, underscoring the
severity of prevention and control challenges (1). The etiology of cancer is rooted in
genetic mutations that trigger uncontrolled cell division, followed
by factors that exacerbate this growth, enabling cancer cells to
penetrate the basement membrane and metastasize to distant organs;
this can result in widespread organ failure and ultimately patient
mortality (2,3). The development of cancer is influenced
by numerous factors, i.e. external factors, including physical
stimuli (such as mechanical stimuli, ultraviolet light, radiation)
and biological factors (such as viruses, bacteria); and internal
factors, including immune dysfunction, endocrine disorders and
genetic factors. All of these factors can increase the risk of
cancer by affecting normal cell growth and genetic stability
(4,5).
Most types of cancer are initially treated with
surgery, which aims to achieve a complete cure. If surgery is not
possible, chemotherapy and radiation therapy are used as
alternative treatments (6);
however, these treatments have poor targeting abilities and can
damage normal cells along with cancer cells, leading to serious
adverse reactions (7).
Recently, tumor immunotherapy has emerged as a major
breakthrough in cancer treatment, which mainly utilizes the human
immune system through active or passive methods to enhance the
specific anticancer immunity of the patient to kill cancer cells
(8). This has achieved notable
therapeutic effects in some patients with advanced cancer. Among
them, immune checkpoint inhibitors (ICIs), a type of immunotherapy,
have markedly prolonged the overall survival (OS) and
progression-free survival (PFS) of patients with various advanced
cancers, and significantly improved the objective response rate
(ORR) (9). Following the success of
nivolumab and pembrolizumab in tumor immunotherapy, sintilimab, a
programmed cell death receptor 1 (PD-1) inhibitor that was
developed in China, stands out for its distinct binding affinity
and epitope.
Unlike nivolumab and pembrolizumab, sintilimab
targets the FG loop of PD-1, exhibiting a ~10 and 50 times stronger
binding affinity, respectively (10). This heightened affinity may endow
sintilimab with greater efficacy in blocking the PD-1/programmed
death-ligand (PD-L)1 pathway, thus amplifying T cell antitumor
activity. As a fully humanized monoclonal antibody, sintilimab also
presents lower immunogenicity, potentially minimizing
immune-related adverse events. While nivolumab has demonstrated
effectiveness across various types of cancer, sintilimab, with its
unique pharmacological profile and potential therapeutic
advantages, may provide superior outcomes in specific patient
cohorts (11).
A single study has revealed that the PFS of
sintilimab in combination with platinum-based doublet chemotherapy
for non-squamous non-small-cell lung cancer (NSCLC) is comparable
to that of pembrolizumab, atezolizumab, tislelizumab, camrelizumab
and nivolumab in combination therapies. Additionally, the rates of
adverse events at any grade were similar among these PD-L1
inhibitors (12). However,
prospective studies and the comparison in animal trials have not
yet been reported in the literature, so it is not discussed in this
paper. Moreover, considering cost-effectiveness and accessibility,
sintilimab is particularly advantageous in resource-limited or
price-sensitive settings, offering an affordable alternative that
could reshape cancer treatment strategies globally.
Introduction to sintilimab
Structure
Sintilimab is a recombinant fully human
immunoglobulin G4 (IgG4) monoclonal antibody against PD-1 that was
developed using yeast technology. Sintilimab has an IgG4 framework,
which is known to have a very low impact on antibody-dependent
cell-mediated cytotoxicity (ADCC) and complement-dependent
cytotoxicity (CDC) and is an ideal choice for therapeutic
antibodies (13). The most
important factor affecting the clinical efficacy of anticancer
drugs is the ability of the antibody to bind to the target with
sufficient strength and duration. Structural analysis has shown
that the epitope of the sintilimab/PD-1 complex is located in the
FG ring of PD-1, which is different from nivolumab or
pembrolizumab. Notably, sintilimab can bind to more PD-1 molecules
on CD3+ T cells than nivolumab or pembrolizumab, with
superior T cell activation properties. Sintilimab has a good
performance in terms of prolonged binding ability, good safety and
observed clinical efficacy (14).
Pharmacokinetics
Pharmacokinetics and anti-drug antibody (ADA)
analyses of sintilimab have been conducted in vitro, in
animal models and in human subjects. A single study has indicated
that sintilimab does not demonstrate antibody-dependent
cell-mediated cytotoxicity or complement-dependent cytotoxicity
(15). In cynomolgus monkeys, serum
concentrations of sintilimab and the area under the curve have been
shown to be increased in a dose-dependent manner within the range
of 1–30 mg/kg. When administered at a dose of 200 mg/kg for 2
weeks, sintilimab was well tolerated and did not result in any
drug-related fatalities (15).
Standard pharmacokinetic evaluations following a single intravenous
dose of 10 mg/kg in PD-1 knockout mice revealed serum half-lives of
35.6 h for sintilimab, compared with 43.5 h for nivolumab and 42.5
h for pembrolizumab (16). Among
381 patients treated with sintilimab, only 0.52% (2/381) tested
positive for ADA, and 0.26% (1/381) developed neutralizing
antibodies following sintilimab infusion (17).
Functional role
By binding to PD-1, sintilimab can block its
interaction with PD-L1 and PD-L2, thereby inhibiting the PD-1/PD-L1
pathway that leads to tumor immune tolerance, and activating T cell
function, enhancing T cell immune surveillance and killing ability
against tumors, generating tumor immune responses, thus achieving
the goal of treating tumors (18).
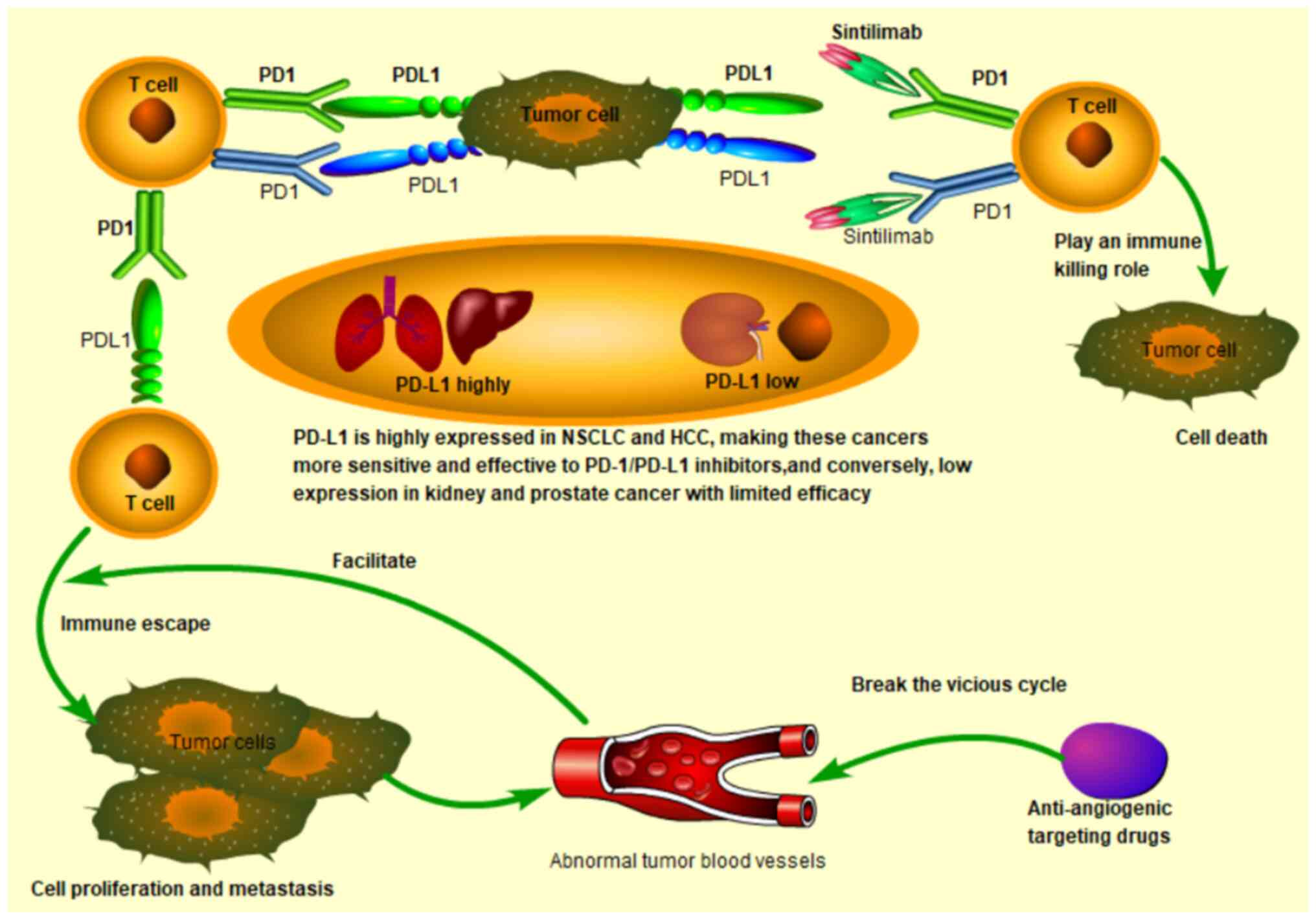
Notably, the invasion and metastasis of tumors occur through
continuous interactions with the surrounding microenvironment.
Previous evidence has shown that abnormal tumor blood vessels in
the tumor microenvironment promote immune-suppressive cells to
evade, thereby promoting tumor angiogenesis. This vicious cycle
leads to the ineffectiveness of single immunotherapy or
anti-angiogenesis single therapy (19). Therefore, the strategy of combining
anti-angiogenesis therapy and immunotherapy seems likely to break
the balance of the tumor microenvironment and to improve treatment
responses (Fig. 1).
Safety
A previous safety assessment of sintilimab included
data from 12 clinical studies involving various tumor types,
including NSCLC, esophageal cancer (EC) and liver cancer, with a
total of 2,461 patients (20). Of
the 568 patients treated with monotherapy, 91.2% experienced an
adverse reaction, rising to 98.0% among the 1,893 patients treated
with the combination. In addition, common adverse reactions and
their incidence in sintilimab treatment have been reported on,
including anemia, fever, thyroid dysfunction and more serious grade
3 and above adverse reactions (Table
I) (21–24). These detailed safety summaries not
only provide a comprehensive view of treatment with sintilimab, but
also offer an important reference for future individualized
treatment strategies and adverse event management.
 | Table I.Sintilimab immune-associated adverse
reactions. |
Table I.
Sintilimab immune-associated adverse
reactions.
|
| Reaction level, n
(%) |
|---|
|
|
|
|---|
| Immune-related
adverse reactions | 1 | 2 | 3 | 4 | 5 |
|---|
| Immune-associated
pneumonia | 2 (0.1) | 58 (2.4) | 33 (1.3) | 4 (0.2) | 12 (0.5) |
| Immune-associated
diarrhea and colitis | - | 4 (0.2) | 11 (0.4) | - | - |
| Immune-associated
hepatitis | 1 (<0.1) | 4 (0.2) | 15 (0.6) | 8 (0.3) | 2 (0.1) |
| Immune-associated
nephritis | 1 (<0.1) | 3 (0.1) | 7 (0.3) | - | - |
| Immune-associated
endocrine diseases | 379 (15.4) | 200 (8.1) | 20 (0.8) | 2 (0.1) | - |
| Thyroid and
parathyroid diseases | 380 (15.4) | 194 (7.9) | 3 (0.1) | - | - |
| Hypothyroidism | 265 (10.8) | 163 (6.6) | 1 (<0.1) | - | - |
|
Hyperthyroidism | 182 (7.4) | 34 (1.4) | 2 (0.1) | - | - |
| Other thyroid
diseases | 28 (1.1) | 6 (0.2) | - | - | - |
| Hypophysitis | 3 (0.1) | 4 (0.2) | 5 (0.2) | 1 (<0.1) | - |
| Adrenal
insufficiency | 2 (0.1) | 5 (0.2) | 3 (0.1) | - | - |
| Immune-related skin
adverse reactions | 57 (2.3) | 55 (2.2) | 22 (0.9) | 1 (<0.1) | - |
| Immune-associated
elevation of amylase and | 44 (1.8) | 26 (1.1) | 17 (0.7) | 6 (0.2) | - |
| lipase and
pancreatitis |
|
|
|
|
|
| Immune-associated
thrombocytopenia | - | 1 (<0.1) | 3 (0.1) | 5 (0.2) | - |
| Immune-related
cardiotoxicity | 1 (<0.1) | 5 (0.2) | 4 (0.2) | - | 2 (0.1) |
| Immune-associated
nervous system adverse reactions | - | 5 (0.2) | 1 (<0.1) | 1 (<0.1) | 2 (0.1) |
| Musculoskeletal and
connective tissue immune-related adverse reactions | 1 (<0.1) | 4 (0.2) | 2 (0.1) | - | - |
| Ocular
immune-related adverse reactions | 2 (0.1) | - | 1 (<0.1) | - | - |
Specific role of sintilimab in various types
of tumors
Sintilimab, as an immunotherapy drug, has shown
broad application potential and efficacy in various types of
cancer, as shown in Table II.
 | Table II.Research progress of Sintilimab in
different cancers. |
Table II.
Research progress of Sintilimab in
different cancers.
| First author,
year | Cancer name | Results |
| (Refs) |
|---|
| Yang et al,
2020; Liu et al, 2024; Dehghani et al, 2023 | NSCLC | Progression-free
survival was significantly longer in the sintilimab + IBI305 +
chemotherapy group than in the chemotherapy alone group | ORIENT-11
ChiCTR-OIC 17013726 | (28–30) |
| Ren et al,
2021 | Liver cancer | Sintilimab combined
with bevacizumab can prolong the median OS, PFS and increase ORR in
advanced HCC associated with chronic hepatitis B | ORIENT32 | (34) |
| Janjigian et
al, 2021; Xu et al, 2023 | GC | Compared with
placebo, sintilimab significantly improved OS in all patients and
in patients with CPS of 5 or more | ORIENT-16 | (42,43) |
| Lu et al,
2022 | EC | The OS and PFS of
sintilimab combined with chemotherapy were better than those of
chemotherapy alone, PD-L1 positive patients showed more significant
improvement | ORIENT-15 | (48) |
| Zeng et al,
2023; Jin et al, 2023 | BTC | Sintilimab +
gemcitabine and cisplatin met pre-specified endpoints and showed an
acceptable safety profile |
ChiCTR2000036652 | (52,53) |
| Qiu et al,
2024 | PC | Advantages of S-1
in combination with sintilimab and anlotinib as second-line therapy
for patients with pancreatic cancer liver metastases to prolong
OS |
ChiCTR2000030659 | (60) |
| Xiao et al,
2024 | CRC | Sintilimab can
significantly increase the CR rate of pMMR LARC in NACT | NCT04304209 | (64) |
| Wang et al,
2023; | Cervical | Treatment with
sintilimab in combination with | NCT04341883 | (69,70) |
| Xu et al,
2022 | cancer | NAB-paclitaxel or
anlotinib has shown good antitumor activity and manageable toxicity
in patients with advanced cervical cancer |
|
|
| Jia et al,
2022 | NEN | Sintilimab was well
tolerated in patients with NENs and the efficacy was
encouraging | NCT02937116 | (72) |
| Liu et al,
2024 | Nasopharynx
cancer | The sintilimab
group had a higher event-free survival rate than the standard
treatment group | NCT03700476 | (73) |
| Tian et al,
2022 | STS | Sintilimab combined
with doxorubicin is a safe and promising treatment for patients
with advanced STS who have failed previous systemic therapy,
including anthracycline chemotherapy |
ChiCTR1900027009 | (74) |
| Lu et al,
2021 | ccRCC | TKIs, together with
6–8 cycles of sintilimab followed by the single use of a TKI, are a
feasible way to treat metastatic ccRCC patients as second-line
treatment | NCT04735861 | (75) |
| Li et al,
2021 | HNSCC | Addition of
sintilimab to IC could provide longer PFS time than traditional
chemotherapy regimen | No.201356HN | (76) |
| Li et al,
2022 | Ovarian clear cell
carcinoma | The combination of
sintilimab and bevacizumab has a good effect in the treatment of
ovarian clear cell carcinoma | NCT04735861 | (77) |
| Wei et al,
2022 | Endometrial
cancer | Sintilimab plus
anlotinib demonstrated robust therapeutic benefits with tolerable
toxicity in endometrial can | NCT04157491 | (78) |
NSCLC
NSCLC is the second most commonly diagnosed cancer
worldwide and a leading cause of cancer-related mortality. NSCLC
accounts for >85% of all lung cancer cases, with a 5-year
survival rate of 26% after diagnosis and 6% for advanced patients
treated with traditional chemotherapy regimens (25). The advent of PD-1/PD-L1
immunotherapy has significantly improved the treatment outlook for
patients with inoperable NSCLC, becoming an important option for
first-line and subsequent treatments (26).
The ORIENT-11 study, a phase III, randomized,
placebo-controlled clinical trial, investigated the efficacy of
sintilimab combined with gemcitabine and cisplatin (GemCis) as
first-line therapy for patients with advanced non-squamous NSCLC
with EGFR or ALK gene mutations. The OS results, published in the
journal ‘Lung Cancer’ in September 2022, demonstrated a median OS
time of 24.2 months for the sintilimab plus chemotherapy group,
which was significantly increased compared with the 16.8 months of
the placebo group, with overall manageable adverse events (27). Furthermore, the combination of
sintilimab with other treatments, such as docetaxel,
cytokine-induced killer cell immunotherapy, radiotherapy and
anlotinib, has shown promising antitumor effects, warranting
further exploration (18,28).
In a phase Ib study (ChiCTR-OIC 17013726), the
efficacy of sintilimab monotherapy for NSCLC was confirmed. The
latest National Comprehensive Cancer Network guidelines recommend
platinum-based regimens combined with PD-1 inhibitors (such as
pembrolizumab) as the preferred first-line treatment for
unresectable or metastatic NSCLC (29); similar combinations with sintilimab
are also under investigation (30).
In summary, the addition of sintilimab to chemotherapy regimens may
significantly prolong the PFS and OS for patients with non-squamous
NSCLC, leading to its approval in China as a first-line treatment
for non-squamous NSCLC in February 2021.
Liver cancer
The global incidence of primary liver cancer ranks
sixth in malignant tumors and its mortality rate ranks third
(31). Due to the hidden onset of
liver cancer, most patients are in the advanced stage when they are
first diagnosed. The effectiveness of surgical resection,
radiofrequency ablation and hepatic arterial chemoembolization
treatment is limited, and the 5-year survival rate is 18% (32). With the improved understanding of
tumor molecular signaling pathways and the tumor microenvironment,
targeted therapy has become an area of focus in advanced
hepatocellular carcinoma (HCC) clinical research. In addition to
targeted therapy, ICIs have made breakthroughs in the treatment of
advanced HCC in recent years (33).
In the ORIENT-32 Chinese multicenter phase III study
of sintilimab combined with bevacizumab, 571 patients with advanced
unresectable liver cancer were randomly assigned to the
sintilimab-bevacizumab group (n=380) or sorafenib group (n=191)
with a median follow-up time of 10 months. The results showed that
the PFS time was 4.6 vs. 2.8 months in the sintilimab-bevacizumab
group compared with in the sorafenib group (P<0.0001), and the
OS time was significantly improved. The ORR of the
sintilimab-bevacizumab group was 21%, which was significantly
higher than the sorafenib group (4%). This previous study showed
that sintilimab combined with bevacizumab can prolong the median OS
and PFS of advanced HCC associated with chronic hepatitis B and
improve ORR, which further confirms that the combination of
immunotherapy and targeted therapy for advanced HCC is highly
effective and suits the clinical reality in China (34). In June 2021, the China National
Medical Products Administration officially approved the innovative
PD-1 inhibitor sintilimab injection combined with bevacizumab
injection for the first-line treatment of unresectable or
metastatic HCC that has not previously received systematic therapy
(35).
Gastric cancer
Gastric cancer, the fifth most common type of cancer
worldwide, is the fourth leading cause of cancer-related mortality
(36). While early-stage disease
can be effectively treated with surgery and adjuvant
chemoradiotherapy, a lack of noticeable symptoms often results in
advanced-stage diagnosis (37).
Despite advancements in chemotherapy, the survival rate for
advanced gastric cancer remains <12 months (38).
The Attract-02 study demonstrated that nivolumab
significantly reduced mortality in Asian patients with advanced or
metastatic gastric cancer (39),
leading to approvals for nivolumab and pembrolizumab as third-line
treatments in Japan and the U.S., respectively (40). In China, sintilimab, a domestically
developed PD-1 inhibitor, has emerged as a promising second-line
treatment, with regulatory approval supported by the ORIENT-16
phase III trial (41). This trial,
conducted across 62 Chinese hospitals, involved 650 patients with
unresectable or metastatic gastric or gastroesophageal junction
adenocarcinoma, randomly assigned to sintilimab or placebo in
combination with capecitabine and oxaliplatin. Sintilimab improved
the OS time to a median of 15.2 months compared with 12.3 months in
the placebo group (42). In
particular, patients with a PD-L1 combined positive score of ≥5
showed an even more pronounced OS benefit with sintilimab at 18.4
vs. 12.9 months for the placebo. These findings underscore the
potential of sintilimab in enhancing survival for specific patient
populations, marking a significant advancement in the second-line
treatment of advanced gastric cancer (43).
EC
EC is a common, aggressive and poorly prognostic
malignant tumor that seriously threatens patient health. Currently,
the main treatment methods include surgery, radiation therapy and
chemotherapy; however, local recurrence and metastasis after
surgery for patients with locally advanced EC are unavoidable
(44). Immunotherapy has great
potential in the treatment of EC (45). To reduce local and distant
recurrence, and to improve survival rates, neoadjuvant
chemoradiotherapy (NACT) has been tested. Based on the CROSS study,
Western countries such as the UK, Germany, France, the US, Canada,
Australia and New Zealand have adopted NACT plus surgery as the
standard treatment for patients with locally advanced EC (46). In Asia, particularly in Japan, NACT
before surgery has been advocated as the standard treatment based
on the results of the JCOG9907 trial (47). ORIENT-15 is the first global phase
III study conducted by Chinese researchers targeting patients with
EC with immunotherapy and chemotherapy. The study enrolled 659
patients, with 327 receiving sintilimab plus chemotherapy
(cisplatin plus paclitaxel or cisplatin plus 5-FU) and 332
receiving a chemotherapy regimen alone (placebo plus cisplatin plus
paclitaxel or cisplatin plus 5-FU). The interim results as of April
9, 2021 showed that for all populations, the OS and PFS of the
sintilimab plus chemotherapy group were greater compared with those
of the chemotherapy alone group (OS, 16.7 vs. 12.5 months; PFS, 7.2
vs. 5.7 months), and the improvement was more obvious in
PD-L1-positive patients (OS, 17.2 vs. 13.6 months; PFS, 8.3 vs. 6.4
months) (48).
Biliary tract cancer (BTC)
BTC is an increasingly prevalent hepatobiliary
malignancy with diverse characteristics across regions. Surgery is
the only curative option for this type of cancer; however, it is
inaccessible to 70% of patients with advanced or metastatic disease
due to asymptomatic early stages, resulting in a poor 5-year
survival rate of <5% and high recurrence rates post-surgery
(49). Monotherapy with
anti-PD-1/PD-L1 antibodies, such as pembrolizumab and nivolumab,
has shown modest efficacy in BTC, with an ORR of 3–22% (50). The combination of durvalumab with
GemCis has demonstrated an improved OS and is now recommended as a
first-line regimen by the National Comprehensive Cancer Network
guidelines (51). A phase II trial
of sintilimab combined with GemCis as a first-line treatment in
advanced BTC showed a median OS and PFS time of 15.9 and 5.1
months, respectively, with an ORR of 36.7%, indicating the efficacy
and safety of the regimen. Furthermore, sintilimab paired with
anlotinib as a second-line therapy for advanced BTC exhibited
promising antitumor activity and a manageable safety profile, with
a median OS time of 12.3 months, offering a potential second-line
treatment option (52,53).
Pancreatic cancer
Pancreatic cancer is a malignant tumor of the
pancreas, which can originate from the exocrine glands of the
pancreas, endocrine glands or non-epithelial tissues. It has a poor
prognosis and a high morbidity and mortality rate compared to most
tumors, which is increasing annually (54). A total of 85% of patients are unable
to undergo radical surgery at initial diagnosis, and the 5-year
survival rate for those who undergo radical resection is <30%
(55). In recent years, PD-1 and
PD-L1 have become the focus of research and development, and have
been shown to have potential as ‘cancer killers’, with notable
results in treating various types of tumors, especially in patients
with positive PD-L1, microsatellite instability-high/deficient
mismatch repair (MSI-H/dMMR) or high tumor mutational burden (TMB)
(56,57). Unfortunately, only a small number of
patients with pancreatic cancer meet these conditions and limited
clinical activity of ICIs has been observed.
In recent years, small-sample clinical trials have
yielded positive results for anti-PD-1 antibodies combined with
chemotherapy drugs in advanced pancreatic cancer (58,59). A
previous study evaluated the efficacy and safety of a combination
of S-1, sintilimab and anlotinib as second-line treatment for
patients with pancreatic cancer and liver metastases. A total of 23
patients were included in the study, 19 of whom underwent objective
efficacy evaluation. In the assessable population, the ORR was
10.5% (95% CI, 0.4–25.7%), PFS was 3.53 months (95% CI, 2.50–7.50)
and OS was 8.53 months (95% CI, 4.97–14.20) (60). This study suggests the advantage of
S-1 in combination with sintilimab and anlotinib as second-line
therapy for prolonging OS in patients with pancreatic cancer and
liver metastases.
Colorectal cancer (CRC)
CRC, the second most frequent malignant tumor in the
digestive system after gastric cancer and EC, is globally on the
rise, with some patients presenting with distant metastases that
preclude surgical intervention (61). The standard treatment for locally
advanced rectal cancer (LARC) involves NACT, followed by total
mesorectal excision and adjuvant chemotherapy, which has shown
improved local control and high pathological complete response
rates (62). The KEYNOTE-177 phase
III trial data have led to the recommendation of anti-PD-1 therapy
as the first-line treatment for dMMR metastatic CRC, with ICIs also
demonstrating benefits in patients with non-metastatic dMMR CRC
(63). In a randomized phase II
trial (ClinicalTrial.gov no. NCT04304209), the
addition of sintilimab, a PD-1 antibody, to NACT in patients with
proficient mismatch repair LARC significantly enhanced the complete
response rate from 26.9 to 44.8%, with a manageable safety profile.
This suggested that PD-L1 positivity can predict which patients may
benefit most from combined therapy (64). Other studies have reported that the
complete response rate may be related to the duration of exposure
to ICIs (65). Therefore, it is
suggested that some patients may require a longer neoadjuvant ICI
regimen to achieve complete remission.
Cervical cancer
Cervical cancer, a leading gynecological malignancy,
often results from the progression of cervical erosion and other
diseases, with early detection and treatment being crucial for
superior outcomes. Globally, this disease is responsible for
>300,000 mortalities annually (66). NACT is now a standard approach to
downstage tumors, eliminate micrometastases and mitigate radiation
complications before radical surgery (67).
Recently, the KEYNOTE 826 study showed that adding
the PD-1 inhibitor pembrolizumab to platinum-based chemotherapy as
a first-line treatment, compared with a placebo, in patients with
PD-L1-positive tumors significantly improved the PFS and OS
(68). A subsequent phase II study
(NCT04341883) treated patients with recurrent or metastatic
cervical cancer who had progressed after at least one systemic
treatment with a combination of sintilimab and nanoparticle
albumin-bound paclitaxel (nab-paclitaxel). Among the 27 patients,
the ORR was 44.4%, with a disease control rate of 88.9%, a median
PFS of 5.2 months and a median OS of 13.1 months, indicating the
promising antitumor activity and manageable toxicity of the
combination (69). In another phase
II study led by Xu et al (70), sintilimab combined with anlotinib
was evaluated in 42 patients with recurrent or metastatic cervical
cancer. The ORR was 54.8%, with 59.0% of the 39 evaluable patients
responding to treatment, and a disease control rate of 94.9%. The
median PFS was 9.4 months, with a subgroup analysis revealing a
median PFS of 11.1 months for squamous cell carcinoma and 5.8
months for adenocarcinoma, suggesting a differential response to
sintilimab combined with antiangiogenic therapy based on tumor
histology (70).
Neuroendocrine neoplasms (NEN)
NENs are tumors that originate from neuroendocrine
cells, which are a large group of cells in the body with a
neuroendocrine phenotype that can produce a variety of hormones.
Neuroendocrine cells are found throughout the body; therefore, NENs
can occur anywhere in the body, but the most common are digestive
neuroendocrine tumors (NETs), such as those in the stomach,
intestines and pancreas, which account for ~2/3 of all NETs
(71). NENs are a group of diseases
with high heterogeneity but limited treatment options.
A phase I study evaluated the safety and efficacy of
the anti-PD-1 monoclonal antibody sintilimab in the treatment of
advanced NENs. This prospective study included patients with
pathologically diagnosed NENs after failure of standard treatment,
and each patient was treated with sintilimab and assessed for
efficacy every 9 weeks. Of the 24 patients included, five had NETs,
one had NET G3, 17 had neuroendocrine cancer (NEC), and one had
adenocarcinoma and neuroendocrine mixed carcinoma. The most common
primary tumor sites were the pancreas and gastrointestinal tract
(seven and 10 cases, respectively). In the phase Ia trial, the ORR
was 20.8% for all enrolled patients and 27.8% for patients with
NEC. The median PFS times for patients with NET and NEC were 2.2
and 2.1 months, respectively. The median OS times for NET and NEC
were not applicable (NA) and 10.8 months (95% CI, 4.3, NA),
respectively. The duration of response was not achieved and the
median follow-up was 20.7 months. Treatment-related adverse events
(TRAEs) occurred in 17 patients (70.8%). The most common TRAE was
thyroid dysfunction (41.7%). In addition, PD-L1 positivity (tumor
proportion score ≥1%) was 18.8% (3 of 16 cases), and PD-L1
expression was not associated with response (72). These results suggested that
sintilimab may be well tolerated in patients with NEC and the
efficacy is encouraging.
Efficacy in other tumors
In other tumors, sintilimab has also been reported
to be effective. In patients with nasopharyngeal carcinoma, a
multicenter, randomized controlled phase III trial at nine
hospitals in China evaluated the effect of adding sintilimab to
standard chemoradiotherapy in patients with locally advanced
nasopharyngeal carcinoma. A total of 425 patients were included and
randomly assigned to sintilimab (n=210) or standard treatment
(n=215) groups. At a median follow-up of 41.9 months, the
sintilimab group had a higher event-free survival rate (86 vs. 76%)
compared with that in the standard treatment group. The OS at 36
months was not significantly different between the two groups (92
vs. 92%) (73).
In patients with soft tissue sarcoma (STS), a
retrospective study analyzed the clinical data of 28 patients with
advanced STS treated with nab-paclitaxel combined with sintilimab.
The ORR, DCR and median PFS were 25%, 50% and 2.25 months,
respectively (74). Overall, the
therapeutic effect of nab-paclitaxel plus PD-1 inhibitors has been
reported to be relatively good.
In a previous study, among patients with clear cell
renal cell carcinoma (ccRCC), 17 patients with advanced ccRCC were
selected from the Shanghai Cancer Center of Fudan University
(Shanghai, China) and were treated with sunitinib as the first line
of treatment. After progression of the disease, patients received
pazopanib alone after 6–8 cycles of immunotherapy with sintilimab
in combination with pazopanib. A total of three patients achieved
partial response after second-line treatment, and 12 patients
remained stable. Notably, two patients progressed, and one died due
to progression. The median PFS time with second-line treatment was
12.2 months (75). This indicates
the long-term survival of patients with metastatic disease using
this treatment regimen and suggests a potential treatment option
for patients with metastatic ccRCC.
In addition, in another study, a total of 163
patients with head and neck squamous cell carcinoma were included;
98 patients received immune checkpoint (IC) therapy alone and 65
patients also received sintilimab. After neoadjuvant therapy,
patients underwent surgery (31.9%) or chemotherapy (68.1%). The
results showed that the ORR in the IC group was significantly lower
compared with that in the IC combined with sintilimab group (68.4
vs 84.6%; P=0.019). The median follow-up time was 28.0 months. In
addition, the 2-year PFS was 27% (95% CI, 18–36%) in the IC group
and 44% (95% CI, 32–56%) in the IC combined with sintilimab group;
the difference was statistically significant (P=0.041) (76). These findings indicated that
sintilimab could provide longer PFS duration than conventional
chemotherapy.
Sintilimab has also shown good antitumor activity in
gynecological tumors, such as ovarian clear cell carcinoma,
endometrial carcinoma and breast cancer. The INOVA study was
designed to evaluate the effect of sintilimab and bevacizumab
combined therapy in patients with recurrent or persistent ovarian
clear cell carcinoma. All 38 participants in the study received
sintilimab plus bevacizumab. As of July 31, 2022, the ORR was 38.5%
and the DCR was 76.9% of the 26 patients included in the evaluation
(77). In addition, a phase II
trial included 23 patients with endometrial cancer that progressed
after platinum chemotherapy. A total of 23 patients received
sintilimab intravenously and anlotinib orally. The median follow-up
time was 15.4 months, the median PFS was not reached and the
12-month PFS rate was 57.1%. ORR was 73.9% (95% CI, 51.6–89.8%),
with four complete responses and 12 partial responses (78).
In a previous study, a 49-year-old woman was
diagnosed with triple-negative breast cancer (TNBC) with extensive
lung and sternal metastases. After first-line chemotherapy failed,
sintilimab in combination with paclitaxel and carboplatin was
revealed to be highly effective. After the necessary investigation
and clinical trials, this combination therapy may be considered for
TNBC (79). In addition, sintilimab
is being tested in various other types of cancer and the findings
of these analyses could lead to new indications for the drug in the
future.
In summary, the present review on sintilimab in
cancer treatment provides a comprehensive and detailed analysis of
its role across various cancer types, including both common and
rare subtypes such as neuroendocrine tumors and biliary tract
cancer. While previous studies have often focused on specific
cancer types or aspects of sintilimab's mechanism, this study
offers a broad overview, detailing its structure, pharmacokinetics
and high binding affinity to PD-1. This detailed analysis helps
explain why sintilimab may be more effective in certain patient
populations and cancer types.
Reflections and prospects
After comprehensive analysis of clinical data and
meta-analyses of sintilimab in the treatment of various tumors, we
have a deeper understanding of its potential in tumor
immunotherapy, as summarized in this section.
Efficacy and patient selection
The current bottleneck in ICB treatment is that
efficacy and patient selection are key considerations for
immunotherapy. For example, in the treatment of NSCLC, a phase III
clinical trial (ORIENT-11) showed that sintilimab combined with
chemotherapy significantly extended PFS and OS, particularly in
patients with positive PD-L1 expression (48). This suggests that the expression
level of PD-L1 is an important biomarker for predicting the
efficacy of sintilimab. Another study (ORIENT-3) in patients with
HCC revealed that sintilimab monotherapy showed a higher ORR in
PD-L1-positive patients (34).
These findings highlight the importance of evaluating PD-L1
expression before treatment to help select patients most likely to
benefit from sintilimab treatment. In addition, TMB, as an emerging
biomarker, has shown potential in predicting the efficacy of
sintilimab, especially in MSI-H/dMMR tumors (80). However, the lack of results from
head-to-head comparisons of sintilimab with other PD-1 antibodies
is also a limitation of the present review.
Combination treatment strategies
As a PD-1 inhibitor, sintilimab has shown potential
in combination with a variety of therapeutic methods in the
treatment of cancer. The aim of the combination treatment strategy
is to enhance antitumor effects by integrating drugs with different
mechanisms of action, while potentially reducing resistance to
monotherapy. For example, the combination of sintilimab with
chemotherapy may take advantage of the immunomodulatory effects of
chemotherapy drugs to enhance the immune response to tumors. In
combination with anti-angiogenic drugs, the aim is to improve the
tumor microenvironment and increase the penetration and activity of
immune cells. In the treatment of NSCLC, a randomized,
double-blind, multicenter phase III trial (ORIENT-11) revealed that
combining sintilimab with chemotherapeutic agents, such as
gemcitabine and cisplatin, significantly improved the PFS and OS in
patients compared with chemotherapy alone (81). In addition, in another study
(ORIENT-32), the combination of sintilimab and bevacizumab
exhibited greater efficacy than sorafenib in patients with advanced
HCC, significantly extending the PFS and OS (34). In addition, the combination of
sintilimab with other ICIs, such as CTLA-4 inhibitors, may enhance
efficacy by targeting different immunosuppressive pathways. These
combination treatment strategies not only provide patients with
more diversified treatment options, but also provide novel
treatment ideas to overcome the complexity and heterogeneity of
tumors. However, this combination therapy also faces several
challenges, such as an increased incidence of adverse events,
particularly immune-related adverse events (irAEs), which require
close monitoring and management. Additionally, the high cost of
combination therapy may limit its widespread application in some
regions. Future research needs to further optimize the combination
therapy regimen to enhance treatment efficacy and reduce adverse
reactions, thereby providing more effective treatment options for
patients with cancer.
Long-term efficacy and safety
The long-term efficacy and safety of sintilimab are
key factors in evaluating its application in the treatment of
cancer. The present review provides some data on its long-term
effects and safety. For example, in a phase III clinical study in
patients with advanced NSCLC, sintilimab combined with chemotherapy
as first-line treatment not only showed good efficacy in the short
term, but long-term follow-up results showed that patients in the
combined treatment group had a significant extension in OS compared
with those receiving chemotherapy alone. These findings suggested
the potential of sintilimab to improve long-term survival in
patients (81). In addition, in a
study of patients with advanced HCC, the treatment regimen of
sintilimab combined with bevacizumab showed sustained efficacy and
manageable safety during long-term follow-up, which further
confirmed the efficacy and safety of sintilimab in long-term
treatment (34). These findings not
only provide a scientific basis for the long-term application of
sintilimab, but also provide important information for clinicians
during treatment planning and patient consultations.
Affordability and accessibility
As an emerging PD-1 inhibitor, sintilimab has
received extensive attention in terms of economic burden and
accessibility. In resource-limited countries and regions,
sintilimab has been considered a better economic choice due to its
relatively low cost. For example, a study from China evaluated the
cost-effectiveness of sintilimab in the treatment of NSCLC and
found that sintilimab combined with chemotherapy had a higher
cost-effectiveness ratio as first-line treatment compared with
standard chemotherapy (82). In
addition, the wide availability of sintilimab in China, due to its
medical insurance coverage, has made this advanced immunotherapy
affordable for more patients. These findings not only highlight the
potential of sintilimab in global immuno-oncology therapy, but also
underscore the importance of improving access to drugs to improve
patient outcomes.
Treatment resistance and
follow-up
Sintilimab, as a PD-1 inhibitor, has made
significant progress in tumor therapy, but treatment resistance
remains a major challenge. Research is currently exploring
strategies to overcome this resistance and options for follow-up
treatments. For example, one study examined patients with NSCLC who
progressed after treatment with PD-1 inhibitors and found that the
subsequent use of tyrosine kinase inhibitors may provide clinical
benefit for this subset of patients (83). Another study evaluated a rechallenge
treatment strategy using antiangiogenic agents in combination with
ICIs in patients with HCC whose disease had progressed after
treatment with PD-1 inhibitors, and showed a modest efficacy and
manageable safety profile (84).
These studies not only provide clues for understanding the
mechanism of treatment resistance of sintilimab, but also provide a
scientific basis for the selection of subsequent treatment
strategies.
Future development of individualized
therapy
Personalized therapy is considered the future
direction of tumor immunotherapy and sintilimab shows great
potential in this area. The latest research has explored how to
optimize the treatment regimen of sintilimab based on the specific
biomarkers and tumor characteristics of patients. For example, one
study used genomic and transcriptomic data to identify specific
gene expression patterns associated with sintilimab response, which
may help predict the patients that are more likely to benefit from
treatment (85). Another study
focused on immune cell subsets in the tumor microenvironment and
found that specific patterns of immune cell infiltration were
associated with the efficacy of sintilimab, providing a new
perspective for individualized therapy (86). These studies not only deepen the
understanding of the mechanism of action of sintilimab, but also
lay the foundation for the development of new biomarkers and
therapeutic strategies.
Conclusions
In summary, sintilimab, as a domestically developed
PD-1 inhibitor in China, has demonstrated significant innovation in
the field of tumor immunotherapy. Its innovative aspects are
reflected in the following areas: Firstly, clinical studies of
sintilimab in Asian populations have provided data different from
Western populations, which is crucial for understanding the
efficacy and safety of PD-1 inhibitors across different ethnicities
and geographical regions. Secondly, the exploration of sintilimab
in combination therapy, such as its use with chemotherapy, targeted
therapy and anti-angiogenic drugs, offers new insights for
enhancing treatment effects and overcoming drug resistance.
Additionally, the application of sintilimab in rare or specific
subtypes of cancer, such as NETs and BTC, has expanded the
therapeutic scope of PD-1 inhibitors, providing new hope to
patients. Finally, the pharmacoeconomic advantages of sintilimab,
especially in resource-limited regions, make its global
accessibility and affordability one of its benefits. These
innovations have not only propelled the application of sintilimab
in clinical practice, but also provide new directions for future
research and development.
Acknowledgements
Not applicable.
Funding
This work was supported by the Nanjing Pharmaceutical
Society-Changzhou Four-Medicine Hospital Pharmaceutical Research
Fund (grant no. 2023YX024), the Bethune Charity Foundation (grant
no. BCF-XD-ZL-20220118-038) and the National Health Commission of
China Pharmaceutical Health Science and Technology Development
Research Center - Innovative Drug Post-Marketing Clinical Research
Research Project - Surface Topic (grant no. WKZX2024CX501213).
Availability of data and materials
Not applicable.
Authors' contributions
YYW wrote the main manuscript text and prepared the
figure and tables. HS reviewed and revised the manuscript. Data
authentication is not applicable. Both authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dhanasekaran R, Deutzmann A,
Mahauad-Fernandez WD, Hansen AS, Gouw AM and Felsher DW: The MYC
oncogene-the grand orchestrator of cancer growth and immune
evasion. Nat Rev Clin Oncol. 19:23–36. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill W, Weeden CE and Swanton C: Tumor
promoters and opportunities for molecular cancer prevention. Cancer
Discov. 14:1154–1160. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilbertson RJ: Mapping cancer origins.
Cell. 145:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curtius K, Wright NA and Graham TA: An
evolutionary perspective on field cancerization. Nat Rev Cancer.
18:19–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mullard A: Addressing cancer's grand
challenges. Nat Rev Drug Discov. 19:825–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boshuizen J and Peeper DS: Rational cancer
treatment combinations: An urgent clinical need. Mol Cell.
78:1002–1018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szeto GL and Finley SD: Integrative
approaches to cancer immunotherapy. Trends Cancer. 5:400–410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoy SM: Sintilimab: First global approval.
Drugs. 79:341–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Li T, Yang W, Wang T, Qin Y, Du Z,
Li Y, Cui P, Hu Y and Liu Z: Comparative efficacy of six programmed
cell death protein-1 inhibitors as first-line treatment for
advanced non-small cell lung cancer: A multicenter retrospective
cohort study. Front Pharmacol. 15:13908722024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Qian Y, Li J, Cui C, Chen L, Qu S
and Lu S: Indirect comparison of sintilimab and other PD-L1
inhibitors for first-line treatment of non-squamous non-small-cell
lung cancer. Future Oncol. 18:1896–1905. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaplon H, Chenoweth A, Crescioli S and
Reichert JM: Antibodies to watch in 2022. MAbs. 14:20142962022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Lin W, Tan F, Li N, Xue Q, Gao S,
Gao Y and He J: Sintilimab for the treatment of non-small cell lung
cancer. Biomark Res. 10:232022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lou B, Wei H, Yang F, Wang S, Yang B,
Zheng Y, Zhu J and Yan S: preclinical characterization of GLS-010
(Zimberelimab), a novel fully human anti-PD-1 therapeutic
monoclonal antibody for cancer. Front Oncol. 11:7369552021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao C, Xiong A, Qian J, Wang W, Liu Y,
Zhang T, Wu Z, Ni H, Lu J, Long S, et al: Dual inhibition of LAG-3
and PD-1 with IBI110 and sintilimab in advanced solid tumors: The
first-in-human phase Ia/Ib study. J Hematol Oncol. 17:1322024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A promising anti-tumor PD-1 antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X and Yi Y: Recent updates on
Sintilimab in solid tumor immunotherapy. Biomark Res. 8:692020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13:8951872022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu J, Li Y, Chen X, Luo C and Zuo X:
Pulmonary fibrosis and cytokine release syndrome after
hyperactivation with sintilimab. J Clin Pharm Ther. 45:1474–1477.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suárez-Almazor ME: Immune-related adverse events of
checkpoint inhibitors. Nat Rev Dis Primers. 6:382020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Zhu L, Ma X, Hong Y, Su X, Lai W
and Gong Z: A case of sintilimab-induced SJS/TEN:Dermatologic
adverse reactions associated with programmed cell death protein-1
inhibitors. Dermatol Ther. 35:e156632022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang M, Dang P, Liu T, Yang K, Wang Y, Tse
G, Liu H, Liu Y, Chan JSK, Liu C and Li G: Risk factors and
outcomes of pericardial effusion in cancer patients receiving PD-1
inhibitors. Int J Cardiol. 407:1320292024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mountzios G, Remon J, Hendriks LEL,
García-Campelo R, Rolfo C, Van Schil P, Forde PM, Besse B, Subbiah
V, Reck M, et al: Immune-checkpoint inhibition for resectable
non-small-cell lung cancer-opportunities and challenges. Nat Rev
Clin Oncol. 20:664–677. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Stefaniak V, et al: Final overall
survival data of sintilimab plus pemetrexed and platinum as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC in the phase 3 ORIENT-11 study. Lung Cancer. 171:56–60. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Zhou H and Zhang L: Response to
letter to the editor: Efficacy and safety of sintilimab plus
pemetrexed and platinum as first-line treatment for locally
advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (ORIENT-11). J Thorac Oncol.
15:e191–e192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu SYM, Huang J, Deng JY, Xu CR, Yan HH,
Yang MY, Li YS, Ke EE, Zheng MY, Wang Z, et al: PD-L1 expression
guidance on sintilimab versus pembrolizumab with or without
platinum-doublet chemotherapy in untreated patients with advanced
non-small cell lung cancer (CTONG1901): A phase 2, randomized,
controlled trial. Sci Bull (Beijing). 69:535–543. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dehghani T, Shahrjerdi A, Kahrizi MS,
Soleimani E, Ravandeh S, Merza MS, Rahnama N, Ebrahimzadeh F and
Bakhshesh M: Targeting programmed cell death protein 1 (PD-1) for
treatment of non-small-cell lung carcinoma (NSCLC); the recent
advances. Pathol Res Pract. 246:1544702023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cuesta ÁM, Palao N, Bragado P,
Gutierrez-Uzquiza A, Herrera B, Sánchez A and Porras A: New and old
key players in liver cancer. Int J Mol Sci. 24:171522023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni
A, Kamel IR, Cloyd JM and Pawlik TM: Management of hepatocellular
carcinoma: A review. JAMA Surg. 158:410–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Llovet JM, Pinyol R, Yarchoan M, Singal
AG, Marron TU, Schwartz M, Pikarsky E, Kudo M and Finn RS: Adjuvant
and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat
Rev Clin Oncol. 21:294–311. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li
Q, Lu Y, Chen Y, Guo Y, et al: Sintilimab plus a bevacizumab
biosimilar (IBI305) versus sorafenib in unresectable hepatocellular
carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study.
Lancet Oncol. 22:977–990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sidaway P: Adjuvant sintilimab effective
in high-risk HCC. Nat Rev Clin Oncol. 21:1682024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shitara K: Chemotherapy for advanced
gastric cancer: Future perspective in Japan. Gastric Cancer. 20
(Suppl 1):S102–S110. 2017. View Article : Google Scholar
|
|
39
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taieb J, Moehler M, Boku N, Ajani JA, Ruiz
EY, Ryu MH, Guenther S, Chand V and Bang YJ: Evolution of
checkpoint inhibitors for the treatment of metastatic gastric
cancers: Current status and future perspectives. Cancer Treat Rev.
66:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mei Y, Shi M and Zhu Z, Yuan H, Yan C, Li
C, Feng T, Yan M, Zhang J and Zhu Z: Addition of sintilimab to
nanoparticle albumin-bound paclitaxel and S-1 as adjuvant therapy
in stage IIIC gastric cancer. Future Oncol. 18:139–148. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L,
Shu Y, Li J, Zhao J, Pan H, et al: Sintilimab Plus chemotherapy for
unresectable gastric or gastroesophageal junction cancer: The
ORIENT-16 randomized clinical trial. JAMA. 330:2064–2074. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mwachiro M and White R: Management of
esophageal cancer treatment in resource-limited settings. Thorac
Surg Clin. 32:397–404. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang FL and Yu SJ: Esophageal cancer:
Risk factors, genetic association, and treatment. Asian J Surg.
41:210–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Q, Liu T and Ding Z: Neoadjuvant
immunotherapy for resectable esophageal cancer: A review. Front
Immunol. 13:10518412022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yokota T, Ando N, Igaki H, Shinoda M, Kato
K, Mizusawa J, Katayama H, Nakamura K, Fukuda H and Kitagawa Y:
Prognostic factors in patients receiving neoadjuvant 5-fluorouracil
plus cisplatin for advanced esophageal cancer (JCOG9907). Oncology.
89:143–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Benson AB, D'Angelica MI, Abrams T, Abbott
DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, et al:
NCCN guidelines® insights: Biliary tract cancers,
version 2.2023. J Natl Compr Canc Netw. 21:694–704. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kalyan A, Khosla H and Kim RD:
Immunotherapy in biliary tract cancers: Where are we? Curr Oncol
Rep. 24:1821–1828. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng L, Wang Y, Xu H and Yi F: Comparison
of different first-line systemic therapies in advanced biliary
tract cancer based on updated random controlled trials: A
systematic review and network meta-analysis. Biomed Res Int.
2022:17206962022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng TM, Yang G, Lou C, Wei W, Tao CJ,
Chen XY, Han Q, Cheng Z, Shang PP, Dong YL, et al: Clinical and
biomarker analyses of sintilimab plus gemcitabine and cisplatin as
first-line treatment for patients with advanced biliary tract
cancer. Nat Commun. 14:13402023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin S, Zhao R, Zhou C, Zhong Q, Shi J, Su
C, Li Q, Su X, Chi H, Lu X, et al: Feasibility and tolerability of
sintilimab plus anlotinib as the second-line therapy for patients
with advanced biliary tract cancers: An open-label, single-arm,
phase II clinical trial. Int J Cancer. 152:1648–1658. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koikawa K, Kibe S, Suizu F, Sekino N, Kim
N, Manz TD, Pinch BJ, Akshinthala D, Verma A, Gaglia G, et al:
Targeting Pin1 renders pancreatic cancer eradicable by synergizing
with immunochemotherapy. Cell. 184:4753–4771.e27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McGrail DJ, Pilié PG, Rashid NU, Voorwerk
L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB and Lim B:
High tumor mutation burden fails to predict immune checkpoint
blockade response across all cancer types. Ann Oncol. 32:661–672.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fu Q, Chen Y, Huang D, Guo C, Zhang X,
Xiao W, Xue X, Zhang Q, Li X, Gao S, et al: Sintilimab plus
modified FOLFIRINOX in metastatic or recurrent pancreatic cancer:
The randomized phase II CISPD3 trial. Ann Surg Oncol. 30:5071–5080.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou SQ, Wan P, Zhang S, Ren Y, Li HT and
Ke QH: Programmed cell death 1 inhibitor sintilimab plus concurrent
chemoradiotherapy for locally advanced pancreatic adenocarcinoma.
World J Clin Oncol. 15:859–866. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qiu X, Lu C, Sha H, Zhu Y, Kong W, Tong F,
Wang Q, Meng F, Liu B and Du J: Efficacy and safety of second-line
therapy by S-1 combined with sintilimab and anlotinib in pancreatic
cancer patients with liver metastasis: A single-arm, phase II
clinical trial. Front Immunol. 15:12108592024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou
Y, Ding R, Yang R, Tan J, Zhang L, et al: Neoadjuvant immunotherapy
for MSI-H/dMMR locally advanced colorectal cancer: New strategies
and unveiled opportunities. Front Immunol. 13:7959722022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xiao WW, Chen G, Gao YH, Lin JZ, Wu XJ,
Luo HL, Lu ZH, Wang QX, Sun R, Cai PQ, et al: Effect of neoadjuvant
chemoradiotherapy with or without PD-1 antibody sintilimab in pMMR
locally advanced rectal cancer: A randomized clinical trial. Cancer
Cell. 42:1570–1581. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW,
Cai PQ, Liu M, Lin JZ, Wang FL, Li C, et al: Neoadjuvant PD-1
blockade with sintilimab in mismatch-repair deficient, locally
advanced rectal cancer: An open-label, single-centre phase 2 study.
Lancet Gastroenterol Hepatol. 8:422–431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN Guidelines® insights: Cervical cancer,
version 1.2024. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gadducci A and Cosio S: Neoadjuvant
chemotherapy in locally advanced cervical cancer: Review of the
literature and perspectives of clinical research. Anticancer Res.
40:4819–4828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Monk BJ, Colombo N, Tewari KS, Dubot C,
Caceres MV, Hasegawa K, Shapira-Frommer R, Salman P, Yañez E, Gümüş
M, et al: First-line pembrolizumab + chemotherapy versus placebo +
chemotherapy for persistent, recurrent, or metastatic cervical
cancer: Final overall survival results of KEYNOTE-826. J Clin
Oncol. 41:5505–5511. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Y, Zhao J, Liang H, Liu J, Huang S,
Zou G, Huang X and Lan C: Efficacy and safety of sintilimab plus
albumin-bound-paclitaxel in recurrent or metastatic cervical
cancer: A multicenter, open-label, single-arm, phase II trial.
EClinicalMedicine. 65:1022742023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xu Q, Wang J, Sun Y, Lin Y, Liu J, Zhuo Y,
Huang Z, Huang S, Chen Y, Chen L, et al: Efficacy and safety of
sintilimab plus anlotinib for PD-L1-positive recurrent or
metastatic cervical cancer: A multicenter, single-arm, prospective
phase II trial. J Clin Oncol. 40:1795–1805. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cuthbertson DJ, Shankland R and
Srirajaskanthan R: Diagnosis and management of neuroendocrine
tumours. Clin Med (Lond). 23:119–124. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jia R, Li Y, Xu N, Jiang HP, Zhao CH, Liu
RR, Shi Y, Zhang YY, Wang SY, Zhou H and Xu JM: Sintilimab in
patients with previously treated metastatic neuroendocrine
neoplasms. Oncologist. 27:e625–e632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu X, Zhang Y, Yang KY, Zhang N, Jin F,
Zou GR, Zhu XD, Xie FY, Liang XY, Li WF, et al:
Induction-concurrent chemoradiotherapy with or without sintilimab
in patients with locoregionally advanced nasopharyngeal carcinoma
in China (CONTINUUM): A multicentre, open-label, parallel-group,
randomised, controlled, phase 3 trial. Lancet. 403:2720–2731. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tian Z, Dong S, Yang Y, Gao S, Yang Y,
Yang J, Zhang P, Wang X and Yao W: Nanoparticle albumin-bound
paclitaxel and PD-1 inhibitor (sintilimab) combination therapy for
soft tissue sarcoma: A retrospective study. BMC Cancer. 22:562022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lu X, Gu W, Shi G and Ye D: Pazopanib
together with 6–8 cycles of sintilimab followed by single use of
pazopanib in the second-line treatment of advanced renal cell
carcinoma. Transl Androl Urol. 10:2078–2083. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li X, Fang Q, Du W, Zhang X, Dai L and
Qiao Y: Induction chemotherapy combined with immunotherapy in
locally advanced head and neck squamous cell carcinoma. BMC Cancer.
21:6222021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li R, Liu X, Song C, Zhang W, Liu J, Jiao
X, Yu Y, Zeng S, Chi J, Zhao Y, et al: Sintilimab combined with
bevacizumab in relapsed/persistent ovarian clear cell carcinoma
(INOVA): An investigator-initiated, multicentre clinical trial-a
study protocol of clinical trial. BMJ Open. 12:e0581322022.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wei W, Ban X, Yang F, Li J, Cheng X, Zhang
R, Huang X, Huang Y, Li Q, Qiu Y, et al: Phase II trial of
efficacy, safety and biomarker analysis of sintilimab plus
anlotinib for patients with recurrent or advanced endometrial
cancer. J Immunother Cancer. 10:e0043382022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yao G, Huang J, Zhang Q, Hu D, Yuan F and
Han G: Excellent response of refractory triple-negative breast
cancer to sintilimab plus chemotherapy: A case report.
Immunotherapy. 15:221–228. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Molife C, Brnabic A, Stefaniak VJ, Belger
MA, Gruver K, Chen JV, Souri S and Blumenschein GR Jr: Sintilimab
plus chemotherapy for first-line treatment of advanced or
metastatic nonsquamous non-small-cell lung cancer: Network
meta-analysis. Immunotherapy. 15:293–309. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li F, Chen Y, Xiao D, Jiang S and Yang Y:
Cost-Effectiveness analysis of sintilimab plus chemotherapy in
advanced non-squamous non-small cell lung cancer: A societal
perspective. Adv Ther. 41:1436–1449. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sangro B, Sarobe P, Hervás-Stubbs S and
Melero I: Advances in immunotherapy for hepatocellular carcinoma.
Nat Rev Gastroenterol Hepatol. 18:525–543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheng J, Li Y, Wang X, Dong Z, Chen Y,
Zhang R, Huang J, Jin X, Yao J, Ge A, et al: Response
stratification in the first-line combined immunotherapy of
hepatocellular carcinoma at genomic, transcriptional and immune
repertoire levels. J Hepatocell Carcinoma. 8:1281–1295. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang S, Yuan P, Mao B, Li N, Ying J, Tao
X, Tang W, Zhang L, Geng X, Zhang F, et al: Genomic features and
tumor immune microenvironment alteration in NSCLC treated with
neoadjuvant PD-1 blockade. NPJ Precis Oncol. 6:22022. View Article : Google Scholar : PubMed/NCBI
|