Introduction
Immune checkpoint inhibitors (ICIs) have
revolutionized the therapeutic approach to cancer treatment. These
agents, which include inhibitors targeting programmed cell death
protein 1 (PD-1), its ligand programmed death ligand 1 (PD-L1) and
cytotoxic T-lymphocyte-associated antigen 4, have significantly
improved survival outcomes across various malignancies (1). The KEYNOTE-024 trial demonstrated that
pembrolizumab significantly improved progression-free survival and
overall survival (OS) times compared with chemotherapy in patients
with PD-L1-positive advanced non-small cell lung cancer (NSCLC)
(2). In the CheckMate 067 clinical
trial, patients with advanced melanoma treated with nivolumab plus
ipilimumab demonstrated durable responses, with a median OS time of
72.1 months (3). However, ICIs can
induce severe immune-mediated toxicities, referred to as
immune-related adverse events (irAEs), which can affect the
functionality of various organ systems, including ICI-related
colitis, pneumonitis, myositis, dermatological toxicity and
endocrine toxicity, among others (4). Although endocrine irAEs occur less
frequently (10–18%) than dermatological (25–70%) or
gastrointestinal (50%) toxicities, they can be severe and, in some
cases, irreversible (5,6). Among these endocrine irAEs,
ICI-induced type 1 diabetes mellitus (ICI-T1DM) is a rare (<1%)
(7) but potentially
life-threatening complication characterized by sudden-onset
hyperglycemia and insulin deficiency, and frequently presenting
with diabetic ketoacidosis (DKA) (8–10). The
present study reports the case of a female patient diagnosed with
lung invasive adenocarcinoma who developed ICI-T1DM during
durvalumab therapy. This case highlights the importance of the
early recognition and management of this rare yet critical adverse
event, while also providing insights into its clinical
presentation, diagnostic challenges and potential underlying
mechanisms.
Case report
Patient
The patient, a 62-year-old woman with no prior
history of diabetes, was asymptomatic and diagnosed with lung mass
lesions by chest computed tomography during a routine health
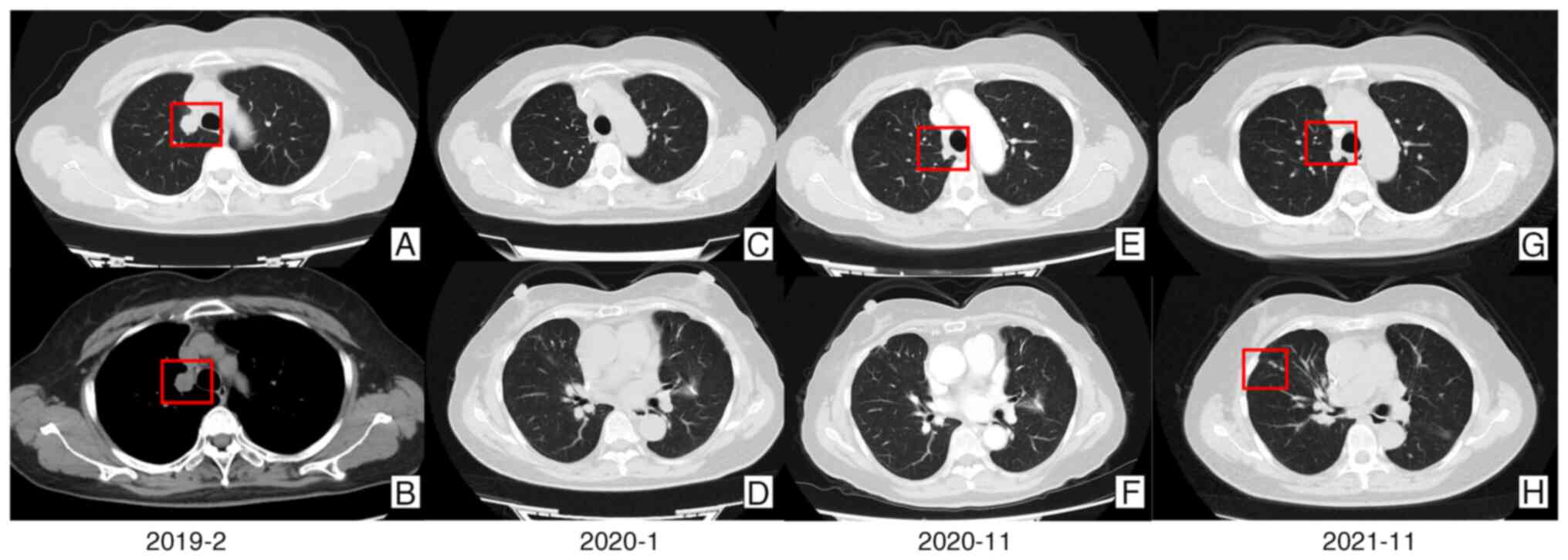
examination (CT) (Fig. 1A and
1B) at China-Japan Friendship
Hospital (Beijing, China) in January 2019. In February 2019, the
patient underwent a right upper lobectomy and left upper lobe wedge
resection. Histopathological examinations confirmed invasive
adenocarcinoma in the upper lobe of the right lung, diagnosed as
stage pT2N0M0 IB according to the 8th edition of the International
Association for the Study of Lung Cancer Tumor-Node-Metastasis
classification (11), without
detectable gene mutations. Additionally, an invasive adenocarcinoma
in the left upper lobe was diagnosed as stage pT1N0M0 IA with an
EGFR21 mutation. The histopathological and immunohistochemical
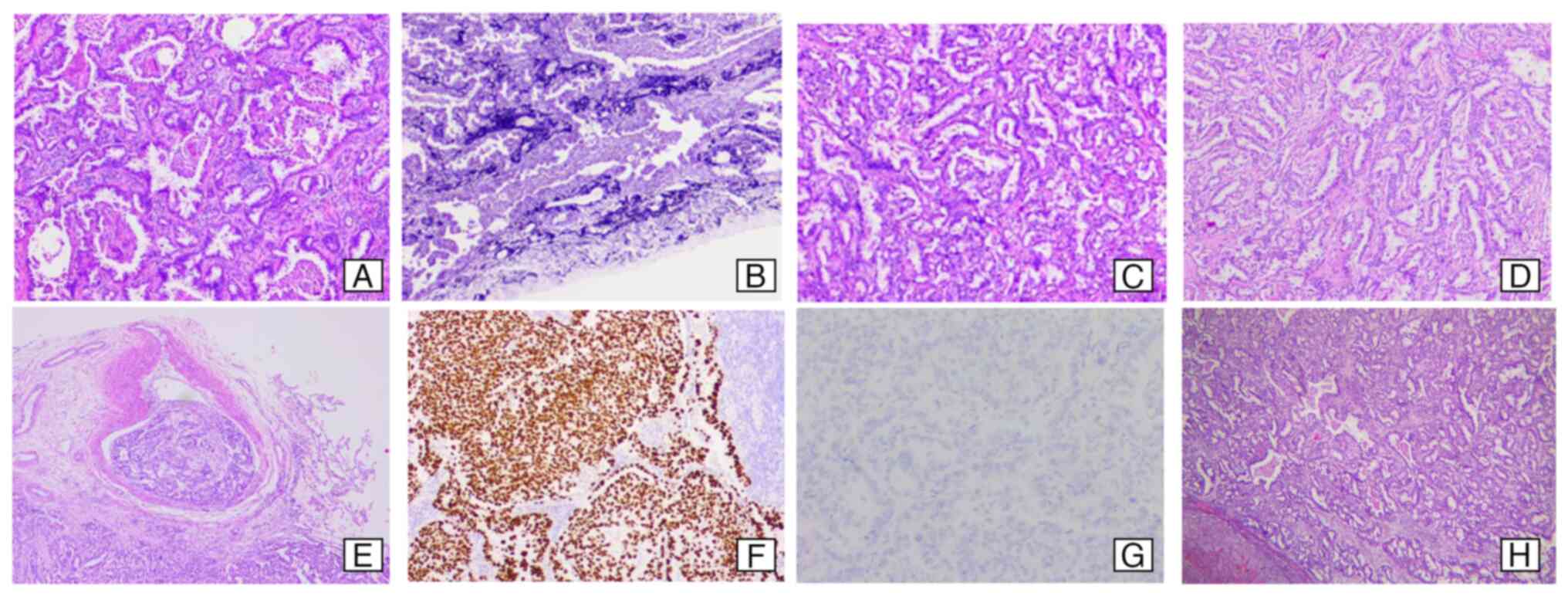
images of the patient tissues are shown in Fig. 2A-G. H&E-stained sections
revealed invasive adenocarcinoma with vascular tumor thrombi.
Elastic fiber staining indicated destruction of the pleural elastic
lamina. Thyroid transcription factor-1 expression was positive,
while anaplastic lymphoma kinase was negative. Targeted therapy
with gefitinib was initiated in early March 2019, with oral
administration of 250 mg once daily. In late September 2019, a left
adrenal tumor resection was performed, with histopathological
examinations (Fig. 2H) revealing
lung adenocarcinoma metastasis without detectable gene mutations.
Following the recurrence of the invasive adenocarcinoma, the
patient received three cycles (21-day cycles) of chemotherapy with
pemetrexed (800 mg on day 1), carboplatin (400 mg on day 2) and
bevacizumab (400 mg on day 1), between November 2019 and ~60 days
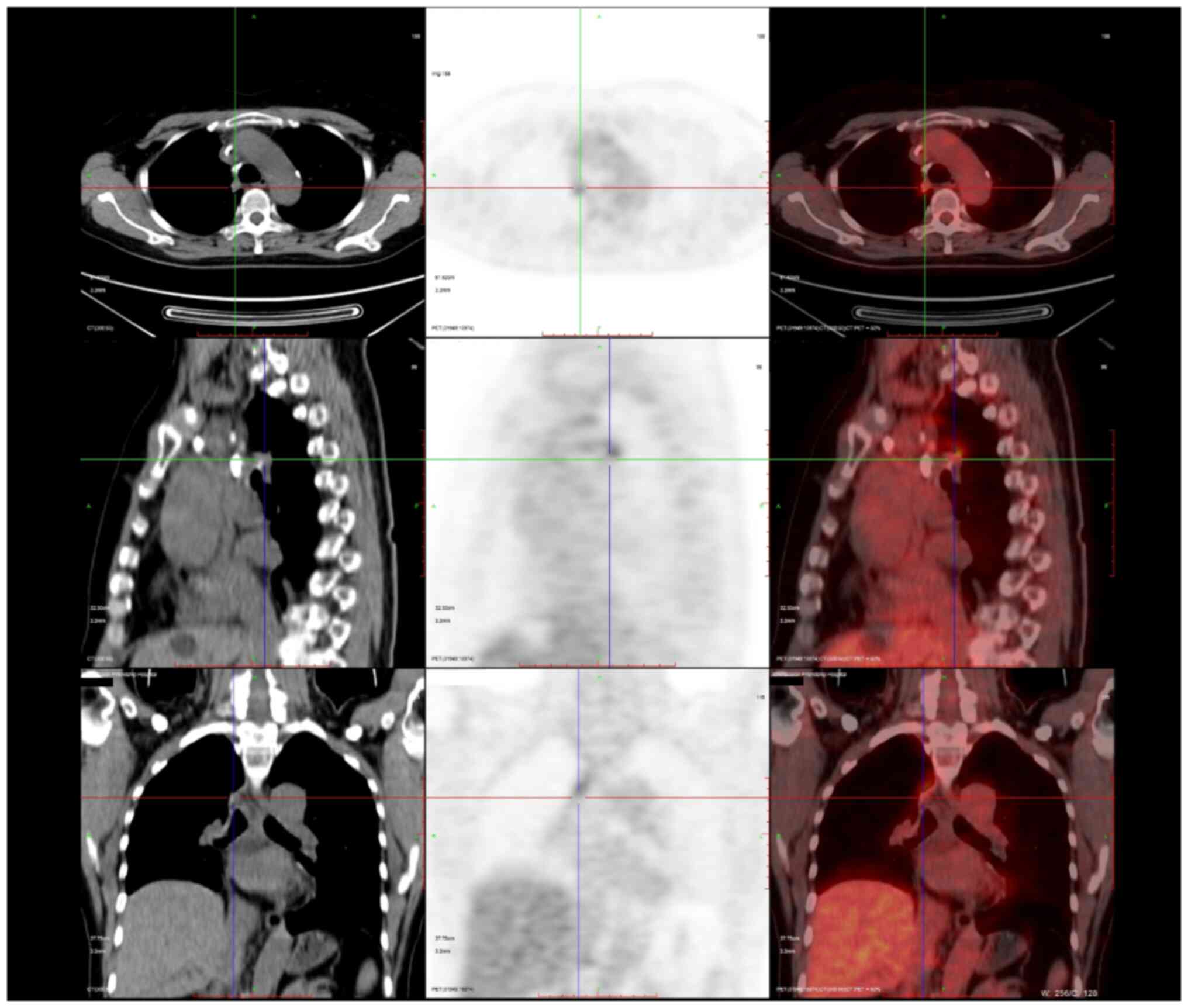
thereafter. In October 2020 and November 2020, positron emission
tomography-CT (Fig. 3) and enhanced
chest CT scans (Fig. 1E and F),
respectively, showed the recurrence of the malignancy in the right
upper hilum. Consequently, the patient was administered 4–6 cycles
(21-day cycles) of the following regimen between November 2020 and
~60 days thereafter: Nedaplatin (120 mg on day 1), pemetrexed (800
mg on day 2) and bevacizumab (400 mg on day 2). After December
2020, the patient opted for gefitinib maintenance therapy (250 mg
once daily). However, in November 2021, a CT scan showed that the
disease had progressed (Fig. 1G and
H). No pathogenic gene mutations were detected.
Subsequently, on November 2021 and 27 days later,
the patient received 1–2 cycles of second-line chemotherapy
combined with immunotherapy involving nedaplatin (120 mg on day 1),
albumin paclitaxel (200 mg on day 8), bevacizumab (400 mg on day 1)
and durvalumab (1,000 mg on day 1). Each cycle lasted 21 days.
After experiencing thirst, polydipsia and polyuria since the end of
December 2021, the patient sought timely medical attention in
January 2022. Given the symptoms and the potential diagnosis of
diabetes, a consultation with the Department of Endocrinology was
requested for further evaluation. The laboratory abnormalities are
shown in Table I. Laboratory tests
revealed significantly elevated fasting and postprandial blood
glucose levels as follows: Fasting plasma glucose, 17.70 mmol/l;
and 2-h postprandial blood glucose, 30.28 mmol/l. The glycated
hemoglobin (HbA1c) level was 7.3%. Additionally, the patient's
C-peptide level was low and the glutamic acid decarboxylase
antibody (GADab) test was positive (35.02 IU/ml), indicating T1DM.
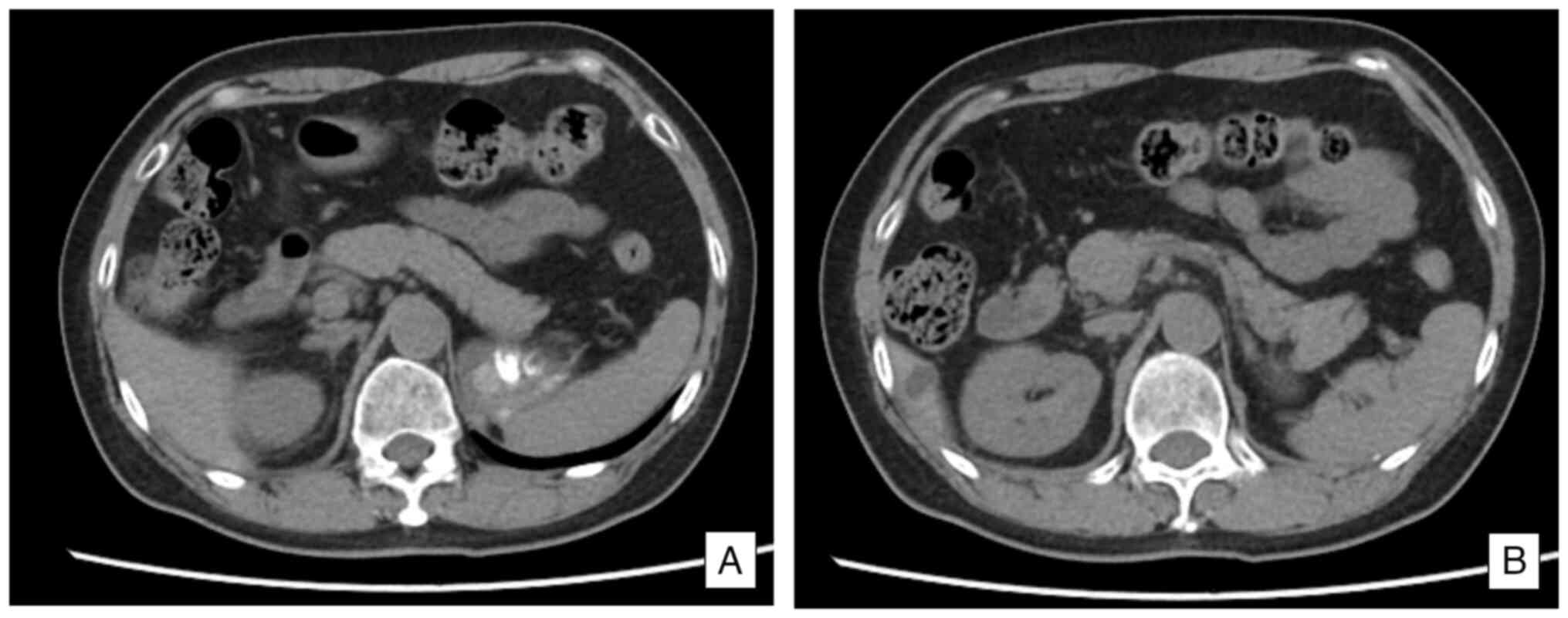
A CT scan (Fig. 4) showed that the
size and morphology of the pancreas were within normal limits, and
the surrounding adipose tissue appeared clear.
 | Table I.Laboratory results. |
Table I.
Laboratory results.
| Laboratory
parameter | Value | Reference |
|---|
| Oral glucose
tolerance test |
|
|
| Fasting
blood-glucose, mmol/l | 17.70 | 3.61–6.11 |
| Glucose
(1 h), mmol/l | 24.56 | <11.10 |
| Glucose
(2 h), mmol/l | 30.28 | <7.84 |
| Insulin release
test |
|
|
| IRI (0
h), µIU/ml | 3.96 | 2.6–24.9 |
| CPS (0
h), ng/ml | 0.96 | 1.1–4.4 |
| IRI (1
h) µIU/ml | 4.14 | 2.6–24.9 |
| CPS (1
h) ng/ml | 0.96 | 1.1–4.4 |
| IRI (2
h) µIU/ml | 4.92 | 2.6–24.9 |
| CPS (2
h) ng/ml | 1.11 | 1.1–4.4 |
| Other blood
laboratory results |
|
|
|
Glycated hemoglobin, % | 7.3 | 4.0–6.0 |
|
Glutamic acid decarboxylase
antibody, IU/ml | 35.02 | <10 |
| Islet
antigen 2 antibody, IU/ml | <0.7 | <10 |
| ALT,
IU/l | 93 | 0-40 |
| AST,
IU/l | 58 | 0-42 |
|
α-amylase, IU/l | 108 | 28-100 |
| Lipase,
U/l | 34 | 0-67 |
| Urine laboratory
results |
|
|
| Urine
routine glucose | Negative |
|
| Ketone
bodies | Negative |
|
Based on the medical history of the patient and the
treatment regimen, the patient was diagnosed with ICI-T1DM
(12). Subsequently, immunotherapy
was discontinued, and the patient was put on insulin replacement
therapy, consisting of insulin degludec/aspart (15 IU
subcutaneously twice daily) as basal insulin and insulin aspart (15
IU subcutaneously as needed) for prandial coverage. Dosages were
titrated according to real-time blood glucose measurements obtained
through capillary blood glucose monitoring. After treatment, the
patient's fasting blood sugar was maintained within the 5.9–7.5
mmol/l range (normal range, 3.61–6.11 mmol/l). Postprandial levels
were as follows: After breakfast, 7.3–11 mmol/l; after lunch,
6.7–8.9 mmol/l; and after dinner, 8.4–10.7 mmol/l (normal range,
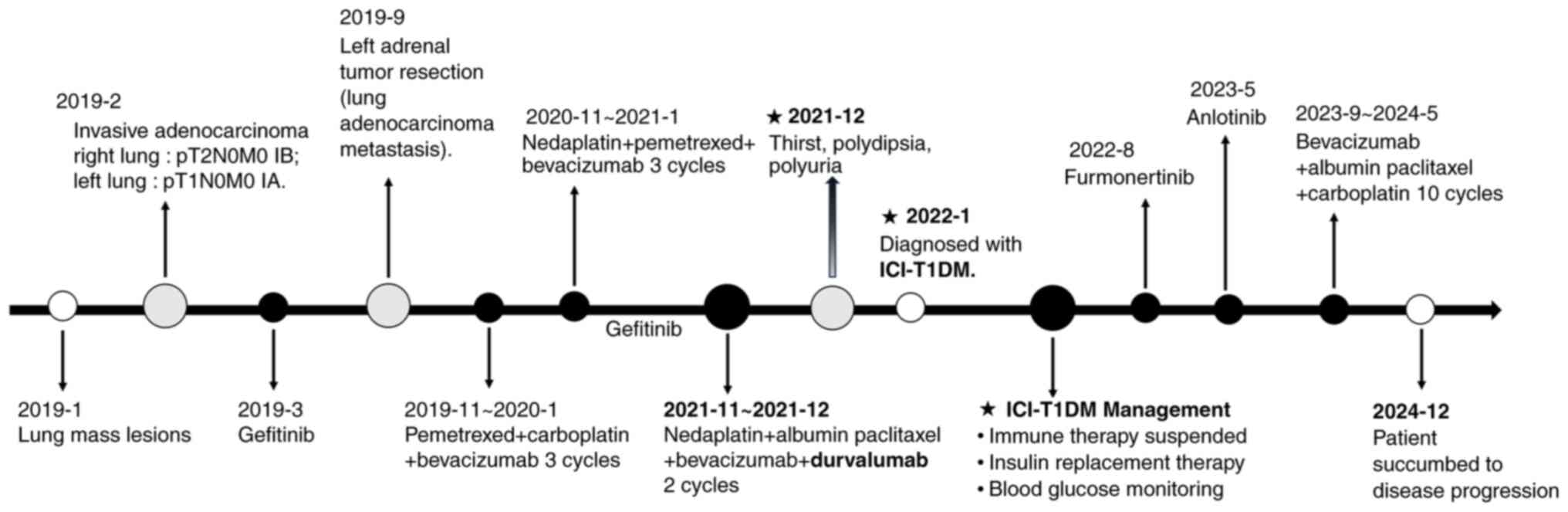
<11.1 mmol/l). A graphical timeline illustrating the patient's
treatment history, durvalumab exposure and the onset of diabetes is
shown in Fig. 5. The patient
resumed anticancer therapy in August 2022 with oral furmonertinib
(80 mg once daily). Monthly follow-ups were conducted at the
Department of Integrative Oncology in the China-Japan Friendship
Hospital. Due to disease progression, the treatment regimen was
subsequently modified to anlotinib (12 mg once daily on days 1–14,
every 21 days), followed by bevacizumab (300 mg on day 1) in
combination with albumin paclitaxel (200 mg on day 1 and 100 mg on
day 8) and carboplatin (300 mg on day 1), administered every 21
days. Ultimately, the patient succumbed to disease progression in
December 2024.
Methods
Tissue staining
Specimens were fixed for 24 to 48 h in 10%
neutral-buffered formalin at room temperature and embedded in
paraffin. The tissue blocks were sliced into 4- or 5-µm thick
sections. H&E staining was performed using hematoxylin for 10
min and eosin for 5 min at room temperature. The elastic fibers
were stained using the iron hematoxylin method, also at room
temperature. 5% Ethanol hematoxylin, 10% ferric chloride and
Verhoeff's iodine solution were mixed at a ratio of 20:8:8 drops,
and then dropped onto the tissue section. Counterstaining was
performed with eosin for 2 min.
Immunohistochemistry was performed by EnVision
system. Antigen retrieval was performed using high pressure at
120°C for 5 min, and endogenous enzyme activity was blocked with 3%
H2O2 for 10 min. The primary antibodies were
ALK (clone D5F3; catalogue number, K18082; Roche Diagnostics) and
TTF-1 (clone SPT24; catalogue number, 18092706; OriGene
Technologies, Inc.), both with a dilution ratio of 1:200, incubated
at room temperature for 1 h. The secondary antibody was horseradish
peroxidase labeled polymer (dilution ratio, 1:2,000; catalogue
number, M00855-M01010; Roche Diagnostics), incubated at 37°C for 30
min. Next, DAB was used for color development, and hematoxylin was
used for counterstaining for 10 min. All sections were observed
using a light microscope.
Literature review
To contextualize this case, a literature review was
conducted in the PubMed database (https://pubmed.ncbi.nlm.nih.gov). The following search
strategy was used: [‘Lung Neoplasms’(Mesh) OR ‘Lung Cancer’ OR
‘NSCLC’ OR ‘SCLC’] AND (‘Nivolumab’ OR ‘Pembrolizumab’ OR
‘Atezolizumab’ OR ‘Durvalumab’) AND (‘Type 1 Diabetes’ OR ‘T1DM’)
AND [‘Case Reports’(Publication Type) OR ‘case report’ OR ‘case
series’]. The literature search was conducted on August 15, 2023,
covering all eligible articles from database inception to the
search date. Inclusion criteria were as follows: Case reports or
case series with a clear diagnostic basis confirming patients with
ICI-T1DM. Exclusion criteria included non-peer-reviewed literature
(e.g., preprints and conference abstracts), case reports with
incomplete data and articles for which the full text was
unavailable. This literature review aimed to collect case reports
of ICI-T1DM associated with anti-PD-1/PD-L1 therapy in patients
with lung cancer. After data retrieval, a total of 25 case reports
involving 27 patients were identified (13–37).
The author, year, patient's age and sex, medical history,
pathological type, ICIs administered, time of disease onset,
symptoms, blood glucose levels, HbA1c percentage, GADab status,
insulinoma-associated antigen 2 antibody (IA-2ab) status,
pancreatic enzyme levels, whether the condition presented with DKA
and treatment modalities, among other parameters. Key clinical
characteristics, including onset time, symptoms and treatment, are
summarized in Table II. The
corresponding information of the patient in this study is also
provided in Table II for
comparison purposes.
 | Table II.ICI-T1DM caused by
anti-PD-1/anti-programmed death ligand 1 treatment in lung
cancer. |
Table II.
ICI-T1DM caused by
anti-PD-1/anti-programmed death ligand 1 treatment in lung
cancer.
| First author | Year | Age at
diagnosis | Sex | Type of tumor | History of
diabetes | ICIs | Onset time of
ICI-T1DM | Clinical symptoms
at onset | Blood glucose,
mmol/l | HbA1c% | DKA occurrence | GADab
positivity | IA-2ab
positivity | Pancreatic enzyme
levels | Treatment | (Refs.) |
|---|
| Alrifai, et
al | 2019 | 69 | Male | NSCLC | T2DM | Pembrolizumab | 4 cycles (21 days
per cycle) | Nausea, vomiting,
polyuria, polydipsia, weakness | 50.4 | 9.20 | Yes | (+) | No data | No data | Insulin, insulin +
metformin | (13) |
| Capitao, et
al | 2018 | 74 | Female | Lung
adenocarcinoma | (−) | Nivolumab | 25 days | Polyuria,
polydipsia, weight loss, vomiting, confusion, asthenia | 58.9 | 8.70 | Yes | (+) | No data | Lipase and amylase
increased | Insulin | (14) |
| Chae, et
al | 2017 | 76 | Male | Lung
adenocarcinoma | (−) | Pembrolizumab | 2 cycles (21 days
per cycle) | Asymptomatic | 34.2 | 6.30 | No | (+) | (+) | No data | Insulin | (15) |
| Chaudry, et
al | 2020 | 75 | Male | NSCLC | (−) | Pembrolizumab | 4 cycles (no cycle
length mentioned) | Fatigue, severe
nausea, weight loss | 35.7 | No data | Yes | (+) | No data | No data | Insulin | (16) |
| Cunha, et
al | 2022 | 59 | Female | Lung
adenocarcinoma | (−) | Pembrolizumab | 3 weeks | Polyuria,
polydipsia, weight loss | 30.8 | 5.60 | Yes | (+) | No data | (−) | Insulin | (17) |
| de Filette, et
al | 2019 | 61 | Male | NSCLC | (−) | Pembrolizumab | 8 weeks | Nausea, vomiting,
diarrhea, generalized weakness | 66.3 | No data | Yes | (+) | (−) | Lipase
increased | Insulin | (18) |
| Delasos, et
al | 2021 | 77 | Male | High-grade
neuroendocrine tumor | (−) | Nivolumab | 15 cycles (14 days
per cycle) | Fatigue, polyuria,
polydipsia | 44.5 | 8.30 | Yes | (−) | (−) | No data | Insulin | (19) |
| Edahiro, et
al | 2019 | 61 | Female | Lung
adenocarcinoma | (−) | Pembrolizumab | 8 cycles (21 days
per cycle) | Emesis, general
malaise, thirst | 31.8 | 8.40 | Yes | (−) | No data | No normal reference
range | Insulin | (20) |
| Godwin, et
al | 2017 | 34 | Female | NSCLC | (−) | Nivolumab | 2 cycles (no cycle
length mentioned) | Abdominal pain,
nausea, weakness | 41.1 | 7.10 | Yes | (+) | (+) | No data | Insulin | (21) |
| Hatakeyama, et
al | 2019 | 60 | Male | Lung
adenocarcinoma | (−) | Nivolumab | 36 cycles (14 days
per cycle) | Asymptomatic | 23.1 | 9.10 | No | (−) | (−) | No data | Insulin | (22) |
| Huang, et
al | 2021 | 59 | Male | SCLC | (−) | Sintilimab | 6 cycles (21 days
per cycle) | Polyuria,
polydipsia | 25.0 | 7.40 | Yes | No data | No data | No data | Insulin | (23) |
| Ishi, et
al | 2021 | 51 | Male | Large cell
carcinoma | T2DM | Nivolumab | 14 cycles (no cycle
length mentioned) | Not mentioned | 57.8 | 6.90 | Yes | (−) | (−) | No data | Insulin | (24) |
|
|
| 62 | Male | SCC | (−) | Atezolizumab | 15 days | Thirst, vomiting,
high fever | 21.9 | 8.90 | No | No data | No data | No data | Insulin +
antibiotics |
|
| Kedzior, et
al | 2021 | 51 | Female | Lung
adenocarcinoma | Not mentioned | Pembrolizumab | 2 cycles (21 days
per cycle) | Abdominal pain,
diarrhea, vomiting, fatigue, dizziness | 62.4 | 8.30 | Yes | (+) | No data | No data | Insulin +
steroid | (25) |
| Lee, et
al | 2018 | 67 | Male | SCC | T2DM | Nivolumab | 2 weeks | Lethargy, polyuria,
polydipsia | 28.6 | 7.60 | Yes | (+) | (−) | No data | Insulin | (26) |
| Li, et
al | 2017 | 63 | Male | SCC | (−) | Nivolumab | 27 days | Palpitations,
fatigue | 32.9 | 7.20 | Yes | (+) | No data | No data | Insulin | (27) |
| Li, et
al | 2020 | 73 | Male | NSCLC | (−) | Anti PD-1 | 10 cycles (21 days
per cycle) | Vomiting, dizzy
tachypnea | 51.0 | 7.60 | Yes | (−) | (−) | No data | Insulin | (28) |
| Lupi, et
al | 2019 | 60 | Male | Lung
adenocarcinoma | Not mentioned | Atezolizumab | 4 cycles (21 days
per cycle) | Not mentioned | 10 (during
L-thyroxine therapy) | No data | Yes | (−) | (−) | No data | Insulin,
hydrocortisone, fludrocortisone | (29) |
| Nishioki, et
al | 2020 | 73 | Female | Lung
adenocarcinoma | (−) | Atezolizumab | >4 months | Dysarthria, gait
disorder, fatigue, vomiting | 53.4 | 7.30 | Yes | (−) | (−) | No data | Insulin | (30) |
| Patel, et
al | 2019 | 49 | Female | Lung
adenocarcinoma | (−) | Durvalumab | 3 months | Lethargy, polyuria,
polydipsia, blurred vision | 21.9 | 7.80 | Yes | (+) | No data | No data | Insulin | (31) |
| Porntharukchareon,
et al | 2020 | 70 | Male | NSCLC | Not | Pembrolizumab +
mentioned | 14 weeks
ipilimumab | Fatigue, nausea,
vomiting | 44.1 | 6.50 | Yes | (−) | (−) | (−) | Insulin | (32) |
| Ren, et
al | 2022 | 71 | Female | SCLC | (−) | Durvalumab | 7 months | Asymptomatic | 13.9 | 9.80 | No | (−) | (−) | (−) | Insulin | (33) |
|
|
| 61 | Female | Lung
adenocarcinoma | Not mentioned | Pembrolizumab | 6 weeks | Vomiting,
difficulty breathing, coma | 29.8 | No data | Yes | No data | No data | No data | Insulin |
|
| Seo, et
al | 2022 | 74 | Male | Lung
adenocarcinoma | (−) | Nivolumab | 8 months | Lower stomach
discomfort, dysarthria, gait disturbance, lethargy, vomiting | 41.0 | 10.60 | Yes | (−) | (+) | No data | Insulin | (34) |
| Sothornwit, et
al | 2019 | 52 | Female | Lung
adenocarcinoma | (−) | Atezolizumab | 24 weeks | Not mentioned | 18.4 | 7.90 | Yes | (+) | (−) | No data | Insulin | (35) |
| Tzoulis, et
al | 2018 | 56 | Female | Lung
adenocarcinoma | (−) | Nivolumab | 3 cycles (14 days
per cycle) | Polyuria,
polydipsia, disorientated, agitated, combative | 47.0 | 8.20 | Yes | (+) | (−) | No data | Insulin | (36) |
| Yang, et
al | 2022 | 78 | Female | SCLC | (−) | Sintilimab | 14 cycles (no cycle
length mentioned) | Polyuria,
polydipsia | 23.4 | 8.20 | No | (−) | (−) | No data | Insulin | (37) |
| Present study |
| 62 | Female | Lung
adenocarcinoma | (−) | Durvalumab | 2 cycles (21 days
per cycle) | Thirst, polydipsia,
polyuria | 17.70 | 7.30 | No | (+) | (−) | Amylase
increased | Insulin |
|
The PubMed database was systematically reviewed to
identify case reports of ICI-T1DM, selecting studies based on
predefined criteria, including confirmed diagnosis and documented
clinical course. The analysis indicated that early-onset cases are
more frequently associated with pancreatic autoantibody positivity
and that PD-1 inhibitors may be linked to a higher incidence of
ICI-T1DM compared with PD-L1 inhibitors. Nearly one-half of the
patients (48.15%) tested positive for GADab. Notably, two patients
with positive GADab and IA-2ab developed ICI-T1DM during the second
cycle. However, further studies are needed to validate these
trends. Collectively, most of the 27 patients were diagnosed with
non-small cell lung cancer. Additionally, 23 patients presented
with a median HbA1c level of 7.90% (mean, 7.95%; range,
5.60–10.60%). Vomiting and polydipsia were the most common,
followed by fatigue, polyuria and consciousness disorder (lethargy,
coma and confusion). Approximately 81.48% of the patients presented
with DKA at the first diagnosis. Statistical analysis was performed
using SPSS version 25 (IBM Corp.). The normality of quantitative
data was assessed using the Shapiro-Wilk test. Normally distributed
data were compared between groups with independent samples t-tests,
while non-normally distributed data were analyzed using the
Mann-Whitney U test. Categorical data were examined with
χ2 or Fisher's exact tests. P<0.05 was considered to
indicate a statistically significant difference. The results
revealed that there was no significant association between GADab
and blood glucose levels (P=0.462) or the occurrence of DKA
(P=0.560). However, GADab positivity was significantly associated
with the onset timing of new-onset ICI-T1DM (P=0.005). The
characteristics of the 27 patients are summarized in Table III.
 | Table III.Data analysis of 27 patients from the
literature review. |
Table III.
Data analysis of 27 patients from the
literature review.
| Characteristic | Value |
|---|
| Median age,
years | 62 |
| Sex, n (%) |
|
|
Male | 15 (55.56) |
|
Female | 12 (44.44) |
| Disease type, n
(%) |
|
|
SCLC | 3 (11.11) |
|
NSCLC | 24 (88.89) |
| History of
diabetes, n (%) |
|
|
T2DM | 3 (11.11) |
|
Negative | 20 (74.07) |
| Not
mentioned | 4 (14.81) |
| ICIs, n (%) |
|
|
Pembrolizumab | 8 (29.63) |
|
Nivolumab | 9 (33.33) |
|
Sintilimab | 2 (7.41) |
|
Atezolizumab | 4 (14.81) |
|
Durvalumab | 2 (7.41) |
| ≥2
types | 1 (3.70) |
| Not
clear | 1 (3.70) |
| Time of onset of
illness, n (%)a |
|
| ≤2
months | 10 (37.04) |
| >2
months | 17 (62.96) |
| Symptoms, n
(%) |
|
|
Vomiting | 11 (40.74) |
|
Polydipsia | 11 (40.74) |
|
Fatigue | 10 (37.04) |
|
Polyuria | 9 (33.33) |
|
Consciousness disorder | 6 (22.22) |
|
Nausea | 5 (18.52) |
| Weight
loss | 3 (11.11) |
|
Abdominal pain | 2 (7.41) |
|
Diarrhea | 2 (7.41) |
| DKA, n
(%)b |
|
|
Yes | 22 (81.48) |
| No | 5 (18.52) |
| Mean HbA1c, % | 7.95 |
| Mean blood glucose,
mmol/lb | 38.83 |
| Antibodies, n
(%) |
|
|
GADab+ | 13 (48.15) |
|
IA-2ab+ | 3 (11.11) |
Despite these novel observations, this literature
review has certain limitations. First, given that this study is
based on case reports, characterized by a small sample size and
lack of a control group, the generalizability of the findings is
limited. Second, variations in diagnostic criteria and follow-up
durations across the included reports introduce potential
heterogeneity, which poses significant challenges to the
interpretation of the results. Additionally, since case reports are
retrospective studies, certain clinical parameters may lack
standardized definitions across different studies. Consequently,
future research should focus on conducting larger-scale cohort
studies, establishing standardized diagnostic criteria and
extending follow-up durations to validate our findings and provide
more reliable clinical guidance.
Discussion
The patient in this study had no prior history of
diabetes or hyperglycemia during previous cancer treatments.
However, after immunotherapy, the patient showed an elevated blood
glucose level, islet dysfunction (with serum C-peptide below the
normal range) and absolute insulin deficiency, among other
complications. These observations exhibited a positive temporal
association with durvalumab. The likelihood of this event was
assessed using the Naranjo Adverse Drug Reaction Probability Scale
(38), which yielded a score of 6,
which indicates a probable relationship. Additionally, according to
a previous report, the incidence of new-onset diabetes associated
with ICIs in patients exposed to PD-L1 inhibitors alone was 0.73%
(8). New-onset diabetes is a rare
adverse effect of durvalumab, occurring in only 0.2% of cases
(39). Among the 27 patients
included in this review, only 2 received durvalumab. All the
patients exhibited a history of ICI treatment. Vomiting and
polydipsia are the most common symptoms (13,14,18,24,30,33,34,40).
The initial symptoms experienced by this patient included thirst,
polydipsia and polyuria without vomiting. Additionally, compared
with the 81.48% of patients who developed DKA, as presented in
Table II, the current patient did
not present with DKA at the time of diagnosis, likely due to the
early recognition of symptoms and timely medical consultation.
Notably, ICI-T1DM is a distinct subtype of T1DM,
primarily triggered by pancreatic β-cell destruction due to ICI
therapy (41). Under normal
physiological conditions, the PD-1/PD-L1 pathway protects
pancreatic β-cells from immune cell toxicity by inducing T-cell
apoptosis. However, previous studies have shown that ICI therapy
disrupts the interaction between PD-L1 molecules on pancreatic
β-cells and PD-1 receptors on autoreactive T cells, thereby
inhibiting their binding (42).
Consequently, autoreactive T cells evade elimination and damage
β-cells. Therefore, PD-1 inhibition leads to T-cell activation and
subsequent destruction of pancreatic β-cells. During this process,
CD8+ T cells function as the primary effector cells.
Previous studies on the pancreatic pathology of patients with
ICI-T1DM have shown an increase in CD8+ T cells and a
reduction in the macrophage population (43–46).
Research indicates that activated autoreactive T cells respond to
PD-1 inhibition by releasing interferons (IFNs). These IFNs
activate monocyte-derived macrophages (44). When treated with anti-PD-L1,
cytotoxic IFN-γ+ CD8+ T cells infiltrate the
islets and induce the dedifferentiation of pancreatic β-cells
(45). These T cells utilize nitric
oxide to kill pancreatic β-cells, leading to insulin deficiency and
the development of ICI-T1DM. This condition, ICI-T1DM, presents
with distinct clinical manifestations, disease features and
pathogenic factors compared to the traditional T1DM, therefore it
warrants differentiation clinically (47). The incidence of DKA in ICI-T1DM is
higher compared with that in traditional T1DM, and its presence is
frequently associated with other irAEs (44,48).
Additionally, in the case of ICI-T1DM, there is no spontaneous
remission period (49). Moreover,
there are inducements of pancreatic autoimmunity before the onset
of ICI-T1DM. Patients with ICI-T1DM may also exhibit elevated
trypsin levels (50,51).
GADab and IA-2ab are biomarkers of pancreatic
autoimmunity and potentially play significant roles in predicting
ICI-T1DM. GADab is an enzyme involved in the synthesis of the
neurotransmitter γ-aminobutyric acid and is also a major pancreatic
islet autoantibody (52). GADab is
the most commonly used diagnostic marker for adult T1DM. In
ICI-T1DM, the interval between the initiation of anti-PD-1/PD-L1
antibody treatment and the onset of ICI-T1DM is associated with the
presence or absence of GADab (52).
The literature review indicated that the onset of ICI-T1DM in
patients with positive GADab results was earlier, ~2 months on
average, compared with the onset in patients with negative results
for the pancreatic islet antibody (52). These observations are consistent
with the present case report, in which the patient was
GADab-positive and the onset time was within 6 weeks. IA-2ab is
also an important marker of pancreatic autoimmunity. Studies have
shown that IA-2ab is present in ~60% of patients with newly
diagnosed T1DM (53). However, in
ICI-T1DM, the significance of IA-2ab is less pronounced compared
with that of GADab. A systematic review revealed that 18% of
patients with ICI-T1DM tested positive for IA-2ab, compared with
51% for GADab (18). In another
retrospective analysis involving 10 patients with ICI-T1DM, IA-2ab
levels were found to be within the normal range (54). This suggests that the predictive
role of IA-2ab in ICI-T1DM requires further investigation. A study
revealed that at least one pancreatic autoantibody was positive in
53% of patients with ICI-T1DM, and two or more autoantibodies were
detected in 15% of patients (18).
Therefore, pancreatic autoantibody serological testing may be
conducted before the initiation of ICI therapy to help predict the
onset of ICI-T1DM.
Given the absence of typical clinical symptoms and
reliable predictors for ICI-T1DM, as well as the life-threatening
risks associated with rapid onset and delayed treatment, clinicians
must remain vigilant. Regular blood glucose monitoring is
essential, and when elevated blood sugar levels are detected, a
timely and accurate differential diagnosis should be made in
conjunction with endocrinologists. Following an accurate diagnosis
of ICI-T1DM, immunosuppressant administration should be
discontinued and replaced with insulin replacement therapy.
Currently, it is believed that the occurrence of ICI-T1DM does not
contraindicate the continuation of ICI therapy, provided that blood
glucose levels can be effectively controlled through insulin
therapy. Therefore, the treatment and follow-up of diabetes should
be continued after the temporary cessation of ICI treatment.
Decisions regarding temporary interruption or permanent
discontinuation of ICI therapy must be guided by an individualized
risk-benefit assessment. Furthermore, the decision to restart ICI
therapy after achieving stable blood glucose control should be made
collaboratively by oncologists and endocrinology experts.
Acknowledgements
Not applicable.
Funding
This study was funded by National High-Level Hospital Clinical
Research Funding (grant no. 2022-NHLHCRF-LX-02-0111), Capital's
Funds for Health Improvement and Research (grant no. 2022-2-4065)
and Noncommunicable Chronic Diseases-National Science and
Technology Major Project (grant no. 2023ZD0502503).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HD and SL participated in study design and wrote the
original manuscript draft. XZ, JZ, YP and XL obtained medical
images and analyzed patient data. CX, YZ and YY analyzed
pathological images and made the diagnosis. ZH contributed to the
follow-up and data analysis. YP participated in language revision
of the manuscript. HC was involved in drafting the manuscript,
revising it critically for important intellectual content, data
analysis and giving final approval of the version to be published.
HD and SL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript
References
|
1
|
Arafat Hossain M: A comprehensive review
of immune checkpoint inhibitors for cancer treatment. Int
Immunopharmacol. 143:1133652024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Long-term outcomes with nivolumab plus ipilimumab
or nivolumab alone versus ipilimumab in patients with advanced
melanoma. J Clin Oncol. 40:127–137. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22:392020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kottschade LA: Incidence and management of
immune-related adverse events in patients undergoing treatment with
immune checkpoint inhibitors. Curr Oncol Rep. 20:242018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elshafie O, Khalil AB, Salman B, Atabani A
and Al-Sayegh H: Immune checkpoint Inhibitors-induced
endocrinopathies: Assessment, management and monitoring in a
comprehensive cancer centre. Endocrinol Diabetes Metab.
7:e005052024. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Zhou H, Zhang Y, Fang W, Yang Y,
Huang Y and Zhang L: Reporting of immune checkpoint inhibitor
therapy-associated diabetes, 2015–2019. Diabetes Care. 43:e79–e80.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchand L, Thivolet A, Dalle S, Chikh K,
Reffet S, Vouillarmet J, Fabien N, Cugnet-Anceau C and Thivolet C:
Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: Description
of pancreatic endocrine and exocrine phenotype. Acta Diabetol.
56:441–448. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Błażowska O, Stróżna K, Dancewicz H,
Zygmunciak P, Zgliczyński W and Mrozikiewicz-Rakowska B: The
Double-edged sword of Immunotherapy-Durvalumab-induced
Polyendocrinopathy-case report. J Clin Med. 13:63222024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thompson JA, Schneider BJ, Brahmer J,
Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M,
Dunnington D, et al: NCCN guidelines insights: Management of
Immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc
Netw. 18:230–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alrifai T, Ali FS, Saleem S, Ruiz DCM,
Rifai D, Younas S and Qureshi F: Immune checkpoint inhibitor
induced diabetes mellitus treated with insulin and metformin:
Evolution of diabetes management in the era of immunotherapy. Case
Rep Oncol Med. 2019:87813472019.PubMed/NCBI
|
|
14
|
Capitao R, Bello C, Fonseca R and Saraiva
C: New onset diabetes after nivolumab treatment. BMJ Case Rep.
2018:20172209992018. View Article : Google Scholar
|
|
15
|
Chae YK, Chiec L, Mohindra N, Gentzler R,
Patel J and Giles F: A case of Pembrolizumab-induced type-1
diabetes mellitus and discussion of immune checkpoint
inhibitor-induced type 1 diabetes. Cancer Immunol Immunother.
66:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudry A, Chaudry M and Aslam J:
Pembrolizumab: An immunotherapeutic agent causing endocrinopathies.
Cureus. 12:e88362020.PubMed/NCBI
|
|
17
|
Cunha C, Silva E, Vieira AC, Saraiva C and
Duarte S: New onset autoimmune diabetes mellitus and hypothyroidism
secondary to pembrolizumab in a patient with metastatic lung
cancer. Endocrinol Diabetes Metab Case Rep. 2022:21–0123.
2022.PubMed/NCBI
|
|
18
|
de Filette JMK, Pen JJ, Decoster L,
Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO,
Aspeslagh S, Neyns B, et al: Immune checkpoint inhibitors and type
1 diabetes mellitus: A case report and systematic review. Eur J
Endocrinol. 181:363–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delasos L, Bazewicz C, Sliwinska A, Lia NL
and Vredenburgh J: New onset diabetes with ketoacidosis following
nivolumab immunotherapy: A case report and review of literature. J
Oncol Pharm Pract. 27:716–721. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edahiro R, Ishijima M, Kurebe H, Nishida
K, Uenami T, Kanazu M, Akazawa Y, Yano Y and Mori M: Continued
administration of pembrolizumab for adenocarcinoma of the lung
after the onset of fulminant type 1 diabetes mellitus as an
Immune-related adverse effect: A case report. Thorac Cancer.
10:1276–1279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Godwin JL, Jaggi S, Sirisena I, Sharda P,
Rao AD, Mehra R and Veloski C: Nivolumab-induced autoimmune
diabetes mellitus presenting as diabetic ketoacidosis in a patient
with metastatic lung cancer. J Immunother Cancer. 5:402017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatakeyama Y, Ohnishi H, Suda K, Okamura
K, Shimada T and Yoshimura S: Nivolumab-induced Acute-onset type 1
diabetes mellitus as an immune-related adverse event: A case
report. J Oncol Pharm Pract. 25:2023–2026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang X, Yang M, Wang L, Li L and Zhong X:
Sintilimab induced diabetic ketoacidosis in a patient with small
cell lung cancer: A case report and literature review. Medicine
(Baltimore). 100:e257952021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishi A, Tanaka I, Iwama S, Sakakibara T,
Mastui T, Kobayashi T, Hase T, Morise M, Sato M, Arima H and
Hashimoto N: Efficacies of programmed cell death 1 ligand 1
blockade in non-small cell lung cancer patients with acquired
resistance to prior programmed cell death 1 inhibitor and
development of diabetic ketoacidosis caused by two different
etiologies: A retrospective case series. Endocr J. 68:613–620.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kedzior SK, Jacknin G, Hudler A, Mueller
SW and Kiser TH: A severe case of diabetic ketoacidosis and
New-onset type 1 diabetes mellitus associated with Anti-glutamic
acid decarboxylase antibodies following immunotherapy with
pembrolizumab. Am J Case Rep. 22:e9317022021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee S, Morgan A, Shah S and Ebeling PR:
Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy.
Endocrinol Diabetes Metab Case Rep. 2018:18–0021. 2018.PubMed/NCBI
|
|
27
|
Li L, Masood A, Bari S, Yavuz S and
Grosbach AB: Autoimmune diabetes and thyroiditis complicating
treatment with nivolumab. Case Rep Oncol. 10:230–234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Wang H, Chen B, Zhao S, Zhang X, Jia
K, Deng J, He Y and Zhou C: Anti PD-1 monoclonal antibody induced
autoimmune diabetes mellitus: A case report and brief review.
Transl Lung Cancer Res. 9:379–388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lupi I, Brancatella A, Cosottini M, Viola
N, Lanzolla G, Sgrò D, Dalmazi GD, Latrofa F, Caturegli P and
Marcocci C: Clinical heterogeneity of hypophysitis secondary to
PD-1/PD-L1 blockade: Insights from four cases. Endocrinol Diabetes
Metab Case Rep. 2019:19–0102. 2019.PubMed/NCBI
|
|
30
|
Nishioki T, Kato M, Kataoka S, Miura K,
Nagaoka T and Takahashi K: Atezolizumab-induced fulminant type 1
diabetes mellitus occurring four months after treatment cessation.
Respirol Case Rep. 8:e006852020. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patel S, Chin V and Greenfield JR:
Durvalumab-induced diabetic ketoacidosis followed by
hypothyroidism. Endocrinol Diabetes Metab Case Rep. 2019:19–0098.
2019.PubMed/NCBI
|
|
32
|
Porntharukchareon T, Tontivuthikul B,
Sintawichai N and Srichomkwun P: Pembrolizumab- and
Ipilimumab-induced diabetic ketoacidosis and isolated
adrenocorticotropic hormone deficiency: A case report. J Med Case
Rep. 14:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren Y, Zhang L, Wang Y and Zhong D: Immune
checkpoint inhibitors related diabetes mellitus: A report of 2
cases and literature review. Zhongguo Fei Ai Za Zhi. 25:61–65.
2022.(In Chinese). PubMed/NCBI
|
|
34
|
Seo JH, Lim T, Ham A, Kim YA and Lee M:
New-onset type 1 diabetes mellitus as a delayed immune-related
event after discontinuation of nivolumab: A case report. Medicine
(Baltimore). 101:e304562022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sothornwit J, Phunmanee A and
Pongchaiyakul C: Atezolizumab-induced autoimmune diabetes in a
patient with metastatic lung cancer. Front Endocrinol (Lausanne).
10:3522019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tzoulis P, Corbett RW, Ponnampalam S,
Baker E, Heaton D, Doulgeraki T and Stebbing J: Nivolumab-induced
fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol
Diabetes Metab Case Rep. 2018:18–0111. 2018.PubMed/NCBI
|
|
37
|
Yang J, Wang Y and Tong XM:
Sintilimab-induced autoimmune diabetes: A case report and review of
the literature. World J Clin Cases. 10:1263–1277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
García-Cortés M, Lucena MI, Pachkoria K,
Borraz Y, Hidalgo R and Andrade RJ: Evaluation of naranjo adverse
drug reactions probability scale in causality assessment of
drug-induced liver injury. Aliment Pharmacol Ther. 27:780–789.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III Non-Small-Cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang LS, Barroso-Sousa R, Tolaney SM,
Hodi FS, Kaiser UB and Min L: Endocrine toxicity of cancer
immunotherapy targeting immune checkpoints. Endocr Rev. 40:17–65.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gardner G and Fraker CA: Natural killer
cells as key mediators in type i diabetes immunopathology. Front
Immunol. 12:7229792021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kani ER, Karaviti E, Karaviti D, Gerontiti
E, Paschou IA, Saltiki K, Stefanaki K, Psaltopoulou T and Paschou
SA: Pathophysiology, diagnosis, and management of immune checkpoint
Inhibitor-induced diabetes mellitus. Endocrine. 87:875–890. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Hou X, Yang Y, Wang C, Zhou J,
Miao J, Gong F, Ge F and Chen W: Immune checkpoint
inhibitors-induced diabetes mellitus (review). Endocrine.
86:451–458. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cho YK and Jung CH: Immune-Checkpoint
Inhibitors-Induced Type 1 diabetes mellitus: From its molecular
mechanisms to clinical practice. Diabetes Metab J. 47:757–766.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perdigoto AL, Deng S, Du KC, Kuchroo M,
Burkhardt DB, Tong A, Israel G, Robert ME, Weisberg SP,
Kirkiles-Smith N, et al: Immune cells and their inflammatory
mediators modify β cells and cause checkpoint Inhibitor-induced
diabetes. JCI Insight. 7:e1563302022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoneda S, Imagawa A, Hosokawa Y, Baden MY,
Kimura T, Uno S, Fukui K, Goto K, Uemura M, Eguchi H, et al:
T-Lymphocyte infiltration to islets in the pancreas of a patient
who developed type 1 diabetes after administration of immune
checkpoint inhibitors. Diabetes Care. 42:e116–e118. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu L, Tsang VHM, Sasson SC, Menzies AM,
Carlino MS, Brown DA, Clifton-Bligh R and Gunton JE: Unravelling
checkpoint inhibitor associated autoimmune diabetes: From bench to
bedside. Front Endocrinol (Lausanne). 12:7641382021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kamitani F, Nishioka Y, Koizumi M,
Nakajima H, Kurematsu Y, Okada S, Kubo S, Myojin T, Noda T, Imamura
T and Takahashi Y: Immune checkpoint Inhibitor-related type 1
diabetes incidence, risk, and survival association. J Diabetes
Investig. 16:334–342. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kyriacou A, Melson E, Chen W and
Kempegowda P: Is immune checkpoint Inhibitor-associated diabetes
the same as fulminant type 1 diabetes mellitus? Clin Med (Lond).
20:417–423. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang AL, Wang F, Chang LS, McDonnell ME
and Min L: Coexistence of immune checkpoint inhibitor-induced
autoimmune diabetes and pancreatitis. Front Endocrinol (Lausanne).
12:6205222021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liao D, Liu C, Chen S, Liu F, Li W,
Shangguan D and Shi Y: Recent advances in immune checkpoint
Inhibitor-induced type 1 diabetes mellitus. Int Immunopharmacol.
122:1104142023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Usui Y, Udagawa H, Matsumoto S, Imai K,
Ohashi K, Ishibashi M, Kirita K, Umemura S, Yoh K, Niho S, et al:
Association of serum Anti-GAD antibody and HLA haplotypes with type
1 diabetes mellitus triggered by nivolumab in patients with
Non-small cell lung cancer. J Thorac Oncol. 12:e41–e43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pardini VC, Mourao DM, Nascimento PD,
Vívolo MA, Ferreira SR and Pardini H: Frequency of islet cell
autoantibodies (IA-2 and GAD) in young Brazilian type 1 diabetes
patients. Braz J Med Biol Res. 32:1195–1198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wei HH, Lai YC, Lin G, Lin CW, Chang YC,
Chang JW, Liou MJ and Chen IW: Distinct changes to pancreatic
volume rather than pancreatic autoantibody positivity: Insights
into immune checkpoint inhibitors induced diabetes mellitus.
Diabetol Metab Syndr. 16:262024. View Article : Google Scholar : PubMed/NCBI
|