Introduction
Lung cancer remains one of the leading causes of
cancer-related mortality worldwide; ~2.2 million new cases of lung
cancer are diagnosed each year, resulting in >1.8 million deaths
annually (1). In China, lung cancer
accounts for ~40% of the global burden with 0.81 million new cases
and 0.71 million deaths reported annually (2). These statistics underscore the urgent
need for more effective treatments to improve the prognosis of
patients with lung cancer (3).
Non-small cell lung cancer (NSCLC) constitutes ~85% of all lung
cancer cases, with a large proportion of patients diagnosed at a
locally advanced or metastatic stage, making them ineligible for
surgical resection (4). The
advanced stage at diagnosis notably limits the effectiveness of
traditional treatments, such as surgery, chemotherapy and
radiotherapy, which contributes to poor overall survival (OS) rates
(5). Despite these challenges,
recent years have witnessed remarkable advancements in the
treatment of advanced NSCLC (6).
The introduction of targeted therapies and immunotherapy agents has
transformed the first-line treatment landscape, positioning
advanced NSCLC as one of most successfully treated types of cancer
with precision medicine (7).
Targeted drugs, particularly those addressing EGFR mutations and
anaplastic lymphoma kinase rearrangements, have demonstrated high
efficacy. For example, the median OS for EGFR-positive patients
with advanced NSCLC is >36 months (8) and the 5-year survival rate for
patients without driver mutations is >15% (9).
In addition to targeted therapies, immunotherapy has
achieved notable breakthroughs in the treatment of advanced NSCLC,
particularly in patients without driver gene mutations (10). Immune checkpoint inhibitors
targeting programmed cell death protein 1 (PD-1), programmed
death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated
protein 4 have delivered notable survival benefits in this
population (11). Consequently,
PD-1/PD-L1 blockades have been established as the standard of care
for second-line or later therapy in patients with advanced NSCLC
(12). Moreover, recent clinical
trials, including the Keynote and IMpower series, have demonstrated
the efficacy of PD-1/PD-L1 blockades (pembrolizumab and
atezolizumab) in combination with chemotherapy as the standard
first-line treatment for advanced NSCLC (13,14).
In China, domestically developed PD-1 blockades such as
tislelizumab, sintilimab, camrelizumab and toripalimab have also
been approved for the treatment of advanced NSCLC, further
expanding the range of therapeutic options (15). These developments have made PD-1
blockades a predominant therapeutic approach for NSCLC in China,
especially as access has improved following reductions in medical
insurance costs (16).
Despite the progress achieved with PD-1/PD-L1
blockades, a significant clinical challenge remains their
relatively low objective response rate (ORR), which is <20%.
While preliminary biomarkers such as PD-L1 expression and tumor
mutational burden (TMB) have shown potential for predicting
immunotherapy efficacy, no other widely accepted biomarkers are
currently available (17). This
highlights the urgent need for additional predictive biomarkers to
identify patients most likely to benefit from PD-1/PD-L1 blockades
therapy in clinical practice (18).
Given the variability in ORR with immunotherapy, identifying
reliable biomarkers is key for guiding treatment decisions.
Biomarkers such as PD-L1 expression, TMB and microsatellite
instability have demonstrated potential in predicting responses to
PD-1 blockades (19). However,
these markers are not universally predictive and often require
invasive tissue biopsies for assessment (20). Consequently, the development of
non-invasive, easily accessible biomarkers is desirable to optimize
clinical decision-making and improve patient outcomes.
The human microbiota is comprised of trillions of
microorganisms residing in various body sites, and has emerged as a
key player in modulating the immune system (21). In particular, the gut microbiota has
been investigated for its role in systemic immune responses and its
potential impact on the efficacy of cancer immunotherapy (22,23).
Preclinical and clinical trials have demonstrated that specific gut
microbiota compositions may potentiate the therapeutic activity of
PD-1/PD-L1 blockades (23,24). The respiratory tract, including the
lungs, also harbors a diverse microbiota that may contribute to
local immune responses (25).
Emerging evidence suggests that the respiratory microbiota may
involve in the tumor microenvironment and the host's immune
response to lung cancer (26).
Moreover, the respiratory microbiota is intricately linked to
respiratory physiology, immune function maturation and the
maintenance of homeostasis (27).
However, whether the respiratory microbiota serves a role in
modulating the efficacy of PD-1 blockades in patients with advanced
NSCLC remains unclear.
The present retrospective exploratory study was
conducted to analyze the respiratory microbiota profile of patients
with advanced NSCLC treated with PD-1 blockades monotherapy. The
primary objectives were to characterize the composition of the
respiratory microbiota in these patients and to investigate its
potential association with clinical outcomes of PD-1 blockades
therapy.
Materials and methods
Study design and eligibility
criteria
A substantial number of patients with advanced NSCLC
are treated with PD-1 blockades monotherapy in clinical practice at
the Tianjin Medical University Cancer Institute and Hospital (Hexi,
China). Consequently, the present study was designed
retrospectively to include patients who received PD-1 blockades
monotherapy in Tianjin Medical University Cancer Institute and
Hospital from May 2019 to May 2023. The inclusion criteria were: i)
Histologically confirmed advanced NSCLC with pathological stage of
IIIb or IV; ii) aged ≥18 years; iii) ECOG performance status score
of 0–2; iv) treatment with PD-1 blockades monotherapy in clinical
practice; and v) available measurable target lesions according to
iRECIST criteria (28). Exclusion
criteria included: i) A history of autoimmune diseases or prior
treatment with steroids or other immunosuppressive therapies; ii)
concurrent diagnosis of another tumor or a life-threatening
disease; iii) lack of suitable specimens for respiratory microbiota
analysis; and iv) EGFR-positive mutation status. Patients with
EGFR-positive NSCLC were excluded from the present study due to
their known lower response rates to PD-1 blockades therapy compared
with that of EGFR wild-type patients (29). Excluding these patients reduced
heterogeneity in treatment responses, allowing for a more focused
analysis of the association between respiratory microbiota
diversity and the efficacy of PD-1 blockades.
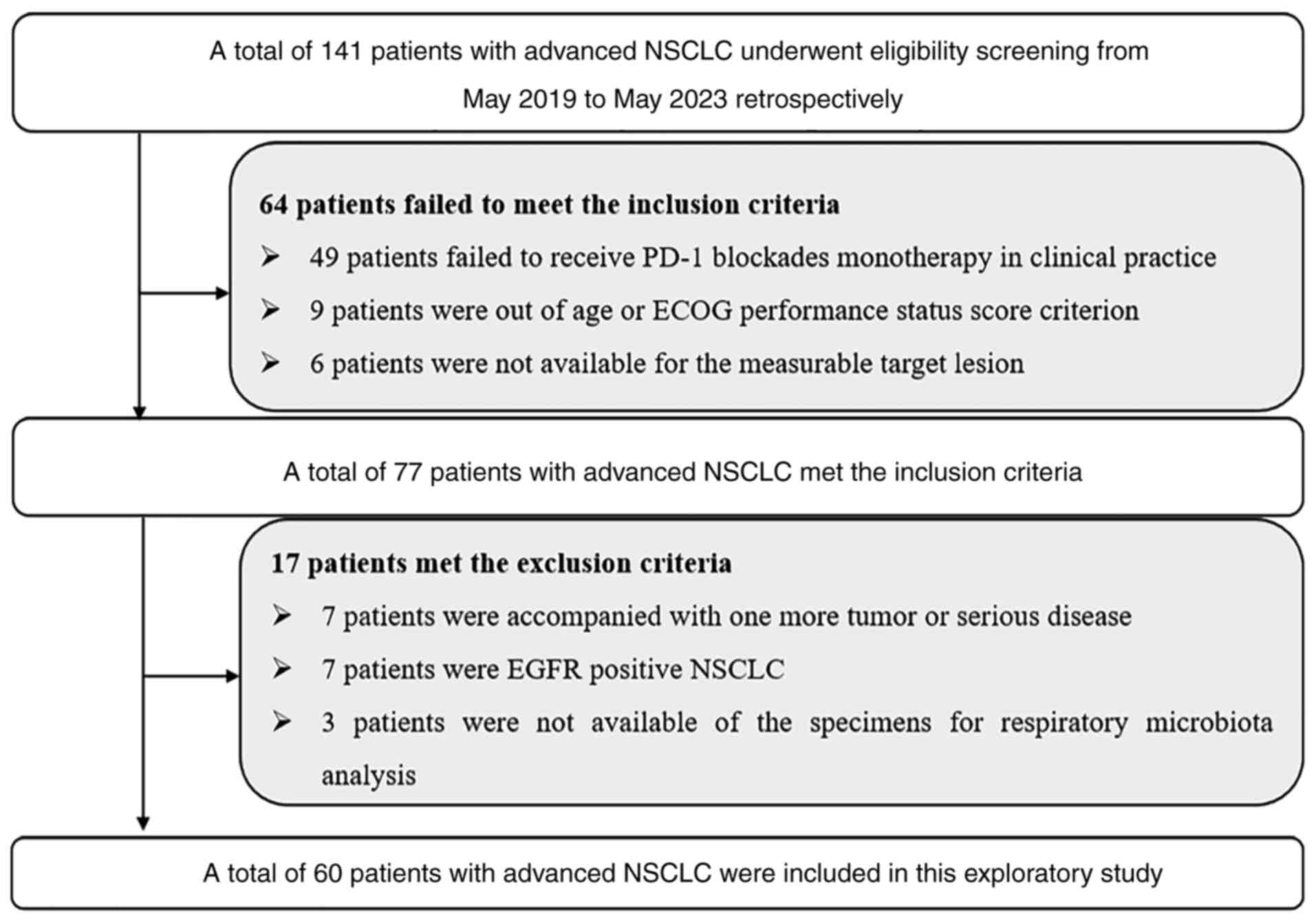
A total of 60 patients with advanced NSCLC who met
the eligible criteria were included in the present study (Fig. 1). The primary endpoint was to
elucidate the association between respiratory microbiota status and
therapeutic outcomes of PD-1 blockades monotherapy in patients with
advanced NSCLC. The present study was approved by the Ethics
Committee of Tianjin Medical University Cancer Institute and
Hospital (approval no. E20230112). Written informed consent was
obtained from all patients included in the present study in
accordance with the recommendations of the Declaration of Helsinki.
Ethics approval was obtained in May 2023, and all data collection
and analysis were performed retrospectively following approval.
Only medical records of previously treated patients were screened
and analyzed, ensuring compliance with ethical guidelines.
Therapeutic regimens and protocol for
efficacy assessment
The PD-1 blockades used in the present study
included tislelizumab (Beigene, Ltd.), sintilimab (Innovent
Biologics, Inc.) and pembrolizumab (Merck Sharp &
Dohme-Hoddesdon), which were chosen based on their availability and
approval for use in clinical practice in China at the time of the
present study. The specific dosage regimen for all three PD-1
blockades was intravenous administration with 200 mg on day 1 over
30 min, every 21 days as 1 therapeutic cycle. Administration of
PD-1 blockades was discontinued upon disease progression or the
occurrence of intolerable adverse reactions. The maximum treatment
duration was set at 2 years. Efficacy of treatment was assessed
using iRECIST criteria (28). Tumor
response was evaluated through radiological CT or MRI scans at
baseline and every 2 cycles when it was feasible. However, due to
the retrospective nature of the present study, not all patients
underwent radiological assessments every 2 cycles; some patients
had efficacy evaluations based on clinical needs. The ORR and
disease control rate (DCR) were assessed based on each patient's
best response during treatment.
Baseline and treatment characteristics were
collected from the hospital's electronic medical record system.
After disease progression following PD-1 blockades treatment,
patients were followed up monthly via telephone to record
subsequent treatments and survival status. Information on death and
exact dates of death was obtained through inquiries with patients'
relatives. The data cut-off date for the present study was 20th
August 2024.
Collection of deep-induced sputum
specimens and analysis of respiratory microbiota
The present study utilized suitable specimens that
were routinely collected from patients for respiratory microbiota
analysis. Respiratory microbiota specimens were collected from
patients immediately before the initiation of PD-1 blockade
therapy. Patients were instructed to cease antibiotic treatments 1
week prior to sample collection. Induced sputum samples were
obtained through the following procedure: Inhalation of hypertonic
saline (4.5% NaCl) via ultrasonic nebulization through the mouth
while exhaling through the nose. The nebulization lasted for 10–15
min. After nebulization, the patient's back was gently tapped to
promote sputum discharge. A total of 3–5 ml of pure sputum was
induced and collected in a sterile disposable sputum collector.
Total DNA was extracted from the sputum specimens using a bacterial
total DNA magnetic bead extraction kit- QIAamp DNA Stool Mini Kit
(QIAGEN, Hilden, Germany), after extraction, the integrity and
quality of DNA were assessed using an Agilent 2100 Bioanalyzer
(Agilent Technologies, USA). and the specimens were stored at −80°C
for subsequent respiratory microbiota analysis. Total DNA specimens
were analyzed using 16S rRNA gene sequencing. The 16S rRNA V3-V4
variable regions of the sputum DNA specimens were amplified using
the following primers: Illumina_16S_341 forward,
5′-CCTAYGGGRBGCASCAG-3′ and Illumina_16S_806 reverse,
5′-GGACTACNNGGGTATCTAAT-3′ (Illumina, Inc.). The final DNA library
concentration was measured using a Qubit fluorometer, and each
library was then diluted to 10 pM prior to sequencing to ensure
optimal cluster generation. Sequencing libraries were prepared
using the NEBNext® Ultra™ DNA Library Prep Kit for
Illumina® (cat. no. E7370; New England BioLabs, Inc.).
Sequencing was performed on an Illumina high-throughput platform
with a paired-end 300 bp configuration, providing high overlap
between forward and reverse reads. The libraries were loaded onto
the MiSeq (Illumina, Inc.) sequencing platform and run in 2×300 bp
paired-end mode. This produced raw paired-end reads for each
sample, with unique index barcodes allowing multiplexing of samples
in a single run. PCR products were sequenced on the
Illumina® platform (PE300; Illumina, Inc.) for analysis.
Reads from each pair were then merged using FLASH software
(v1.2.7), which overlapped the forward and reverse reads to
reconstruct the full V3-V4 amplicon sequence. These merged reads
(raw tags) underwent stringent quality filtering using QIIME
(v1.7.0, http://qiime2.org/). These primers
incorporated degenerate bases to enable broad coverage across
diverse bacterial taxa. This design ensured inclusivity of various
bacterial genomes present in respiratory microbiota samples, as
validated by a previous study (30). The high-quality reads were then
grouped into operational taxonomic units (OTUs) based on sequence
similarity. OTU clustering was performed using Uparse software
(v7.0.1001, http://drive5.com/uparse/)
16S rRNA gene sequencing targeting the V3-V4 regions
was conducted using the Illumina MiSeq (Illumina, Inc.) platform
with a target sequencing depth of at least 15,000 reads per sample.
Quality filtering was applied to remove reads with a Phred score
<20, and chimeric sequences were identified and excluded using
the UCHIME algorithm. Reads were clustered into operational
taxonomic units at 97% similarity using the QIIME pipeline, and
rarefaction was performed at 15,000 reads per sample to standardize
sequencing depth (31).
Based on the sequencing results of the 16S rRNA from
respiratory microbiota, the 60 patients with advanced NSCLC were
divided into high (H) α diversity group and low (L) α diversity
group based on the median Shannon diversity index value of the
cohort, which was calculated to be 5.09. The Shannon index, which
combined richness and evenness of species within a sample, is a
commonly used metric for microbiota diversity (32). The median cut-off was chosen rather
than an arbitrary or literature-based threshold, because no
universal clinical cut-off for the Shannon index in relation to
PD-1 blockade efficacy was established currently (33). Additionally, to complement the
findings from the Shannon diversity index, the Simpson diversity
index was also used (34), and the
60 patients were also divided into H α diversity group and L α
diversity group based on the median Simpson diversity index value
(0.91). Among the 60 subjects, the Simpson diversity index was
similar with and correlated with the Shannon diversity index in 55
patients (91.7%), indicating agreement between the two indices.
The H group was consisted of 30 patients, while the
L group included 30 patients. Subsequent analyses were conducted to
compare the therapeutic outcomes of PD-1 blockades monotherapy
between the two groups.
Statistical analysis
The ORR in the present study was calculated as the
proportion of patients who achieved complete response (CR) or
partial response (PR) during treatment. The DCR was calculated as
the proportion of patients who achieved CR, PR or stable disease
(SD) during treatment. Data analysis was performed using SPSS
(version 25.0; IBM Corp.). The association between respiratory
microbiota groups and ORR or DCR was analyzed using the
χ2 test, with Fisher's exact test applied for sparse
data (cut-off value of <5 samples). PFS and OS were analyzed
using Kaplan-Meier curves plotted using the Stata software (v14.0;
Stata Corp LP). The association between respiratory microbiota
groups and PFS was compared using the log-rank test. PFS was
defined as the time from the initiation of PD-1 blockade treatment
to tumor progression or death, and OS was defined as the time from
the initiation of PD-1 blockade treatment to death from any cause
(35). Patients who had not
experienced disease progression or death by the data cut-off were
considered censored. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline clinical characteristics,
demographics and respiratory microbiota profiles of 60 patients
with advanced NSCLC
Baseline clinical characteristics of the 60 patients
with advanced NSCLC included in the present study were summarized
in Table I. Patients enrolled were
representative of common clinical presentations of advanced NSCLC
with a median age of 64 years (range, 22–81 years). Adenocarcinoma
and squamous cell carcinoma were diagnosed in 31 and 29 patients,
respectively. All patients had previously treated advanced NSCLC,
with prior first-line therapy reported in 13 patients and
subsequent-line therapy in 47 patients. As described, three PD-1
blockades were administered: Tislelizumab, sintilimab and
pembrolizumab, used in 28, 22 and 10 patients, respectively. The
baseline clinical characteristics of patients in H and L α
diversity groups were comparable, as no statistically significant
differences were observed (P>0.05; Table I). Similarly, there was no
statistically significant difference in the proportion of
adenocarcinoma and squamous cell carcinoma cases between the H and
L groups (χ2=139; P=0.710).
 | Table I.Baseline characteristics of the 60
patients with advanced NSCLC according to respiratory microbiology
H (n=30) and L (n=30) α diversity status. |
Table I.
Baseline characteristics of the 60
patients with advanced NSCLC according to respiratory microbiology
H (n=30) and L (n=30) α diversity status.
| Baseline
characteristics | Overall, n (%) | L, n (%) | H, n (%) | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
| Median
(range) | 64 (22–81) |
|
| 0.271 | 0.602 |
|
≥64 | 34 (56.7) | 18 (60.0) | 16 (53.3) |
|
|
|
<64 | 26 (43.3) | 12 (40.0) | 14 (46.7) |
|
|
| ECOG performance
status score |
|
|
| 0.071 | 0.791 |
|
0-1 | 23 (38.3) | 12 (40.0) | 11 (36.7) |
|
|
| 2 | 37 (61.7) | 18 (60.0) | 19 (63.3) |
|
|
| Sex |
|
|
| 0.287 | 0.592 |
|
Male | 38 (63.3) | 20 (66.7) | 18 (60.0) |
|
|
|
Female | 22 (36.7) | 10 (33.3) | 12 (40.0) |
|
|
| Pathological
staging |
|
|
| 0.741 | 0.389 |
|
IIIb | 6 (10.0) | 4 (13.3) | 2 (6.7) |
|
|
| IV | 54 (90.0) | 26 (86.7) | 28 (93.3) |
|
|
| Smoking status |
|
|
| 0.884 | 0.347 |
|
Non-smoker | 13 (21.7) | 8 (26.7) | 5 (16.7) |
|
|
|
Former/current smoker | 47 (78.3) | 22 (73.3) | 25 (83.3) |
|
|
| Histological
category |
|
|
| 0.067 | 0.796 |
|
Adenocarcinoma | 31 (51.7) | 16 (53.3) | 15 (50.0) |
|
|
|
Squamous cell carcinoma | 29 (48.3) | 14 (46.7) | 15 (50.0) |
|
|
| Lines of previous
treatment |
|
|
| 0.884 | 0.347 |
|
First-line | 13 (21.7) | 5 (16.6) | 8 (26.7) |
|
|
|
Subsequent line | 47 (78.3) | 25 (83.4) | 22 (73.3) |
|
|
| History of surgical
resection |
|
|
| 0.317 | 0.573 |
|
Yes | 18 (30.0) | 8 (26.7) | 10 (33.3) |
|
|
| No | 42 (70.0) | 22 (73.3) | 20 (66.7) |
|
|
| No. of metastatic
lesions |
|
|
|
|
|
| ≤3 | 38 (63.3) | 18 (60.0) | 20 (66.7) | 0.287 | 0.592 |
|
>3 | 22 (36.7) | 12 (40.0) | 10 (33.3) |
|
|
| PD-1 blockades |
|
|
| 0.543 | 0.762 |
|
Tislelizumab | 28 (46.7) | 13 (43.3) | 15 (50.0) |
|
|
|
Sintilimab | 22 (36.7) | 11 (36.7) | 11 (36.7) |
|
|
|
Pembrolizumab | 10 (16.6) | 6 (20.0) | 4 (13.3) |
|
|
Regarding the respiratory microbiota landscape
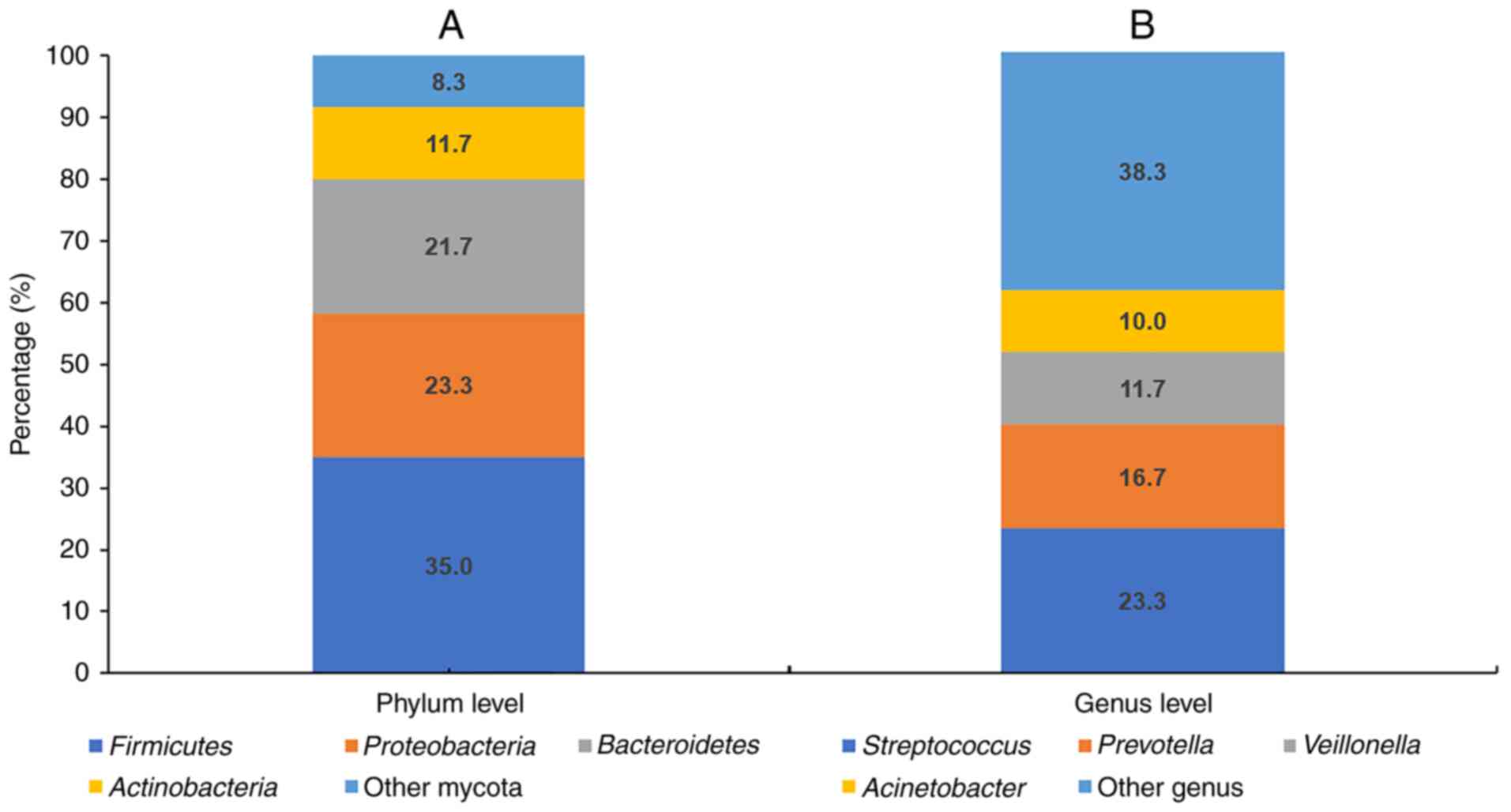
(Fig. 2), the most abundant phyla
identified at the phylum level among the 60 patients with advanced
NSCLC were firmicutes (35.0%), proteobacteria (23.3%),
bacteroidetes (21.7%), actinobacteria (11.7%) and other mycota
(8.3%). At the genus level, the most abundant genera were
Streptococcus (23.3%), Prevotella (16.7%),
Veillonella (11.7%), Acinetobacter (10.0%) and other
genera (38.3%). Based on the Shannon diversity index, patients were
categorized into H group and L group, with 30 and 30 patients in
each group, respectively.
Association between efficacy of PD-1
blockades monotherapy and respiratory microbiota α diversity
status
Radiological assessment results for all 60 patients
were collected and recorded. Treatment efficacy was evaluated based
on the best response observed during therapy. Among the 60
patients, no CR or PR were observed in 14 patients, SD was found in
21 patients and progressive disease was documented in 25 patients,
yielding an ORR of 23.3% (95% CI, 13.4–36.0%) and a DCR of 58.3%
(95% CI, 44.9–70.9%).
Subsequent analysis of the association between
respiratory microbiota α diversity status and therapeutic outcomes
suggested that the ORR in H group and L group was 26.7 and 20.0%,
respectively, which showed no statistical significance
(χ2=0.373; P=0.542) and the odds ratio (OR) was 1.46
(95% CI, 0.44–4.86). To further explore this, the microbiota
profiles of patients in the L group who achieved PR, were analyzed.
The results indicated no significant correlation between these
cases and the microbiota characteristics typical of the H group
(P>0.05). The PR values in the L group suggested that factors
beyond respiratory microbiota diversity might contribute to PD-1
blockade efficacy. These factors might include genetic variations,
baseline immune system status or other unmeasured clinical factors.
Such findings emphasize the need for a multifactorial approach to
identify robust biomarkers for predicting PD-1 blockade
efficacy.
Furthermore, the DCRs in H group and L group were
70.0 and 46.7%, respectively, which demonstrated a notable
difference (χ2=3.360; P=0.067) and the OR was 2.67 (95%
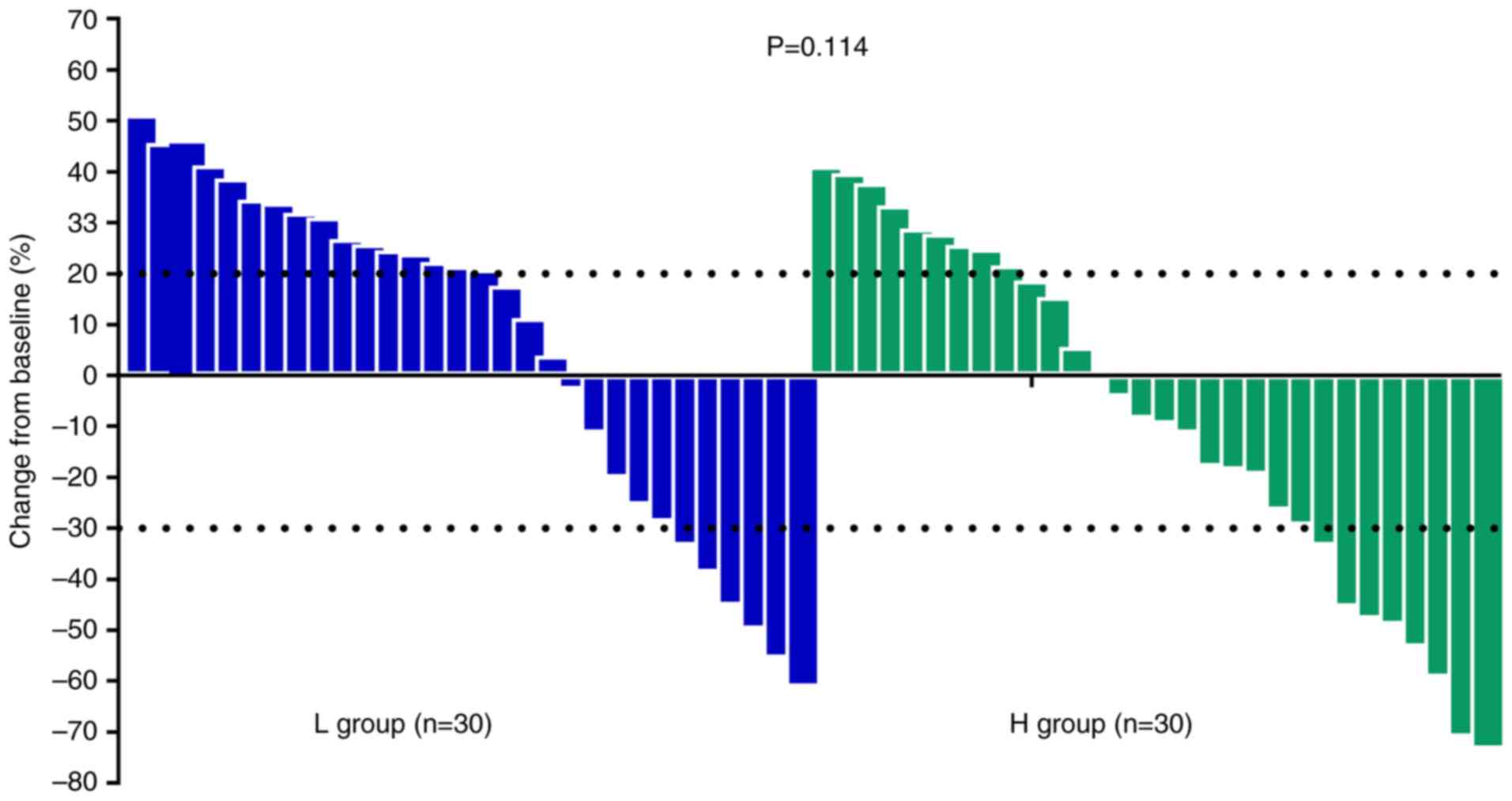
CI, 0.92–7.70). The waterfall plot illustrating the maximum
reduction of target lesion during treatment in target lesions for
patients in the H and L groups is presented in Fig. 3. Although target lesion shrinkage
appeared more pronounced in the H group compared with that of the L
group, the difference did not reach statistical significance
(P=0.114).
Association between prognosis of PD-1
blockades monotherapy and respiratory microbiota α diversity
status
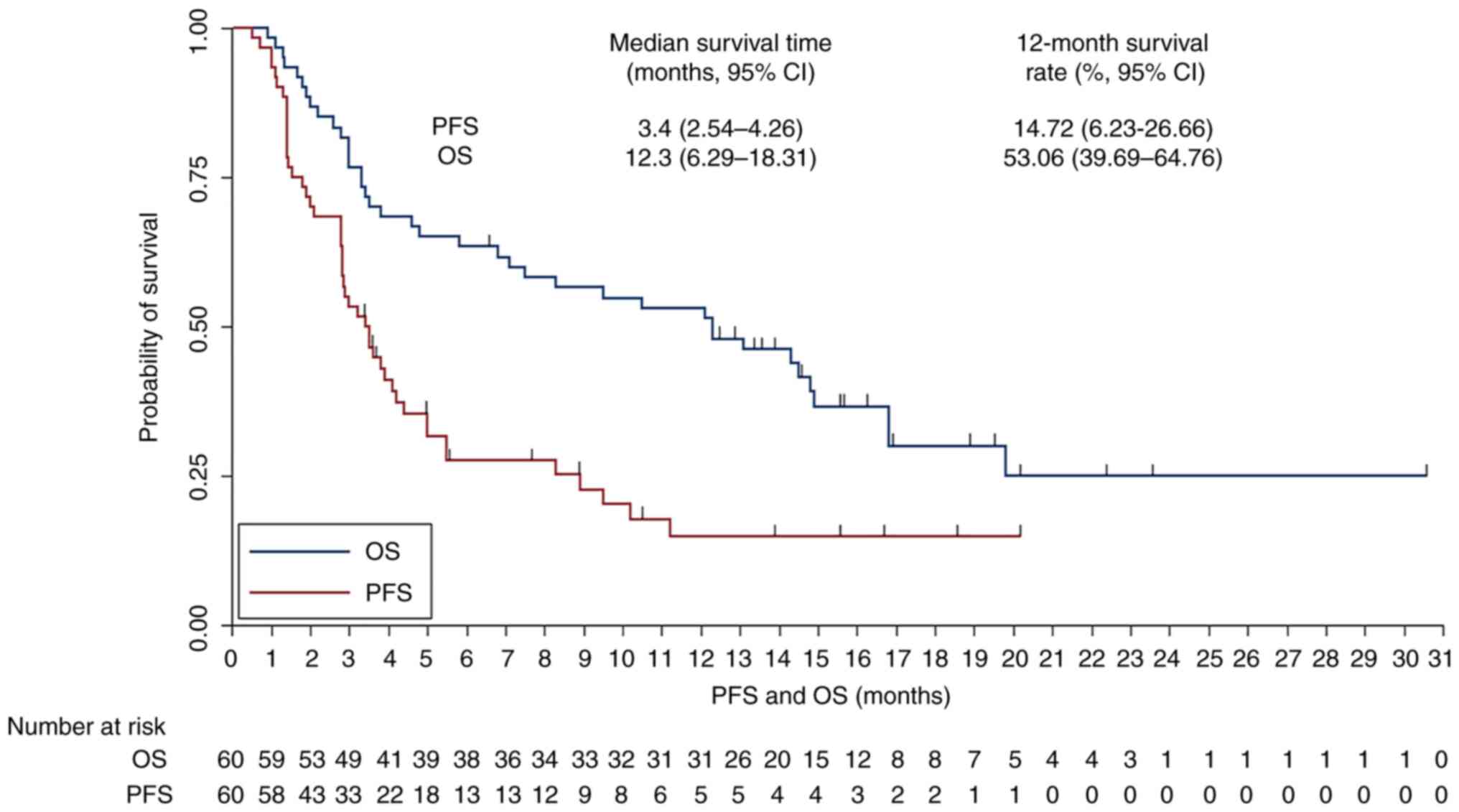
The median follow-up duration of the present study
was 10.3 months (range, 0.5–30.5 months) for all enrolled patients.
The PFS and OS survival curves were presented in Fig. 4. The median PFS for the 60 patients
with advanced NSCLC receiving PD-1 blockades monotherapy was 3.4
months (95% CI, 2.54–4.26) with a 6-month PFS rate of 27.53% (95%
CI, 16.57–39.63%) and a 12-month PFS rate of 14.72% (95% CI,
6.23–26.66%). Additionally, the median OS for the 60 patients with
advanced NSCLC was 12.3 months (95% CI, 6.29–18.31) with a 12-month
OS rate of 53.06% (95% CI, 39.69–64.76%) and a 24-month OS rate of
24.95% (95% CI, 12.19–40.02%).
The association between respiratory microbiota α
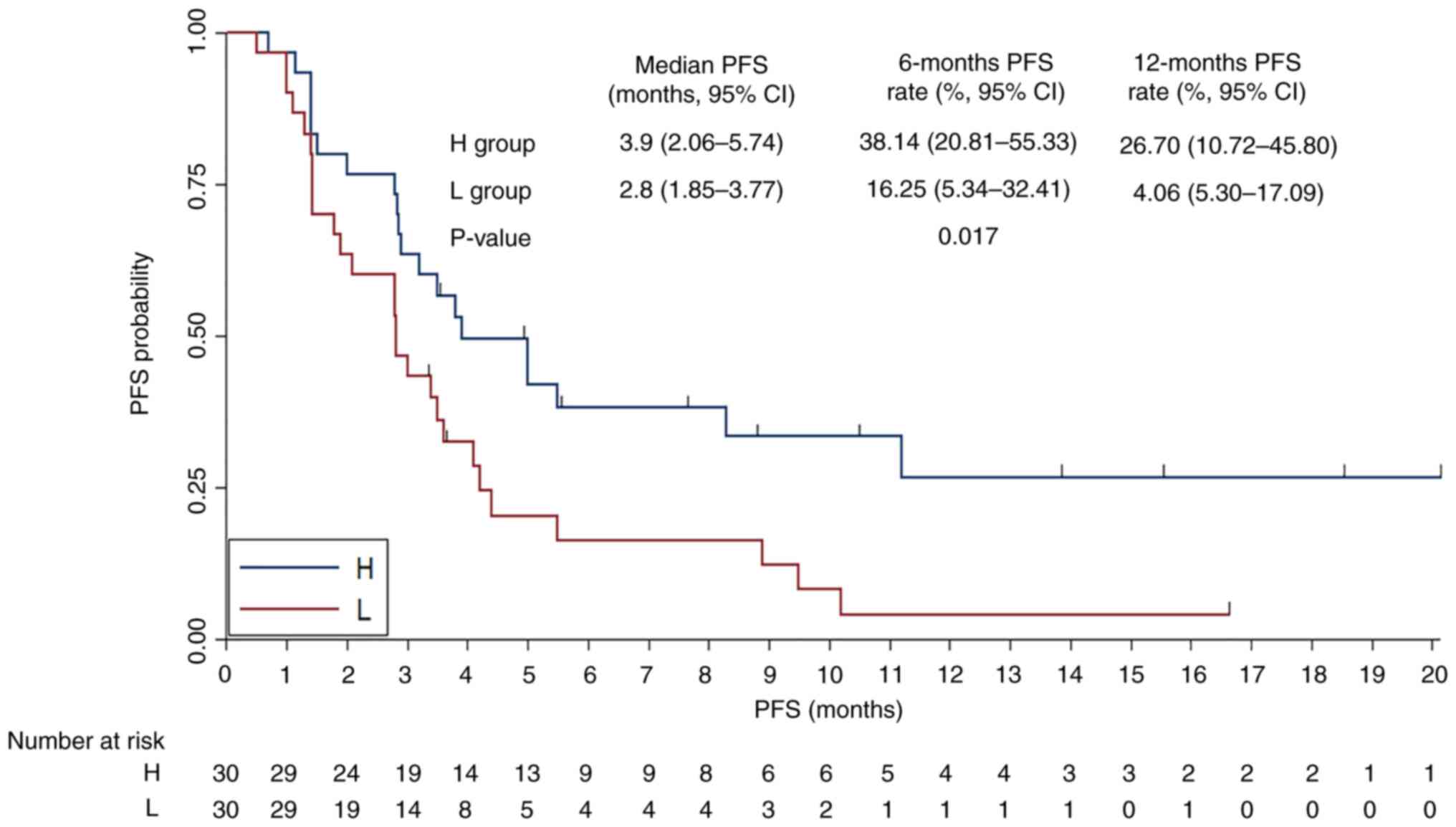
diversity status and PFS was subsequently analyzed (Fig. 5). The median PFS was 3.9 months (95%
CI, 2.06–5.74) for the H α diversity group and 2.8 months (95% CI,
1.85–3.77) for the L group. The 6-month PFS rates were 38.14% for
the H group and 16.25% for the L group, while the 12-month PFS
rates were 26.70 and 4.06%, respectively. The differences
demonstrated statistical significance (χ2=5.670;
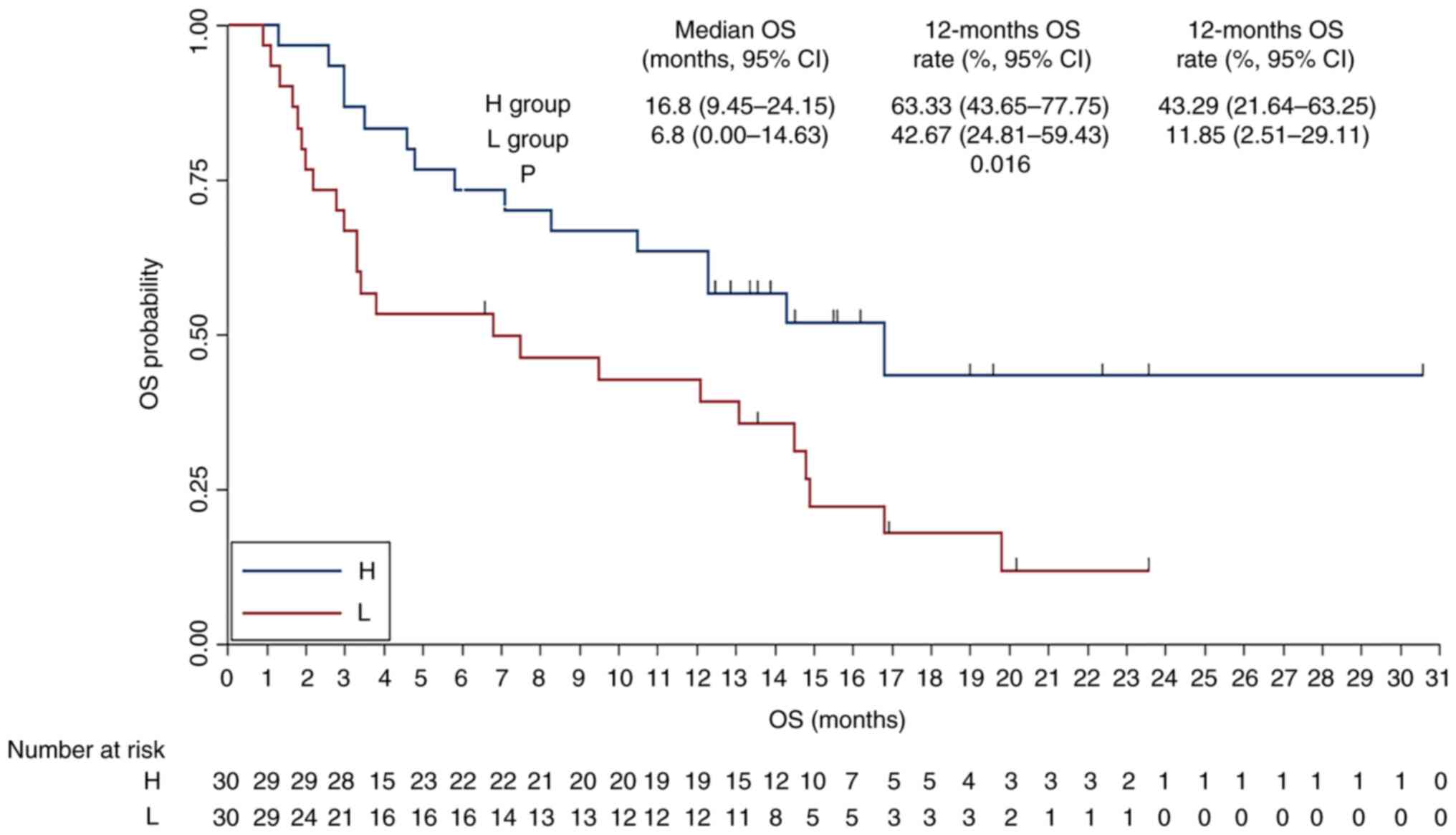
P=0.017). The association between respiratory microbiota α
diversity status and OS was also analyzed (Fig. 6), the median OS was 16.8 months (95%
CI, 9.45–24.15) for the H group and 6.8 months (95% CI, 0.00–14.63)
for the L group. The 12-month OS rates were 63.33% for the H group
and 42.76% for the L group, while the 24-month OS rates were 43.29
and 11.85%, respectively and the difference was statistically
significant (χ2=5.809; P=0.016).
Discussion
The present study retrospectively analyzed the
efficacy of PD-1 blockades monotherapy in 60 patients with advanced
NSCLC. The preliminary findings suggested that PD-1 blockades might
provide survival benefits for patients with advanced NSCLC in
clinical practice. Furthermore, the association analysis between
respiratory microbiota status and therapeutic outcomes demonstrated
that higher α diversity of respiratory microbiota was associated
with increased PFS and OS. These findings indicated that
respiratory microbiota diversity could be used as a potential
biomarker to predict the efficacy of PD-1 blockades. Nevertheless,
these results warrant further confirmation with prospective
clinical trials in the future.
As a highly heterogeneous respiratory malignancy,
lung cancer demonstrates a rising incidence in the Chinese
population and remains the leading cause of cancer-related deaths
in China currently (36). Despite
breakthroughs in therapy of advanced NSCLC with targeted drugs and
PD-1/PD-L1 blockades offering substantial survival benefits in
first-line treatment (37), PD-1
blockades have only been available in China since 2018.
Consequently, a considerable number of patients who had previously
undergone chemotherapy and experienced disease progression have
been able to receive PD-1 monotherapy, achieving notable survival
benefits in clinical practice (38). In the present study, only 22% of
patients had previously received first-line treatment, while 78% of
the 60 patients with advanced NSCLC included were treated with PD-1
blockades therapy as third-line or later treatment. Given the
demonstrated efficacy of PD-1 blockades in both lung adenocarcinoma
and squamous cell carcinoma in prior clinical trials (39,40),
~52% of the patients in the present study were diagnosed with
either lung adenocarcinoma or squamous cell carcinoma, consistent
with findings from previous research (41). Furthermore, the inclusion of both
imported and domestic PD-1 blockades such as tislelizumab,
sintilimab and pembrolizumab, reflects the current clinical
therapeutic trend, which highlights the widespread use of various
PD-1 blockades in the treatment of advanced NSCLC in China,
aligning with findings from a previous exploratory analysis of PD-1
blockades in NSCLC (42).
Although designed as a retrospective analysis, the
present study evaluated the therapeutic outcomes and prognosis of
PD-1 blockades monotherapy in 60 patients with advanced NSCLC in
clinical practice. Therapeutic outcomes suggested that in a
real-world setting, the ORR of PD-1 blockades monotherapy in
patients with advanced NSCLC was 23.3%, with a DCR of 58.3% and a
median PFS of 3.4 months. These outcomes were where increased in
comparison to those reported for second-line monotherapy with
docetaxel in advanced NSCLC, which demonstrated an ORR of ~10%, a
DCR of 55% and a median PFS of 3 months (43). However, it seemed that the efficacy
and PFS results in the present study were slightly decreased to
those reported for pembrolizumab as the second-line treatment in
advanced NSCLC in the Keynote-010 trial, which achieved an ORR of
~20% and a median PFS of 4 months (44). It could be considered that the
discrepancy might be due to two main factors; first, the
Keynote-010 trial recruited only patients with PD-L1 positive
expression (>1%), while the present retrospective study did not
perform PD-L1 expression testing on enrolled patients potentially
including some patients with PD-L1-negative expression, which might
result in worse therapeutic outcomes. Second, as a retrospective
study, patient management and medication adherence in this analysis
were relatively inferior compared with the rigorously controlled
conditions of phase III clinical trials. A previous study also
concluded that therapeutic outcomes from retrospective study tended
to be slightly worse than those from clinical trials (45). Due to the relatively long follow-up
period, OS analysis was also carried out in the present study. The
results highlighted that the median OS for the 60 patients with
advanced NSCLC was 12.3 months with a 12-month OS rate of 53.06%
(95% CI, 39.69–64.76%), which was slightly longer compared with
those previously reported for docetaxel monotherapy and increased
in comparison with the OS outcomes reported for pembrolizumab
monotherapy in patients with advanced NSCLC (46). This improvement may be attributed to
the introduction of various new targeted therapies and
immunotherapy agents since 2018, particularly the approval of
anlotinib as a third-line therapy for advanced NSCLC, which has
provided significant survival benefits (47). As a result, patients in the present
study who experienced disease progression after PD-1 blockades
treatment were also able to receive subsequent treatments such as
anlotinib or PD-L1 blockades, which may have contributed to
survival benefits.
Furthermore, the present study also investigated the
association between respiratory microbiota and therapeutic
outcomes. The respiratory microbiota analysis suggested that the
most common phylum level among patients with advanced NSCLC were
Firmicutes (35.0%), Proteobacteria (23.3%), Bacteroidetes (21.7%)
and Actinobacteria (11.7%). And the most common genera in genus
level were Streptococcus (23.3%), Prevotella (16.7%),
Veillonella (11.7%) and Acinetobacter (10.0%). These
microbiota findings were consistent with previous research by Apopa
et al (48), who analyzed
lung biopsy specimens from 29 patients with lung cancer using 16S
rRNA sequencing and reported that Bacteroidetes and Proteobacteria
were predominant phyla in patients with lung cancer. Besides,
another study highlighted that the significant differences existed
in lung microbiota between patients with lung cancer and healthy
individuals, noting that tumor tissue microbiota exhibited lower α
diversity compared with healthy lung tissue (49). These findings underscored a
potential role of respiratory microbiota in the occurrence and
development of lung cancer. Moreover, correlation between
respiratory microbiota α diversity and prognosis observed in the
present study suggested that higher α diversity of respiratory
microbiota was associated with superior therapeutic outcomes among
patients receiving PD-1 blockades therapy (median PFS, 3.8 vs. 2.8
months; median OS, 16.8 vs. 6.8 months). To the best of our
knowledge, the present results indicated for the first time that α
diversity of respiratory microbiota might be used as a potential
biomarker to predict the therapeutic outcomes of PD-1 blockades
clinically. The higher ORR and DCR observed in the high α diversity
group might be explained by the role of microbiota diversity in
modulating immune responses. A diverse microbiota composition was
thought to support a more balanced and robust immune
microenvironment, which might enhance the efficacy of PD-1
blockades by promoting regulatory T-cell function, pro-inflammatory
cytokine production and effective antigen presentation (50). A previous study also reported
similar findings, where microbiota diversity was associated with
improved responses to immunotherapy, likely due to its influence on
systemic immune priming (51). Jin
et al (52) explored the
relationship between gut microbiota diversity and therapeutic
outcomes of PD-1 blockades in patients with advanced NSCLC who
included 37 patients with advanced NSCLC and found that higher gut
microbiota diversity was significantly associated with higher ORR
and longer PFS. Additionally, an exploratory study Jang et
al (53) included a total of 84
patients with advanced NSCLC to investigate the relationship of
lung microbiome with immunotherapy response in lung cancer. It was
found that abundances of Neisseria and Veillonella
differed significantly in relation to PD-L1 expression levels and
immunotherapy response, suggesting that human microbiome might
serve a certain role in the immune system in vivo.
Collectively, these results were consistent with the present
findings, highlighting that the respiratory microbiota might
involve in immune responses and mediate differences in the
therapeutic outcomes of PD-1 blockades in clinical practice.
However, the specific mechanisms by which respiratory microbiota
contributed to the efficacy of PD-1 blockades remained unclear and
warranted further investigation. Understanding these mechanisms
might provide valuable insights into optimizing immunotherapy
strategy and improving patient outcomes. While the present study
focused on the association between respiratory microbiota diversity
and clinical efficacy outcomes of PD-1 blockades therapy, the
potential influence of microbiota on immune-related adverse events
remained an area for future exploration. It has been suggested that
that certain microbial profiles might affect immune modulation and
inflammatory responses (33).
Future studies should examine the relationship between respiratory
microbiota diversity and adverse events of immunotherapy, which
might offer predictive insights for immune-related adverse events
risk management in clinical practice.
The present study had several limitations that
should be acknowledged. Firstly, designed as a real-world study,
the sample size was relatively small and the conclusion remains to
be confirmed in larger patient cohorts. Additionally, the
retrospective nature of this analysis meant that patient management
was less rigorous compared with that of well-controlled clinical
trials, potentially introducing biases. Besides, as the present
study collected microbiota specimens only at baseline, it was not
possible to track potential changes in bacterial flora during PD-1
blockades therapy. Future prospective trials should consider
longitudinal sampling of microbiota to assess dynamic changes
during PD-1 blockades therapy and their association with treatment
outcomes. In addition, targeting specific bacterial taxa, such as
Akkermansia muciniphila and bifidobacterium species,
might provide valuable insights, as these had been previously
linked to enhanced immunotherapy responses. Finally, incorporating
immune-related biomarkers, such as cytokine levels, regulatory
T-cell counts and PD-L1 expression, could offer a more
comprehensive understanding of the interactions between microbiota
and the immune system in the context of PD-1 blockades.
Furthermore, the present study was limited to a diversity analysis
due to data constraints, and b diversity analyses could not be
performed. Future studies should include b diversity metrics, such
as Bray-Curtis or UniFrac distances, to assess differences in
microbial composition between patients and explore their potential
association with treatment outcomes of PD-1 blockades. Despite
these limitations, the present study provided preliminary insights
into the therapeutic outcomes of PD-1 blockades monotherapy in
patients with advanced NSCLC and found a significant association
between respiratory microbiota α diversity and therapeutic
outcomes. These findings may have potential clinical implications
in the selection of PD-1 blockade therapy for advanced NSCLC in
clinical practice.
The present findings suggested that microbiota
diversity testing might have potential as a predictive biomarker
for PD-1 blockades efficacy. If further validated, this testing
could be integrated into clinical practice to guide treatment
selection and stratify patients in immunotherapy trials in the
future. Additionally, microbiome-targeted interventions to increase
microbial diversity could be explored as a novel approach to
enhance treatment outcomes for patients with low diversity in
clinical practice.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from Tianjin Science
and Technology Fund (grant no. 20JCYBJC00600).
Availability of data and materials
The data generated in the present study may be found
in the figshare repository (accession no. 10.6084/m9.figshare.
28102844.v1) at the following URL: https://doi.org/10.6084/m9.figshare.28102844.v1.
Authors' contributions
LZ, PW and WDZ designed the study, performed the
data of this study and wrote this manuscript. MJL, XPL, BY and TX
collected the data, conducted the experiment and participated in
the patients' follow-up. PW and WDZ guided the design and
supervised the present study, LZ and WDZ confirm the authenticity
of all the raw data. All agreed to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work were appropriately investigated and resolved. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University Cancer Institute and
Hospital (approval no. E20230112; Hexi, China). Written informed
consent was obtained by each enrolled patient according to the
recommendations of the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rong Y, Bentley JP, Bhattacharya K, Yang
Y, Chang Y, Earl S and Ramachandran S: Incidence and risk factors
of immune-related adverse events induced by immune checkpoint
inhibitors among older adults with non-small cell lung cancer.
Cancer Med. 13:e68792024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito K, Hashimoto K, Kaira K, Yamaguchi O,
Mouri A, Shiono A, Miura Y, Kobayashi K, Imai H, Kuji I and Kagamu
H: Clinical impact of inflammatory and nutrition index based on
metabolic tumor activity in non-small cell lung cancer treated with
immunotherapy. Oncol Lett. 27:1102024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González-Cao M, Cai X, Bracht JWP, Han X,
Yang Y, Pedraz-Valdunciel C, Morán T, García-Corbacho J, Aguilar A,
Bernabé R, et al: hmgb1 expression levels correlate with response
to immunotherapy in non-small cell lung cancer. Lung Cancer
(Auckl). 15:55–67. 2024.PubMed/NCBI
|
|
5
|
Zhang CL, Gao MQ, Jiang XC, Pan X, Zhang
XY, Li Y, Shen Q, Chen Y and Pang B: Research progress and value of
albumin-related inflammatory markers in the prognosis of non-small
cell lung cancer: A review of clinical evidence. Ann Med.
55:1294–1307. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorin M, Prosty C, Ghaleb L, Nie K,
Katergi K, Shahzad MH, Dubé LR, Atallah A, Swaby A, Dankner M, et
al: Neoadjuvant chemoimmunotherapy for NSCLC: A systematic review
and meta-analysis. JAMA Oncol. 10:621–633. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrera-Juárez M, Serrano-Gómez C,
Bote-de-Cabo H and Paz-Ares L: Targeted therapy for lung cancer:
Beyond EGFR and ALK. Cancer. 129:1803–1820. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Remon J, Besse B, Aix SP, Callejo A,
Al-Rabi K, Bernabe R, Greillier L, Majem M, Reguart N, Monnet I, et
al: Osimertinib treatment based on plasma T790M monitoring in
patients with EGFR-mutant non-small-cell lung cancer (NSCLC): EORTC
Lung Cancer Group 1613 APPLE phase II randomized clinical trial.
Ann Oncol. 34:468–476. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun L, Bleiberg B, Hwang WT, Marmarelis
ME, Langer CJ, Singh A, Cohen RB, Mamtani R and Aggarwal C:
Association between duration of immunotherapy and overall survival
in advanced non-small cell lung cancer. JAMA Oncol. 9:1075–1082.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang Y, Liu X, Wang Y, Zheng D, Meng Q,
Jiang L, Yang S, Zhang S, Zhang X, Liu Y and Wang B: Mechanisms of
resistance to targeted therapy and immunotherapy in non-small cell
lung cancer: Promising strategies to overcoming challenges. Front
Immunol. 15:13662602024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu M, Yi M, Li N, Luo S and Wu K:
Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp
Hematol Oncol. 10:182021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen
L and Zhou J: The progress and challenge of anti-PD-1/PD-L1
immunotherapy in treating non-small cell lung cancer. Ther Adv Med
Oncol. 13:17588359219929682021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Castro G Jr, Kudaba I, Wu YL, Lopes G,
Kowalski DM, Turna HZ, Caglevic C, Zhang L, Karaszewska B,
Laktionov KK, et al: Five-year outcomes with pembrolizumab versus
chemotherapy as first-line therapy in patients with non-small-cell
lung cancer and programmed death ligand-1 tumor proportion score ≥
1% in the KEYNOTE-042 study. J Clin Oncol. 41:1986–1991. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Socinski MA, Nishio M, Jotte RM, Cappuzzo
F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D,
Moro-Sibilot D, Thomas CA, et al: IMpower150 final overall survival
analyses for atezolizumab plus bevacizumab and chemotherapy in
first-line metastatic nonsquamous NSCLC. J Thorac Oncol.
16:1909–1924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Yang Y, Yu J, Zhang S, Li X, Wu X,
Nie X, Liu W, Zhang P, Li Y, et al: Efficacy and safety of
anti-PD-1/PD-L1 in combination with chemotherapy or not as
first-line treatment for advanced non-small cell lung cancer: A
systematic review and network meta-analysis. Thorac Cancer.
13:322–337. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Wang W, Yuan Q, Li L, Wang YC,
Chi CZ and Xu CH: Correlation between immune-related adverse events
and the efficacy of PD-1/PD-L1 inhibitors in the treatment of
non-small cell lung cancer: Systematic review and meta-analysis.
Cancer Chemother Pharmacol. 89:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma YT, Hua F, Zhong XM, Xue YJ, Li J, Nie
YC, Zhang XD, Ma JW, Lin CH, Zhang HZ, et al: Clinicopathological
characteristics, molecular landscape, and biomarker landscape for
predicting the efficacy of PD-1/PD-L1 inhibitors in Chinese
population with mismatch repair deficient urothelial carcinoma: A
real-world study. Front Immunol. 14:12690972023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bie F, Tian H, Sun N, Zang R, Zhang M,
Song P, Liu L, Peng Y, Bai G, Zhou B and Gao S: Research progress
of anti-PD-1/PD-L1 immunotherapy related mechanisms and predictive
biomarkers in NSCLC. Front Oncol. 12:7691242022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zurlo IV, Schino M, Strippoli A, Calegari
MA, Cocomazzi A, Cassano A, Pozzo C, Di Salvatore M, Ricci R,
Barone C, et al: Predictive value of NLR, TILs (CD4+/CD8+) and
PD-L1 expression for prognosis and response to preoperative
chemotherapy in gastric cancer. Cancer Immunol Immunother.
71:45–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanus M, Parada-Venegas D, Landskron G,
Wielandt AM, Hurtado C, Alvarez K, Hermoso MA, López-Köstner F and
De la Fuente M: Immune system, microbiota, and microbial
metabolites: The unresolved triad in colorectal cancer
microenvironment. Front Immunol. 12:6128262021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu Y, Yuan X, Wang M, He Z, Li H, Wang J
and Li Q: Gut microbiota influence immunotherapy responses:
Mechanisms and therapeutic strategies. J Hematol Oncol. 15:472022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou CB, Zhou YL and Fang JY: Gut
microbiota in cancer immune response and immunotherapy. Trends
Cancer. 7:647–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao G, Shen S, Zhang T, Zhang J, Huang S,
Sun Z and Zhang H: Lacticaseibacillus rhamnosus Probio-M9 enhanced
the antitumor response to anti-PD-1 therapy by modulating
intestinal metabolites. EBioMedicine. 91:1045332023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mifsud EJ, Kuba M and Barr IG: Innate
immune responses to influenza virus infections in the upper
respiratory tract. Viruses. 13:20902021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiesweg M, Mairinger F, Reis H, Goetz M,
Kollmeier J, Misch D, Stephan-Falkenau S, Mairinger T, Walter RFH,
Hager T, et al: Machine learning reveals a PD-L1-independent
prediction of response to immunotherapy of non-small cell lung
cancer by gene expression context. Eur J Cancer. 140:76–85. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai J, Capik SF, Kegley B, Richeson JT,
Powell JG and Zhao J: Bovine respiratory microbiota of feedlot
cattle and its association with disease. Vet Res. 53:42022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Wu F, Li X, Zhao C, Jia Y, Jia K,
Han R, Qiao M, Li W, Yu J, et al: Patients with short PFS to
EGFR-TKIs predicted better response to subsequent anti-PD-1/PD-L1
based immunotherapy in EGFR common mutation NSCLC. Front Oncol.
11:6399472021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klindworth A, Pruesse E, Schweer T,
Peplies J, Quast C, Horn M and Glöckner FO: Evaluation of general
16S ribosomal RNA gene PCR primers for classical and
next-generation sequencing-based diversity studies. Nucleic Acids
Res. 41:e12013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weiss S, Xu ZZ, Peddada S, Amir A,
Bittinger K, Gonzalez A, Lozupone C, Zaneveld JR, Vázquez-Baeza Y,
Birmingham A, et al: Normalization and microbial differential
abundance strategies depend upon data characteristics. Microbiome.
5:272017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin
Y, Wang W, Tang W, Tan Z, Shi J, et al: Altered fecal microbiota
composition in patients with major depressive disorder. Brain Behav
Immun. 48:186–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tiffeau-Mayer A: Unbiased estimation of
sampling variance for Simpson's diversity index. Phys Rev E.
109:0644112024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dou XJ, Ma RY, Ren DW, Liu Q and Yan P:
Effectiveness and safety of anlotinib combined with PD-1 blockades
in patients with previously immunotherapy treated advanced
non-small cell lung cancer: A retrospective exploratory study. Lung
Cancer (Auckl). 15:29–40. 2024.PubMed/NCBI
|
|
36
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023.PubMed/NCBI
|
|
37
|
Brozos-Vázquez EM, Díaz-Peña R,
García-González J, León-Mateos L, Mondelo-Macía P, Peña-Chilet M
and López-López R: Immunotherapy in nonsmall-cell lung cancer:
Current status and future prospects for liquid biopsy. Cancer
Immunol Immunother. 70:1177–1188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Himuro H, Nakahara Y, Igarashi Y, Kouro T,
Higashijima N, Matsuo N, Murakami S, Wei F, Horaguchi S, Tsuji K,
et al: Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC
patients treated with immune checkpoint inhibitors. Cancer Immunol
Immunother. 72:2829–2840. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herbst RS, Garon EB, Kim DW, Cho BC,
Gervais R, Perez-Gracia JL, Han JY, Majem M, Forster MD, Monnet I,
et al: Five year survival update from KEYNOTE-010: Pembrolizumab
Versus docetaxel for previously treated, programmed death-ligand
1-positive advanced NSCLC. J Thorac Oncol. 16:1718–1732. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shah MA, Kojima T, Hochhauser D, Enzinger
P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT,
et al: Efficacy and safety of pembrolizumab for heavily pretreated
patients with advanced, metastatic adenocarcinoma or squamous cell
carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA
Oncol. 5:546–550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li XP, Zhang WD, Li MJ, Wang J, Lian J and
Zhou HG: Effectiveness and Safety of PD-1 Inhibitor Monotherapy for
Elderly Patients with Advanced Non-Small Cell Lung Cancer: A
Real-World Exploratory Study. J Oncol. 2022:17102722022.PubMed/NCBI
|
|
42
|
Peng L, Wang Y, Liu F, Qiu X, Zhang X,
Fang C, Qian X and Li Y: Peripheral blood markers predictive of
outcome and immune-related adverse events in advanced non-small
cell lung cancer treated with PD-1 inhibitors. Cancer Immunol
Immunother. 69:1813–1822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomised phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng JD, Chai LX, Zhao ZP, Hao YY and Li
S: Efficacy and safety of anlotinib for patients with advanced
NSCLC who progressed after standard regimens and the preliminary
analysis of an efficacy predictor. Cancer Manag Res. 12:5641–5650.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Velcheti V, Chandwani S, Chen X, Pietanza
MC and Burke T: First-line pembrolizumab monotherapy for metastatic
PD-L1-positive NSCLC: Real-world analysis of time on treatment.
Immunotherapy. 11:889–901. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Apopa PL, Alley L, Penney RB, Arnaoutakis
K, Steliga MA, Jeffus S, Bircan E, Gopalan B, Jin J,
Patumcharoenpol P, et al: PARP1 Is up-regulated in non-small cell
lung cancer tissues in the presence of the cyanobacterial toxin
microcystin. Front Microbiol. 9:17572018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cameron SJS, Lewis KE, Huws SA, Hegarty
MJ, Lewis PD, Pachebat JA and Mur LAJ: A pilot study using
metagenomic sequencing of the sputum microbiome suggests potential
bacterial biomarkers for lung cancer. PLoS One. 12:e01770622017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, Xu
X, Zhao Z, Lv J and Li Z: Gegen Qinlian decoction enhances the
effect of PD-1 blockade in colorectal cancer with microsatellite
stability by remodelling the gut microbiota and the tumour
microenvironment. Cell Death Dis. 10:4152019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen
Y, Zheng H, Yao C, Wang Y and Lu S: The diversity of gut microbiome
is associated with favorable responses to anti-programmed death 1
immunotherapy in Chinese patients With NSCLC. J Thorac Oncol.
14:1378–1389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jang HJ, Choi JY, Kim K, Yong SH, Kim YW,
Kim SY, Kim EY, Jung JY, Kang YA, Park MS, et al: Relationship of
the lung microbiome with PD-L1 expression and immunotherapy
response in lung cancer. Respir Res. 22:3222021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|