Introduction
According to recent data from GLOBOCAN, colorectal
cancer (CRC) is the third most common cancer (after lung cancer and
female breast cancer) and the second leading cause of
cancer-related death (after lung cancer). Furthermore, there were
1,926,118 newly diagnosed CRC cases and 903,859 CRC-related deaths
in 2022 worldwide (1). Moreover,
owing to the greater proportion of older individuals and changes in
lifestyle, the incidence of CRC continues to rise (2). Screening and early diagnosis are
highly challenging, and improvements could help reduce the
morbidity and mortality associated with CRC (3,4). Owing
to the large population and limited health care resources in China,
colonoscopy is only used as a screening method for high-risk
individuals (5–7). In three studies utilizing patient
questionnaires, age, family history of cancer, history of smoking,
diabetes and frequency of intake of certain foods (such as
vegetables, fried food, pickled food and white meat) were noted as
being associated with the development of CRC (8–10).
Laxatives are widely used to treat constipation and
can be abused in eating disorders (particularly bulimia nervosa and
binge-eating disorder), and include stimulant agents, saline and
osmotic products (11). However, it
has been reported that the prevalence of laxative abuse ranges from
10 to 60% (12), which might lead
to disorders of electrolytes and acid-base; these disorders involve
the renal and cardiovascular systems and can become
life-threatening (13,14). Therefore, more precautions are
required to prevent the harm caused by laxative use.
Some laxatives including phenolphthalein laxatives
and magnesium laxatives have been reported to significantly
increase the incidence of CRC (15–17).
However, macrogol and fiber laxatives have been reported to have
the opposite effects (18,19). Moreover, other studies have
demonstrated that laxative use is not associated with CRC (20–26).
Therefore, there is still controversy regarding the influence of
laxative use on the incidence of CRC.
Materials and methods
Reporting guidelines
The present study was based on prior established
studies (15–26). The findings were reported following
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses statement (27).
Inclusion and exclusion criteria
The inclusion criteria for studies and patients were
as follows: i) Patients who had completed any questionnaire
including self-reported laxative use; ii) at least one type of
laxative was reported; and iii) the incidence of CRC was reported.
The exclusion criteria were as follows: i) The type of study was
case reports, reviews, meta-analysis, letters to the editor,
comments or conference abstracts/proceedings (due to typically
limited peer review and incomplete datasets); ii) only had
pediatric populations; and iii) data were insufficient including
missing core variables, incomplete metrics or non-extractable
data.
Search strategy and study
selection
A comprehensive search strategy was developed to
identify relevant studies, which included terms such as ‘laxative’
and ‘CRC’. For laxative, the additional search terms were
‘cathartics’ OR ‘laxative’. For CRC, the additional search terms
were ‘colorectal cancer’ OR ‘colon cancer’ OR ‘rectal cancer’ OR
‘colorectal neoplasm’ OR ‘colon neoplasm’ OR ‘rectal neoplasm’ OR
‘colorectal tumor’ OR ‘colon tumor’ OR ‘rectal tumor’. The search
scope was limited to the title, abstract or key words, and only
studies published in English were included. The search strategy was
implemented by two independent authors across three databases
including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/) and the Cochrane Central
Register of Controlled Trials (https://www.cochranelibrary.com/central). After
removing duplicated studies, the titles and abstracts were screened
to identify potentially relevant studies. Then, full texts were
reviewed to determine eligible studies based on the inclusion and
exclusion criteria. Additionally, the reference lists of included
studies were examined to identify additional relevant articles. In
cases of disagreement between the two authors, a group discussion
with a third individual was consulted to reach a consensus.
Data collection
Data extraction was performed independently by two
authors at the same time. The study characteristic data extracted
were as follows: The first author, publication year, country where
the study was conducted, study period, sample size, study type,
laxative subtype, and conclusion. Patients who used laxatives were
categorized into the laxative use group, and those who had no
history of laxative use were categorized into the non-laxative use
group. The incidence of CRC in each group was recorded. To ensure
accuracy and completeness, the extracted data were cross-checked by
both authors, and any discrepancies were resolved through
discussion.
Quality assessment
The quality of the included studies was assessed
using the Newcastle-Ottawa Scale (NOS) score (28). Then, two independent authors
assigned ratings based on the following criteria: 9 points
indicated high quality, 7–8 points indicated median quality and
<7 points indicated low quality. Any discrepancies in scoring
were resolved through discussion or consultation with a third
individual.
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs)
were calculated to compare the incidence of CRC between the
laxative use and the non-laxative use groups. Heterogeneity was
assessed using the I2 statistic and the χ2
test (29,30). According to the Cochrane handbook,
an I2<30% was considered non-important, an
I2 range of 30–60% was considered moderate and an
I2>60% was considered substantial (30). The random-effect model was applied
as the default model, and P<0.05 indicated a statistically
significant difference. Publication bias was evaluated using funnel
plot. All statistical analyses were performed using Stata V18.0
(StataCorp LP).
Results
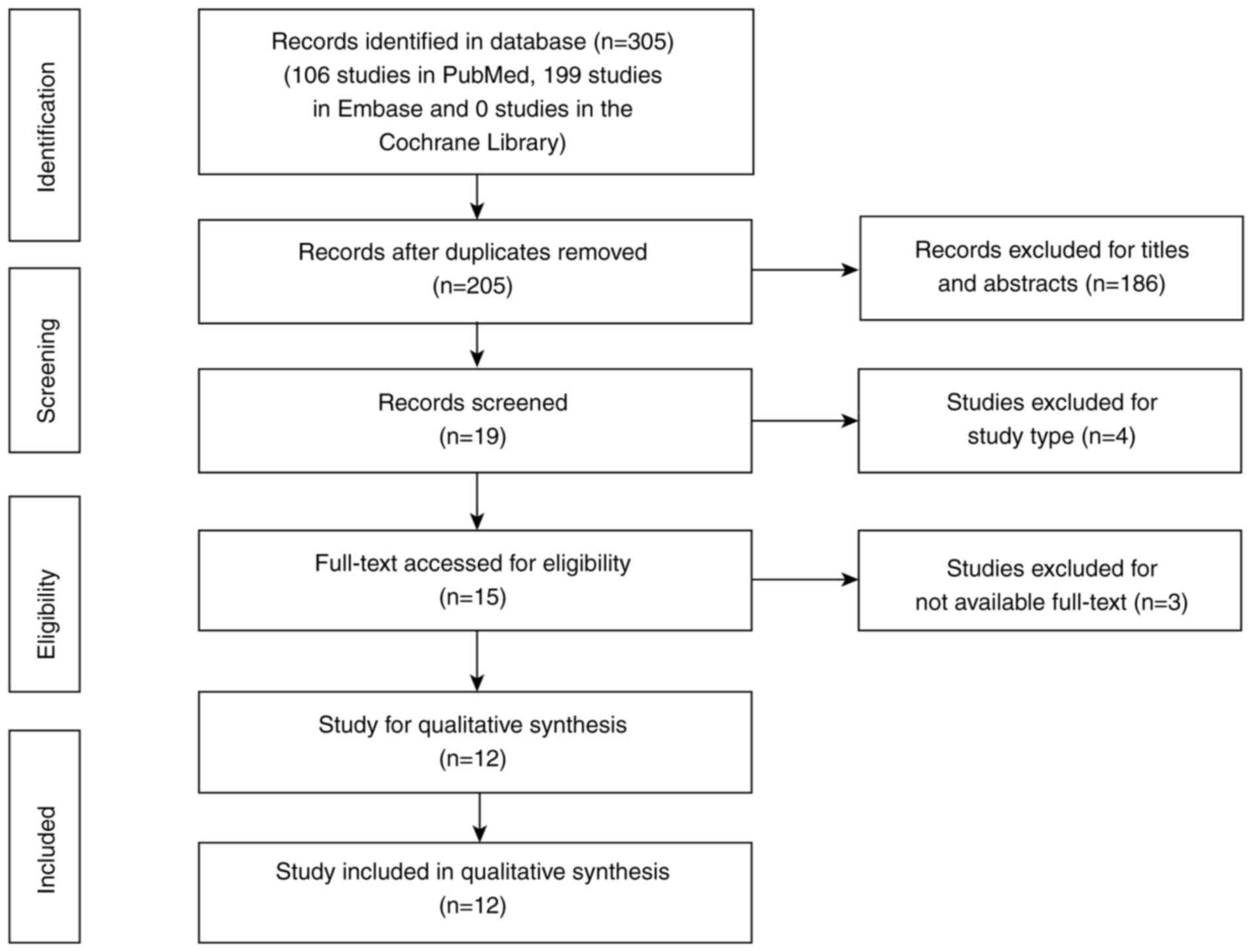
Study selection
Following the initial search, 305 studies were
identified (106 studies in PubMed, 199 studies in Embase and 0
studies in the Cochrane Library). After removing duplicates, 205
studies remained. Screening of the titles and abstracts identified
19 studies with potentially relevant content. Finally, 12
observational studies with sufficient data were included in the
pooled analysis after full-text review (Fig. 1). Additionally, the reference lists
of these studies were screened, but no further eligible studies
were identified.
Baseline characteristics
The 12 included studies were published between 1988
and 2019, spanning a period of 30 years. These studies were
conducted in seven countries, with 5 studies originating from the
United States. Among the included studies, 7 were case-control
studies and the remaining 5 were cohort studies. The primary
laxative types investigated included anthraquinone, fiber,
phenolphthalein and magnesium laxatives. Detailed information on
the laxative types and the conclusions of each study are shown in
Table I.
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
| First author/s,
year | Country | Study date | No. of
patients | Study type | Laxative type | Conclusion | NOS | (Refs.) |
|---|
| Kune et al,
1988 | Australia | 1980-1981 | 1,442 | Case-control | NR | Laxative use was
unlikely to be an etiological factor in the development of
CRC. | 8 | (20) |
| Jacobs and White,
1998 | United States | 1985-1989 | 838 | Case-control | Phenolphthalein,
fiber, magnesium and other commercial laxatives | All types of
commercial laxatives, except for fiber laxatives, seemed to be
associated with increased risk of CRC. | 7 | (15) |
| Dukas et al,
2000 | United States | 1984-1996 | 84,577 | Cohort | Softeners, bulk
agents and suppositories | The findings did
not support an association between laxative use and the risk of
CRC. | 7 | (21) |
| Nusko et al,
2000 | Germany | 1993-1996 | 554 | Case-control | Anthranoid
laxative | Anthranoid laxative
use was not associated with any significant risk of developing
colorectal adenoma or carcinoma. | 7 | (22) |
| Nascimbeni et
al, 2002 | Italy | 1997-1999 | 192 | Case-control |
Anthranoid-containing laxatives were
defined as herbal drugs containing senna, cascara, frangula, aloe
and rheum, or as laxative drugs containing danthrone and purified
sennosides. | The findings did
not support the hypothesis of a cause-effect relationship of CRC
with laxative use. | 6 | (23) |
| Roberts et
al, 2003 | United States | 1996-2000 | 1,691 | Case-control | Phenolphthalein,
fiber, magnesium, other commercial and non-commercial or unknown
laxatives. | There was no
association between laxative use and colon cancer. | 7 | (24) |
| Watanabe et
al, 2004 | Japan | 1990-1997 | 41,670 | Cohort | NR | Laxative use
increased the risk of colon cancer. | 9 | (16) |
| Park et al,
2009 | United Kingdom | 1993-1997 | 889 | Cohort | NR | Laxative use was
not associated with CRC risk. | 8 | (25) |
| Charlton et
al, 2013 | United Kingdom | 2000-2009 | 33,138 | Case-control | All stimulant,
osmotic and. bulk laxatives, fecal softeners and bowel cleansing
preparations | A reduced CRC risk
associated with macrogol use was observed after accounting for the
lead time for CRC. | 7 | (18) |
| Zhang et al,
2013 | United States | 1982-2010 | 88,173 | Cohort | Softeners, bulking
agents and suppositories. | Laxative use seemed
to not be associated with CRC risk. | 9 | (26) |
| Citronberg et
al, 2014 | New Zealand | 2000-2010 | 144,455 | Cohort | Fiber-based
(Metamucil, Citrucel, FiberCon or Fiberall) and non-fiber (Ex-lax,
Correctol or milk of magnesia) laxatives | The risk of CRC
increased with non-fiber laxative use and decreased with fiber
laxative use. | 9 | (19) |
| Citronberg et
al, 2018 | United States | 1997-2008 | 17,694 | Case-control | Fiber (Metamucil,
Citrucel, Fibercon, Serutan and psyllium) and non-fiber (Ex-Lax,
Correctol, Dulcolax, Senokot, Colace, castor oil, cod liver oil,
mineral oil, milk of magnesia, lactulose and Epsom salts)
laxatives. | Individuals who
reported using non-fiber-based laxatives regularly were at a
significantly increased risk for CRC compared with those who
reported no laxative use. No statistically significant associations
were observed between fiber-based laxative use and CRC. | 9 | (17) |
History of laxative use and the risk
of CRC
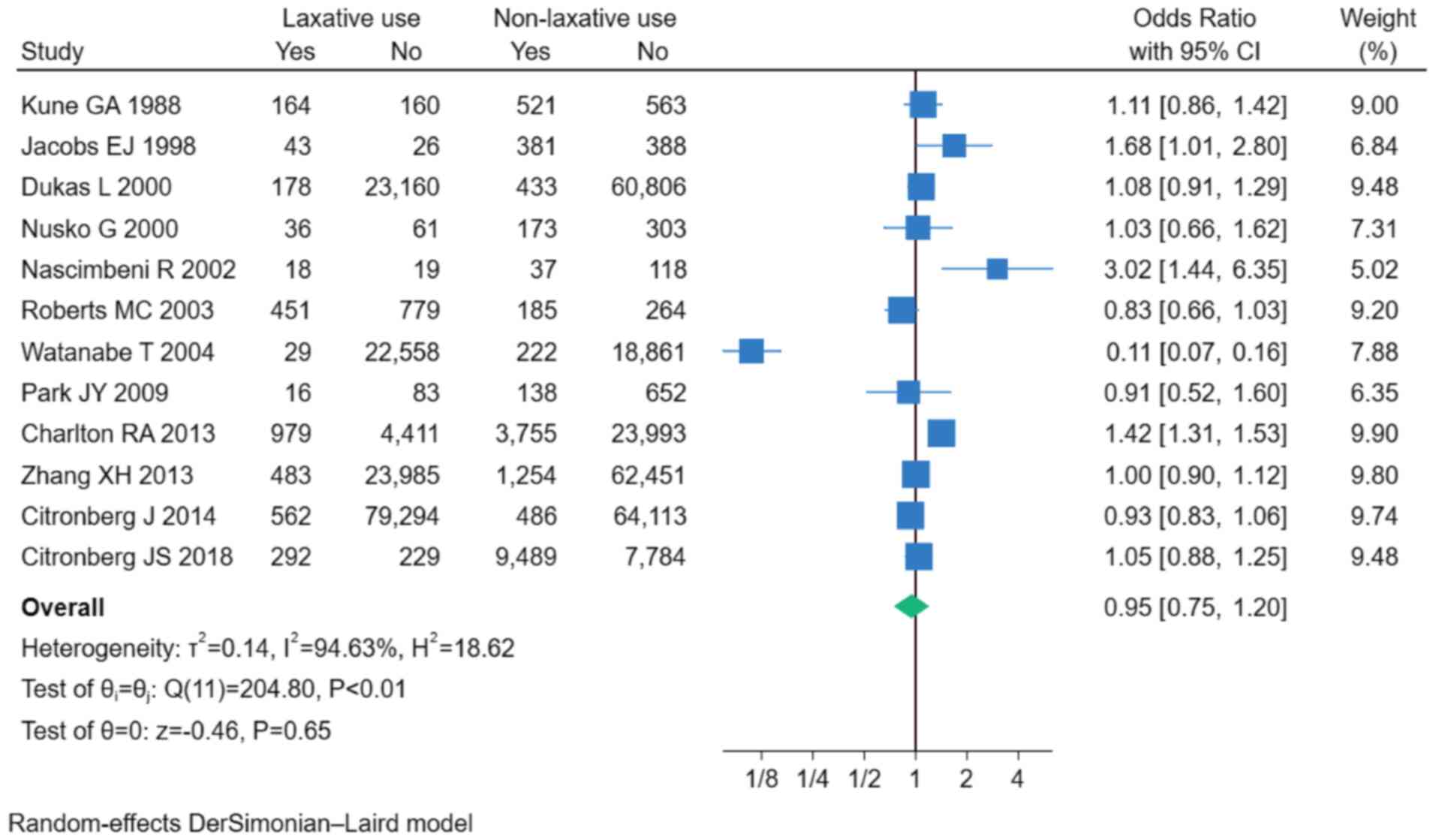
The results from the 12 included studies indicated
that a history of laxative use was not significantly associated
with the incidence of CRC (OR, 0.95; 95% CI, 0.75–1.20; P=0.65;
I2=94.63%; Fig. 2) To
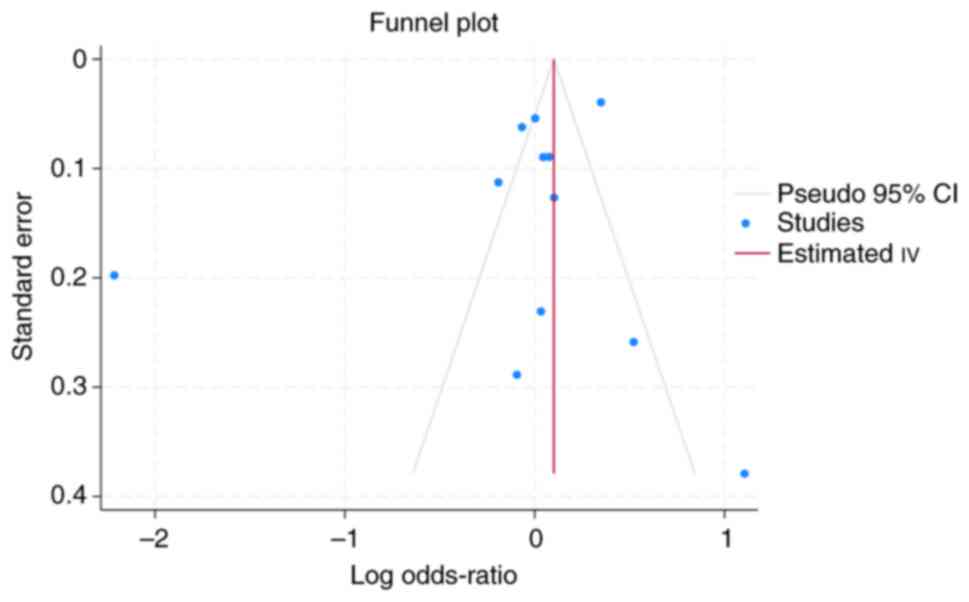
assess potential publication bias, a funnel plot was generated
(Fig. 3). Visual inspection of the
funnel plot revealed asymmetry, suggesting the possibility of
publication bias.
History of different laxative type use
and the risk of CRC
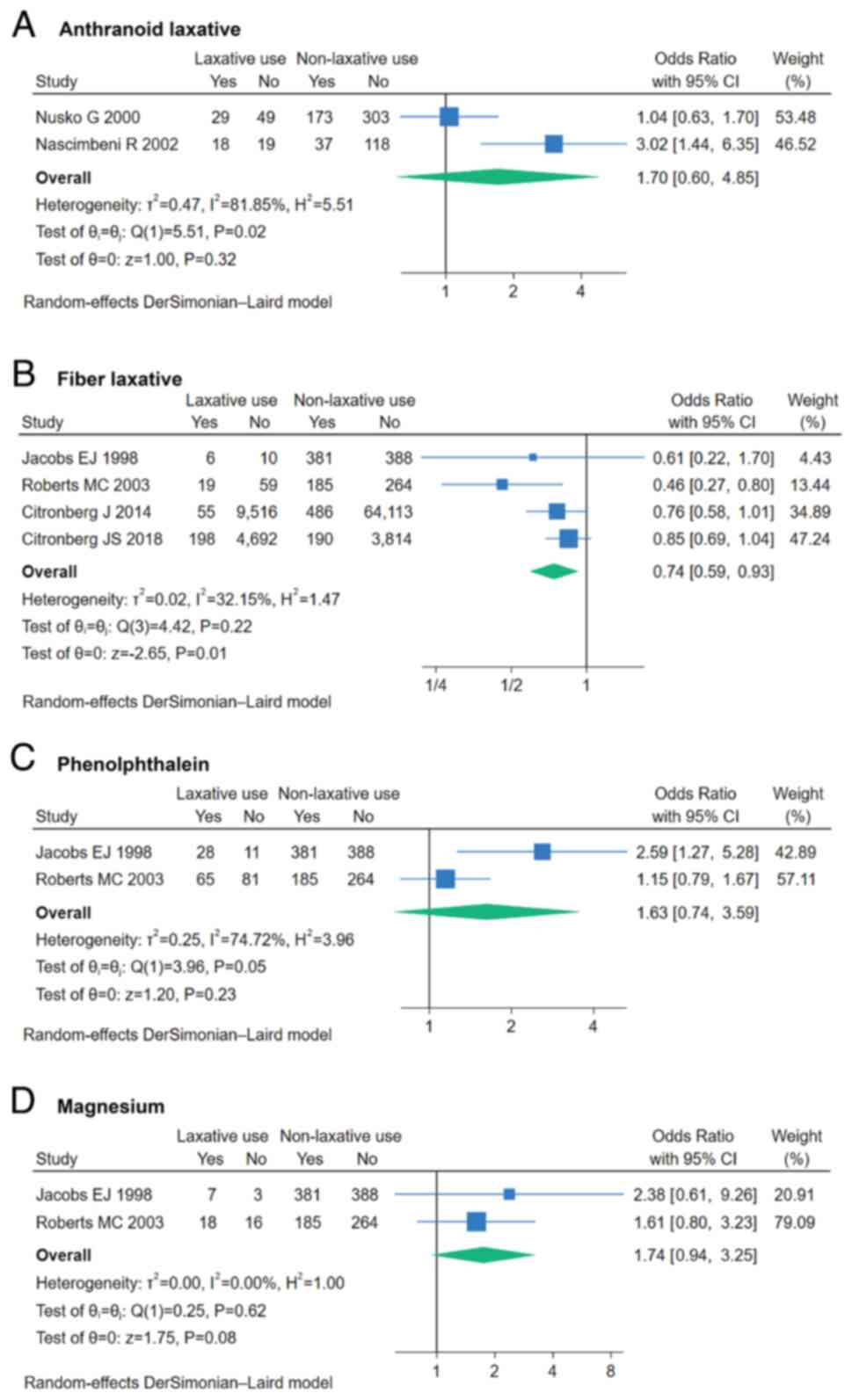
In total, 4 studies reported data on fiber
laxatives. Pooled analysis revealed that fiber laxative use was
associated with a reduced risk of CRC (OR, 0.74; 95% CI, 0.59–0.93;
P=0.01; I2=32.15%; Fig.
4B). Additionally, 3 other types of laxatives were each
reported in 2 studies. However, anthranoid, phenolphthalein and
magnesium laxatives showed no significant association with CRC risk
(Fig. 4A, C and D).
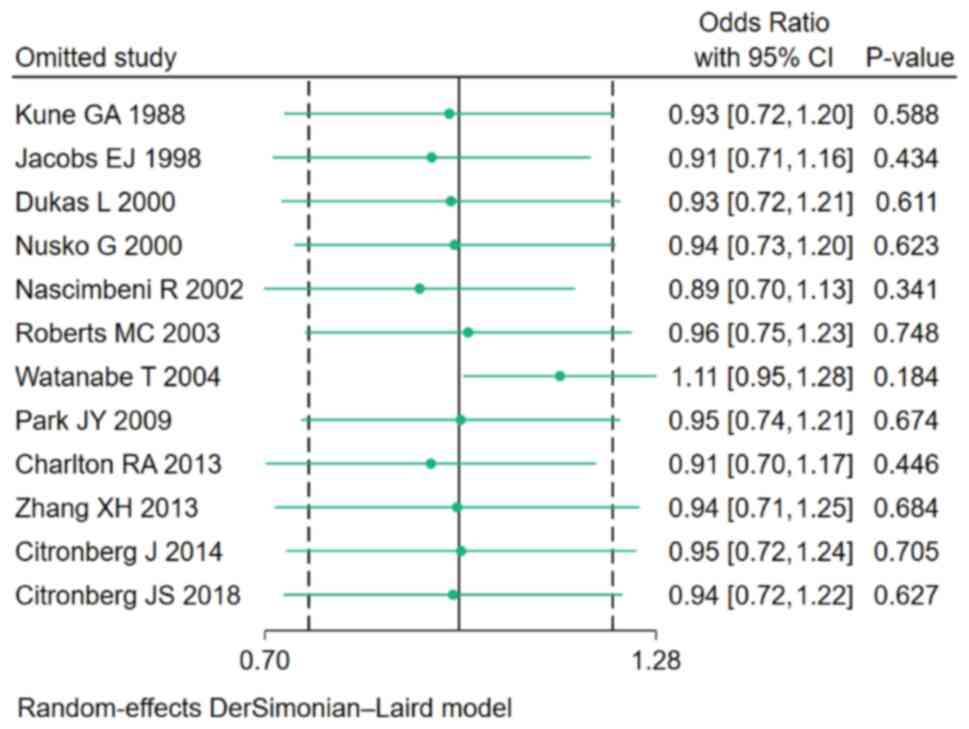
Sensitivity analysis
To evaluate the robustness of the study findings, a
sensitivity analysis was performed by sequentially excluding each
study and re-running the pooled analysis. The results remained
consistent across all iterations, indicating that the findings were
not significantly altered by any single study (Fig. 5).
Discussion
In the present study, 12 eligible observational
studies involving 415,313 patients were identified for analysis.
After pooling the data, no association between laxative use and the
risk of CRC was found. Subgroup analyses of different laxative
types revealed that the fiber laxative group had fewer patients
with CRC than the non-fiber laxative group. Other types of
laxatives, including anthranoid, phenolphthalein and magnesium
laxatives, had no identified effect on the incidence of CRC.
To diagnose patients with CRC earlier, both
CRC-related symptoms and strong risk factors for CRC should be
considered. Staging at detection dramatically impacts survival
outcomes, with 5-year survival rates of 91% for localized disease
(Stage I) versus merely 14% for metastatic cases (Stage IV)
(31). Then, late diagnosis
increases surgical morbidity risks and permanent ostomy rates,
severely compromising quality of life (32). In addition to commonly examined
characteristics, including age, family history of cancer, history
of smoking, diabetes and the frequency of food intake (8–10),
drug use, including macrolide and lincosamide use, has also been
reported to be a risk factor for CRC (33). Drugs might change the gut microbiota
composition and destroy immune responses, which could lead to
chronic inflammation and tumor progression (34–36).
Additionally, quinolones (a type of antibiotic) can damage DNA,
which increases the risk of cancer (37).
Laxatives are also widely used and might influence
CRC. Certain studies have reported that non-fiber laxatives are
associated with an increased risk of CRC (15,17,19). A
previous study examining 41,670 cases demonstrated that laxative
use increased the risk of colon cancer (16). Conversely, other studies revealed
that the risk of CRC decreased with fiber laxative use (15,19),
and Charlton et al (18)
reported that macrogol reduces the risk of CRC. Additionally, other
studies did not find an overall association between laxative use
and CRC (20–26), and a previous systematic review and
meta-analysis indicated that there was no association between
anthraquinone laxative use and the development of CRC (38). Therefore, there is notable
controversy regarding whether laxatives have harmful effects,
protective effects or no effect on the incidence of CRC.
Long-term medication or drug abuse might damage the
intestinal environment. Some biological evidence has shown that
ecological imbalance, especially in some bacterial strains, might
promote cancer by altering cell growth, differentiation and
apoptosis (39,40). Some non-fiber laxatives including
anthranoid laxatives and phenolphthalein were found to have
mutagenic and genotoxic effects, which might be the mechanisms
underlying the development of CRC risk (19,41,42).
The potential mechanism underlying the protective effect of fiber
laxatives might be that high dietary fiber intake could dilute
carcinogens and bind carcinogenic bile acids. Additionally, fiber
helps produce short-chain fatty acids, which can normalize cell
proliferation and differentiation as well as promote
anticarcinogenic action (43–45).
However, the association between laxative use and CRC risk is
complex, and the present study, as a synthesis of observational
data, could not clarify the mechanisms underlying various laxative
types.
To the best of our knowledge, the present study
represented the largest and most comprehensive pooled analysis to
date on the association between laxative use and CRC risk. The
findings of the present study resolved existing controversies, that
have notable implications for clinical practice and may pave the
way for future investigations into the mechanisms underlying
laxative effects on CRC risk. However, the present study has
several limitations. First, the results might have a bias
accounting for the frequency of laxative intake. However, there
were not enough data for a dose-response meta-analysis. Second, 3
of the 12 included studies only analyzed colon cancer cases,
although both colon cancer and rectal cancer originate from
epithelial cells (46), which may
have led to the high heterogeneity of the studies. Third, some of
the included studies were old, which might limit the universality
of the study findings. Fourth, while funnel plot asymmetry
suggested potential publication bias, statistical confirmation was
limited by the small number of included studies (n=7). This pattern
may reflect the selective reporting of positive outcomes.
Therefore, additional long-term randomized controlled trials that
investigate the relationship between the dosage of laxatives
administered and the incidence of CRC are needed to determine the
potential mechanisms and effects of different types of
laxatives.
In conclusion, the standardized use of laxatives for
treatment is safe. Moreover, fiber laxatives may decrease the risk
of CRC. However, more studies are needed to determine whether
laxative abuse increases the risk of CRC.
Acknowledgements
We extend our gratitude to Dr Tianwu Chen
(Department of Radiology, The Second Affiliated Hospital of
Chongqing Medical University) for his expert arbitration in
resolving methodological disagreements during data extraction and
eligibility assessment.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
LXR conceived the study. LXR and ZXM designed the
search strategy, performed the literature search and data
extraction and assessed the risk of bias. LXR and ZXM confirm the
authenticity of all the raw data. ZXM performed the data analysis
and wrote the first draft of the manuscript. LXR and ZXM revised
the manuscript. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards BK, Ward E and Kohler BA: Annual
report to the nation on the status of cancer, 1975–2006, featuring
colorectal cancer trends and impact of interventions (risk factors,
screening, and treatment) to reduce future rates. Cancer.
116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JS, Perdue LA, Henrikson NB, Bean SI
and Blasi PR: Screening for Colorectal Cancer: Updated Evidence
Report and Systematic Review for the US Preventive Services Task
Force. JAMA. 325:1978–1998. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knudsen AB, Rutter CM, Peterse EFP, Lietz
AP, Seguin CL, Meester RGS, Perdue LA, Lin JS, Siegel RL,
Doria-Rose VP, et al: Colorectal cancer screening: An updated
modeling study for the US preventive services task force. JAMA.
325:1998–2011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L,
Li X, Guo L, Zheng Z, Zou S, et al: Participation and yield of a
population-based colorectal cancer screening programme in China.
Gut. 68:1450–1457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao M, Li H, Sun D, He S, Yu Y, Li J, Chen
H, Shi J, Ren J, Li N and Chen W: Cancer screening in China: The
current status, challenges, and suggestions. Cancer Lett.
506:120–127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Lu B and Dai M: Colorectal cancer
screening in China: Status, challenges, and prospects-China, 2022.
China CDC Wkly. 4:322–328. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai QC, Yu ED, Xiao Y, Bai WY, Chen X, He
LP, Yang YX, Zhou PH, Jiang XL, Xu HM, et al: Derivation and
validation of a prediction rule for estimating advanced colorectal
neoplasm risk in average-risk Chinese. Am J Epidemiol. 175:584–593.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong MC, Lam TY, Tsoi KK, Hirai HW, Chan
VC, Ching JY, Chan FK and Sung JJ: A validated tool to predict
colorectal neoplasia and inform screening choice for asymptomatic
subjects. Gut. 63:1130–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sung JJY, Wong MCS, Lam TYT, Tsoi KKF,
Chan VCW, Cheung W and Ching JYL: A modified colorectal screening
score for prediction of advanced neoplasia: A prospective study of
5744 subjects. J Gastroenterol Hepatol. 33:187–194. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Streatfeild J, Hickson J, Austin SB,
Hutcheson R, Kandel JS, Lampert JG, Myers EM, Richmond TK,
Samnaliev M, Velasquez K, et al: Social and economic cost of eating
disorders in the United States: Evidence to inform policy action.
Int J Eat Disord. 54:851–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roerig JL, Steffen KJ, Mitchell JE and
Zunker C: Laxative abuse: Epidemiology, diagnosis and management.
Drugs. 70:1487–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Linardon J: Rates of abstinence following
psychological or behavioral treatments for binge-eating disorder:
Meta-analysis. Int J Eat Disord. 51:785–797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hazzard VM, Simone M, Austin SB, Larson N
and Neumark-Sztainer D: Diet pill and laxative use for weight
control predicts first-time receipt of an eating disorder diagnosis
within the next 5 years among female adolescents and young adults.
Int J Eat Disord. 54:1289–1294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacobs EJ and White E: Constipation,
laxative use, and colon cancer among middle-aged adults.
Epidemiology. 9:385–3891. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe T, Nakaya N, Kurashima K,
Kuriyama S, Tsubono Y and Tsuji I: Constipation, laxative use and
risk of colorectal cancer: The Miyagi Cohort Study. Eur J Cancer.
40:2109–2115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Citronberg JS, Hardikar S, Phipps A,
Figueiredo JC and Newcomb P: Laxative type in relation to
colorectal cancer risk. Ann Epidemiol. 28:739–741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charlton RA, Snowball JM, Bloomfield K and
de Vries CS: Colorectal cancer risk reduction following macrogol
exposure: A cohort and nested case control study in the UK. PLoS
One. 8:e832032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Citronberg J, Kantor ED, Potter JD and
White E: A prospective study of the effect of bowel movement
frequency, constipation, and laxative use on colorectal cancer
risk. Am J Gastroenterol. 109:1640–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kune GA, Kune S, Field B and Watson LF:
The role of chronic constipation, diarrhea, and laxative use in the
etiology of large-bowel cancer. Data from the Melbourne Colorectal
Cancer Study. Dis Colon Rectum. 31:507–512. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dukas L, Willett WC, Colditz GA, Fuchs CS,
Rosner B and Giovannucci EL: Prospective study of bowel movement,
laxative use, and risk of colorectal cancer among women. Am J
Epidemiol. 151:958–964. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nusko G, Schneider B, Schneider I,
Wittekind C and Hahn EG: Anthranoid laxative use is not a risk
factor for colorectal neoplasia: Results of a prospective case
control study. Gut. 46:651–655. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nascimbeni R, Donato F, Ghirardi M,
Mariani P, Villanacci V and Salerni B: Constipation, anthranoid
laxatives, melanosis coli, and colon cancer: A risk assessment
using aberrant crypt foci. Cancer Epidemiol Biomarkers Prev.
11:753–757. 2002.PubMed/NCBI
|
|
24
|
Roberts MC, Millikan RC, Galanko JA,
Martin C and Sandler RS: Constipation, laxative use, and colon
cancer in a North Carolina population. Am J Gastroenterol.
98:857–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JY, Mitrou PN, Luben R, Khaw KT and
Bingham SA: Is bowel habit linked to colorectal cancer?-Results
from the EPIC-Norfolk study. Eur J Cancer. 45:139–145. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wu K, Cho E, Ma J, Chan AT, Gao
X, Willett WC, Fuchs CS and Giovannucci EL: Prospective cohort
studies of bowel movement frequency and laxative use and colorectal
cancer incidence in US women and men. Cancer Causes Control.
24:1015–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ioannidis JP: Interpretation of tests of
heterogeneity and bias in meta-analysis. J Eval Clin Pract.
14:951–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brenner H, Bouvier AM, Foschi R, Hackl M,
Larsen IK, Lemmens V, Mangone L and Francisci S; EUROCARE Working
Group, : Progress in colorectal cancer survival in Europe from the
late 1980s to the early 21st century: the EUROCARE study. Int J
Cancer. 131:1649–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simin J, Fornes R and Liu Q: Antibiotic
use and risk of colorectal cancer: A systematic review and
dose-response meta-analysis. Br J Cancer. 123:1825–1832. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sobhani I, Tap J, Roudot-Thoraval F,
Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J and
Furet JP: Microbial dysbiosis in colorectal cancer (CRC) patients.
PLoS One. 6:e163932011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong SH and Yu J: Gut microbiota in
colorectal cancer: Mechanisms of action and clinical applications.
Nat Rev Gastroenterol Hepatol. 16:690–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Francescone R, Hou V and Grivennikov SI:
Microbiome, inflammation, and cancer. Cancer J. 20:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bush NG, Diez-Santos I, Abbott LR and
Maxwell A: Quinolones: Mechanism, lethality and their contributions
to antibiotic resistance. Molecules. 25:56620202
|
|
38
|
Lombardi N, Crescioli G, Maggini V,
Bellezza R, Landi I, Bettiol A, Menniti-Ippolito F, Ippoliti I,
Mazzanti G, Vitalone A, et al: Anthraquinone laxatives use and
colorectal cancer: A systematic review and meta-analysis of
observational studies. Phytother Res. 36:1093–1102. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hibberd AA, Lyra A, Ouwehand AC, Rolny P,
Lindegren H, Cedgård L and Wettergren Y: Intestinal microbiota is
altered in patients with colon cancer and modified by probiotic
intervention. BMJ Open Gastroenterol. 4:e0001452017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gagnière J, Raisch J, Veziant J, Barnich
N, Bonnet R, Buc E, Bringer MA, Pezet D and Bonnet M: Gut
microbiota imbalance and colorectal cancer. World J Gastroenterol.
22:501–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Gorkom BA, de Vries EG, Karrenbeld A
and Kleibeuker JH: Review article: Anthranoid laxatives and their
potential carcinogenic effects. Aliment Pharmacol Ther. 13:443–452.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dunnick JK and Hailey JR: Phenolphthalein
exposure causes multiple carcinogenic effects in experimental model
systems. Cancer Res. 56:4922–4926. 1996.PubMed/NCBI
|
|
43
|
Park Y, Hunter DJ, Spiegelman D, Bergkvist
L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim
JL, Fuchs CS, et al: Dietary fiber intake and risk of colorectal
cancer: A pooled analysis of prospective cohort studies. JAMA.
294:2849–2857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alabaster O, Tang Z and Shivapurkar N:
Dietary fiber and the chemopreventive modelation of colon
carcinogenesis. Mutat Res. 350:185–197. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lipkin M, Reddy B, Newmark H and Lamprecht
SA: Dietary factors in human colorectal cancer. Annu Rev Nutr.
19:545–586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barker N, Ridgway RA, van Es JH, van de
Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR,
Sansom OJ and Clevers H: Crypt stem cells as the cells-of-origin of
intestinal cancer. Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|