Introduction
Gastrointestinal stromal tumors (GIST) are the most
common mesenchymal tumors occurring in the gastrointestinal tract
(1), with an annual incidence is up
to 10 to 15 cases per million individuals (2–6). The
prognosis of GIST is associated with the tumor size and mitotic
index, and median overall survival ranged from 47 to 57 months
(2–6). Curative resection is advised for most
patients with GISTs but not for those with locally advanced GISTs
(LAGISTs), which are unsuitable for radical resection, those for
whom resection presents risks of substantial organ dysfunction or
those whose GISTs are borderline unresectable (2–6).
Mutations in the genes encoding the receptor
tyrosine-protein kinase KIT (KIT) and platelet-derived
growth factor receptor α (PDGFRα) may prompt considerations
of treatment with first-line imatinib therapy (2–7).
Imatinib inhibits GIST progression by targeting KIT and
PDGFRα. This preoperative treatment modality can be employed
in cases of LAGISTs that are unresectable or borderline
unresectable. First-line imatinib therapy can enable surgical
resection and minimize the risk of tumor spillage or bleeding
during surgery. However, whether imatinib can be used to treat
LAGIST with exon mutations of KIT and PDGFRα genes
(4,8) remains to be elucidated. KIT
gene mutations occur in ~80% of GISTs (8). Additionally, a higher occurrence of
primary imatinib resistance was observed in GISTs with KIT
exon 9 mutations, PDGFRα D842V mutations and wild-type
KIT and PDGFRα compared with other types of gene
mutation (9). Tyrosine kinase
inhibitors (TKIs) such as imatinib have transformed therapeutic
approaches for advanced GISTs. Currently, four TKIs, imatinib,
sunitinib, regorafenib and ripretinib, are approved for use as
first-line, second-line, third-line and fourth-line therapies,
respectively (5,10). Ripretinib, a broad-spectrum
KIT and PDGFRα inhibitor, is approved for the
treatment of adult patients with LAGISTs who have received prior
treatment with three or more kinase inhibitors, including imatinib.
Furthermore, avapritinib, a type I kinase inhibitor, is approved
for the treatment of adults with unresectable or metastatic GISTs
harboring a PDGFRα exon 18 mutation, including PDGFRα
D842V mutation (11). The present
study specifically examined treatment outcomes for patients with
LAGISTs with several gene mutations following first-line therapy
with imatinib.
Materials and methods
Patient demographics
There are no clear criteria to define LAGIST at
present. In the present study, LAGIST that was initially diagnosed
was defined as being unsuitable for radical resection, risk of
substantial organ dysfunction or borderline unresectable according
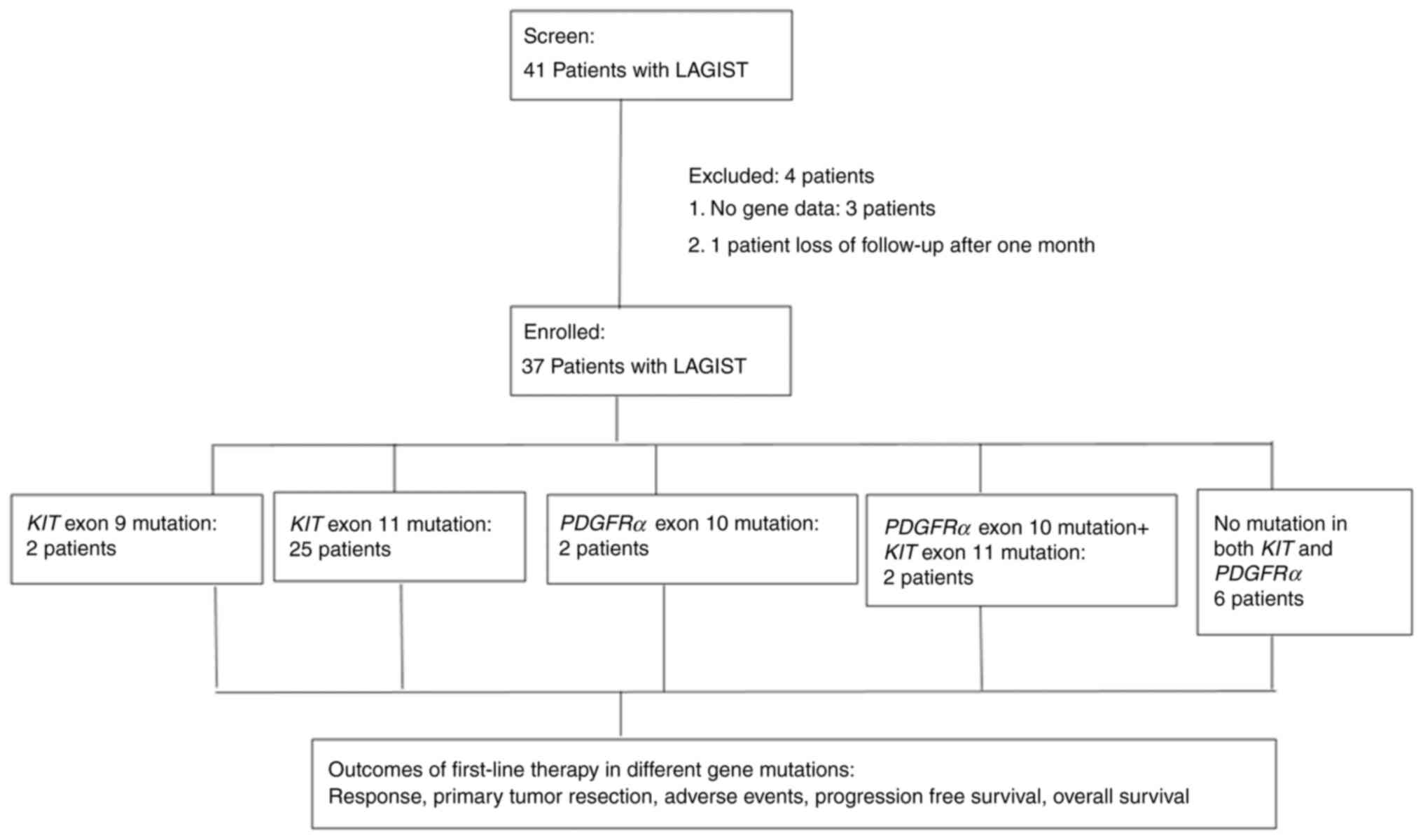
to a previous study by our group (3). Fig. 1
depicts a flowchart of the patient recruitment process. A total of
41 patients who had received a diagnosis of a LAGIST and who
underwent first-line treatment at a single institution (Kaohsiung
Medical University Hospital, Kaohsiung, Taiwan) between December
2010 and July 2023 were included. A total of 3 of the 41 patients
were excluded for having no gene data and 1 patient was excluded
for being lost to follow-up after 1 month, leaving a total of 37
patients for final enrolment. Enrolled patients were closely
monitored until January 2024. The inclusion criteria were as
follows: i) Having an LAGIST that was initially diagnosed as
unsuitable for radical resection; and ii) being at risk of
substantial organ dysfunction or borderline unresectable (3,12–14).
Following the administration of first-line therapy with imatinib,
the included patients were followed for a median period of 41
months (range, 10 to 183 months). Tumor responses were assessed
through concurrent analysis of computed tomography (CT) images and
the genetic mutation profiles of KIT and PDGFRα
genes. The present study was approved by the Institutional Review
Board of Kaohsiung Medical University Hospital [Kaohsiung, Taiwan;
approval no. KMUHIRB-E(I)-20240084] and the requirement for patient
consent was waived due to the respective nature of the study.
Treatment
Each patient received a prescription for imatinib at
a daily dose of 400 mg, with a treatment duration ranging from 3 to
56 months (median, 15 months). In cases where patients experienced
grade 3 or 4 toxicities, the imatinib dose was reduced to 300 mg
per day. Sunitinib was administered as a second-line therapy at a
daily dose of 37.5 mg when the first-line imatinib treatment failed
and disease progression was evident on CT scans performed every 3
months. Sunitinib was administered to improve the response after
consultation with a multidisciplinary team (MDT), which comprised
surgeons, radiologists, gastroenterologists and oncologists.
Regorafenib was employed as a third-line treatment at a daily dose
of 120 mg. After the radical resection, the adjuvant treatment was
continued for a total of 36 months under close supervision by the
MDT.
Evaluation of tumor response and
toxicities
Tumor dimensions and density were verified through
assessment of abdominal CT scans by two radiologists. Any
discrepancies were resolved through a joint re-examination of the
images by both radiologists. Tumor responses were assessed using CT
images in accordance with the response evaluation criteria in solid
tumors 1.1 (RECIST 1.1) (15).
Adverse events (AEs) were categorized based on the Common
Terminology Criteria for AEs, version 3.0 (16). In evaluating surgical resectability
in patients with LAGISTs, the timing followed the method of
combined CT-measured tumor density and RECIST 1.1 described in a
previous study by our group (3).
All enrolled patients were followed up by CT and laboratory data
every 3 months for efficacy evaluation until surgical intervention.
The decision to proceed with surgery was made using combined
CT-measured tumor density and RECIST for evaluating surgical
timing. With either a tumor size (tumor dimensions) reduction of
>30% or a reduction of >30% of tumor density, surgery was
considered (3). Surgical timing was
confirmed by the MDT, which comprised surgeons, radiologists,
gastroenterologists and oncologists. The clinical condition of the
patients (such as age, Eastern Cooperative Oncology Group
performance status and willingness of the patient to undergo
surgery) was also considered.
KIT and PDGFRα gene mutations
CT-guided core biopsies were performed prior to the
initiation of the first-line imatinib therapy to collect tumor
tissue specimens. Biopsy specimens were carefully embedded in
paraffin, fixed using formalin and subsequently sectioned into
slices measuring 4 µm in thickness. DNA extraction was performed
using the Qiagen DNA extraction kit (Qiagen, Inc.), following the
manufacturer's instructions. The concentration and quality of the
extracted DNA were assessed using a NanoDrop 2000
spectrophotometer. The optical density at either 260 or 280 nm for
DNA extracted from all patient specimens fell within the range of
1.8 to 2.0, indicating that the DNA samples were of suitable
quality for subsequent experiments. The DNA samples were subjected
to analysis using polymerase chain reaction (PCR) in a PCR
instrument from Applied Biosystems (Thermo Fisher Scientific,
Inc.). Subsequently, the KIT or PDGFRα primers (at a
concentration of 100 µM) and the 2X PCR Master Mix (Thermo Fisher
Scientific, Inc.) were introduced and mutations confirmed by Sanger
sequencing. The thermocycling conditions were as follows: 95°C for
10 min, then 40 cycles of 95°C denaturation for 30 sec, 58°C
annealing for 45 sec and 72°C extension for 45 sec, followed by a
final step of 72°C for 7 min. The primers for the KIT gene
were as follows: Forward (exon 9), 5′-GATGCTCTGCTTCTGTACT-3′ and
reverse (exon 9), 5′-GCCTAAACATCCCCTTAAATTGG-3; forward (exon 11),
5′-CTCTCCAGAGTGCTCTAATGAC-3 and reverse (exon 11),
5′-AGCCCCTGTTTCATACTGACC-3′; forward (exon 13),
5′-CGGCCATGACTGTCGCTGTAA-3′ and reverse (exon 13),
5′-CTCCAATGGTGCAGGCTCCAA-3′; forward (exon 17),
5′-TCTCCTCCAACCTAATAGTG-3′ and reverse (exon 17),
5′-GGACTGTCAAGCAGAGAAT-3′; forward (exon 18),
5′-CATTTCAGCAACAGCAGCAT-3′ and reverse (exon 18),
5′-CAAGGAAGCAGGACACCAAT-3′. The primers for PDGFRα gene were
as follows: Forward (exon 10), 5′-GACTCTCAGGAATTGGCC-3′; reverse
(exon 10), 5′-CAGCTGATGAGTTGTCCTG-3′; forward (exon 12),
5′-GAACGTTGTTGGACTCTACTGTG-3′ and reverse (exon 12),
5′-GCAAGGGAAAAGGGAGTCT-3′; forward (exon 14),
5′-GTAGCTCAGCTGGACTGATA-3′ and reverse (exon 14):
5′-AATCCTCACTCCAGGTCAGT-3′; forward (exon 18),
5′-CTTGCAGGGGTGATGCTAT-3′ and reverse (exon 18),
5′-AGAAGCAACACCTGACTTTAGAGATTA-3′.
Statistical analysis
All statistical analyses were performed using SPSS
version 21 (IBM Corp.). Progression-free survival (PFS) was
calculated from the treatment initiation date to the date of any
form of progression or the last recorded follow-up. Overall
survival (OS) was defined as the duration from the commencement of
treatment to either mortality from any cause or the last follow-up
date. PFS and OS were evaluated using the Kaplan-Meier method and
the log-rank test was employed to compare time-to-event
distributions. Treatment response rates, resection rates and AE
rates were compared using Fisher's exact test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient series, tumor characteristics
and mutation status
The demographic and clinicopathological
characteristics of the enrolled patients are presented in Table I. The median age of the cohort was
65 years, with total ages ranging from 33 to 87 years. Among the
patients, 25 (67.6%) were men and 12 (32.4%) were women. The
LAGISTs were located in various sites: Stomach (21 patients;
56.8%), omentum (1 patient; 2.7%), pancreas (1 patient; 2.7%),
small intestine (5 patients; 13.5%), mesocolon (1 patient; 2.7%),
pelvic area (1 patient; 2.7%) and rectum (7 patients; 18.9%).
 | Table I.Demographics of 37 patients with
locally advanced gastrointestinal stromal tumor. |
Table I.
Demographics of 37 patients with
locally advanced gastrointestinal stromal tumor.
| Characteristic | Patients, n
(%) |
|---|
| Sex |
|
|
Male | 25 (67.6) |
|
Female | 12 (32.4) |
| Median age (years,
range) | 65 (33–87) |
| Tumor location |
|
|
Stomach | 21 (56.8) |
|
Omentum | 1 (2.7) |
|
Pancreas | 1 (2.7) |
| Small
intestine (including duodenum) | 5 (13.5) |
|
Mesocolon | 1 (2.7) |
| Pelvic
area | 1 (2.7) |
|
Rectum | 7 (18.9) |
| Type of gene
mutation |
|
|
KIT exon 9
insertion | 2 (5.4) |
|
KIT exon 11
insertion | 5 (13.5) |
|
KIT exon 11
deletion | 14 (37.8) |
|
KIT exon 11-point
mutation | 5 (13.5) |
|
KIT exon 11
duplication | 1 (2.7) |
|
PDGFRα exon 10-point
mutation | 2 (5.4) |
|
KIT exon 11 deletion;
PDGFRα exon | 2 (5.4) |
|
10-point mutation |
|
| No
mutation in both KIT and PDGFRα | 6 (16.2) |
| Mutation
numbers | 31 (83.8) |
|
KIT exon 9 | 2 (5.4) |
|
KIT exon 11 | 27 (73.0) |
|
PDGFRα exon 10 | 4 (10.8) |
Regarding genetic mutations, 29 patients (78.4%) had
KIT mutations and 4 (10.5%) had PDGFRα mutations. A
total of 2 patients (5.4%) had KIT exon 9 insertions, five
(13.5%) had KIT exon 11 insertions, 14 patients (37.8%) had
KIT exon 11 deletions, 5 (13.5%) had KIT exon 11
point mutations and 1 (2.7%) had KIT exon 11 duplications. A
total of 2 patients (5.4%) had PDGFRα exon 10 point
mutations and 2 patients (5.4%) had PDGFRα exon 10 point
mutations with a KIT exon 11 deletion. A total of 6 patients
(16.2%) had wild-type KIT and PDGFRα (Table I). Representative Sanger sequencing
images depicting each respective mutation are shown in Fig. S1, Fig.
S2, Fig. S3, Fig. S4, Fig.
S5, Fig. S6, Fig. S7.
Treatment outcomes
Analysis of tumor responses using the RECIST 1.1
revealed that 20 of the 37 patients (54.1%) achieved a partial
response (PR) and 15 (40.5%) exhibited stable disease (SD). After
therapy, 24 of the 37 patients (64.9%) with unresectable LAGISTs
underwent primary tumor R0 resection (Table II). The median duration of
first-line therapy was 16 months, with the total therapy duration
ranging from 3 to 56 months. A total of 21 of the 24 patients
eligible for resection were treated with imatinib; 3 patients
subsequently switched to second-line sunitinib to achieve an
improved response. However, the treatment response and surgical
resectability rates of the 37 patients with LAGISTs following
first-line therapy did not differ significantly in tumors with
varying mutations of the KIT and PDGFRα genes (both
P>0.05; Table II). As of the
final follow-up performed in January 2024, 21 of the patients were
still alive. Sunitinib was administered for 4–52 months (median, 9
months) and regorafenib was administered for 5 months (case no. 5)
and 24 months (case no. 21), up to the end of treatment or
follow-up (Table III). Of the 21
patients with stomach LAGIST, 10 (47.6%) patients achieved PR, 9
(42.9%) patients SD and 2 (9.5%) patients progressive disease. Of
the 5 patients with small intestine LAGIST, 3 (60%) patients
achieved PR and 2 (40%) patients achieved SD. Of the 7 patients
with rectal LAGIST, 6 (85.7%) patients achieved PR and 1 (14.3%)
patient achieved SD (Table III).
A total of 21 patients received adjuvant treatment with imatinib
after the operation and 3 patients had adjuvant treatment with
sunitinib (Table III). The
adjuvant regimen was the same as that previously used in the
first-line setting or the second-line setting. The median PFS of
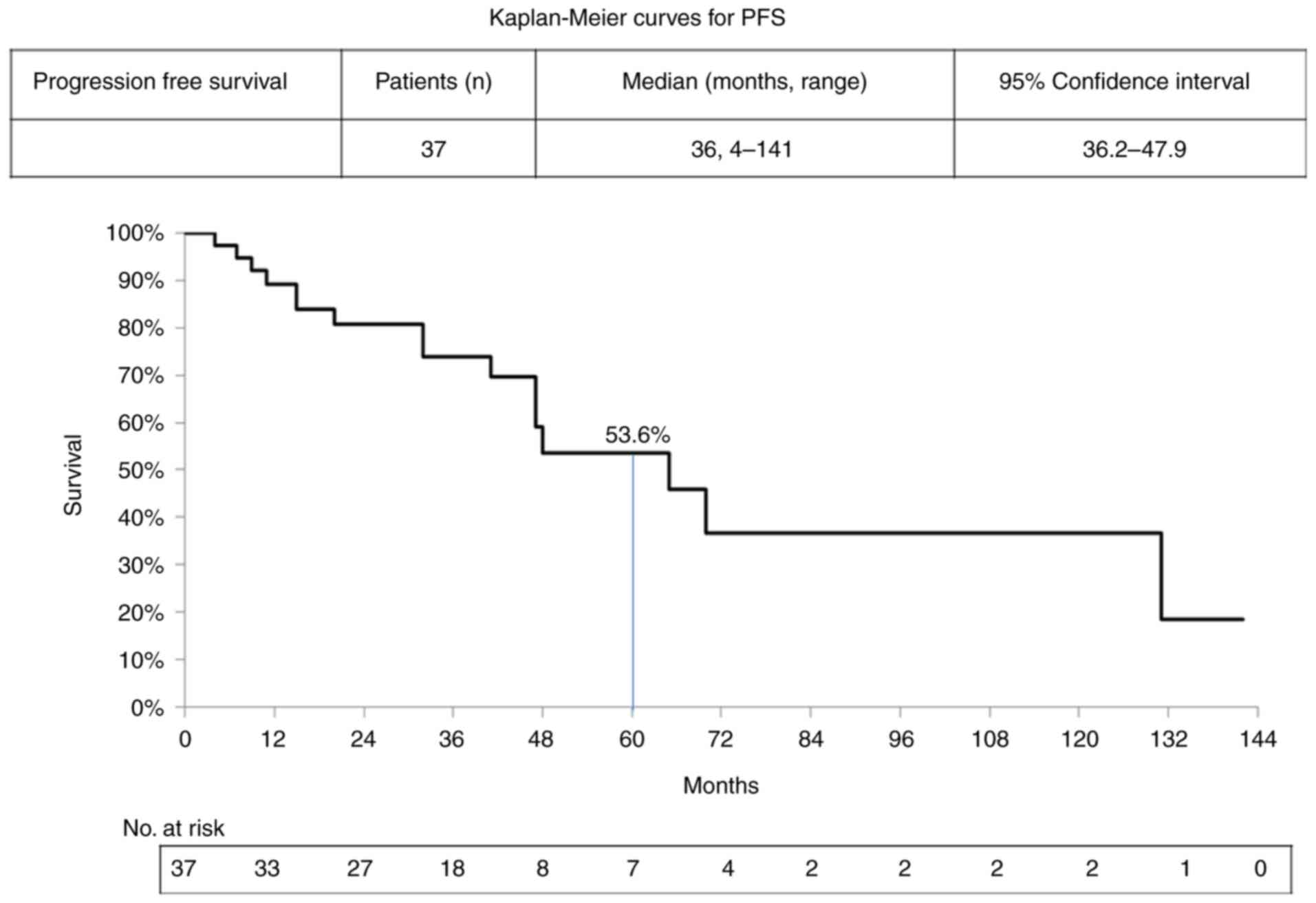
the 37 patients was 36 months, with the total PFS ranging from 4 to
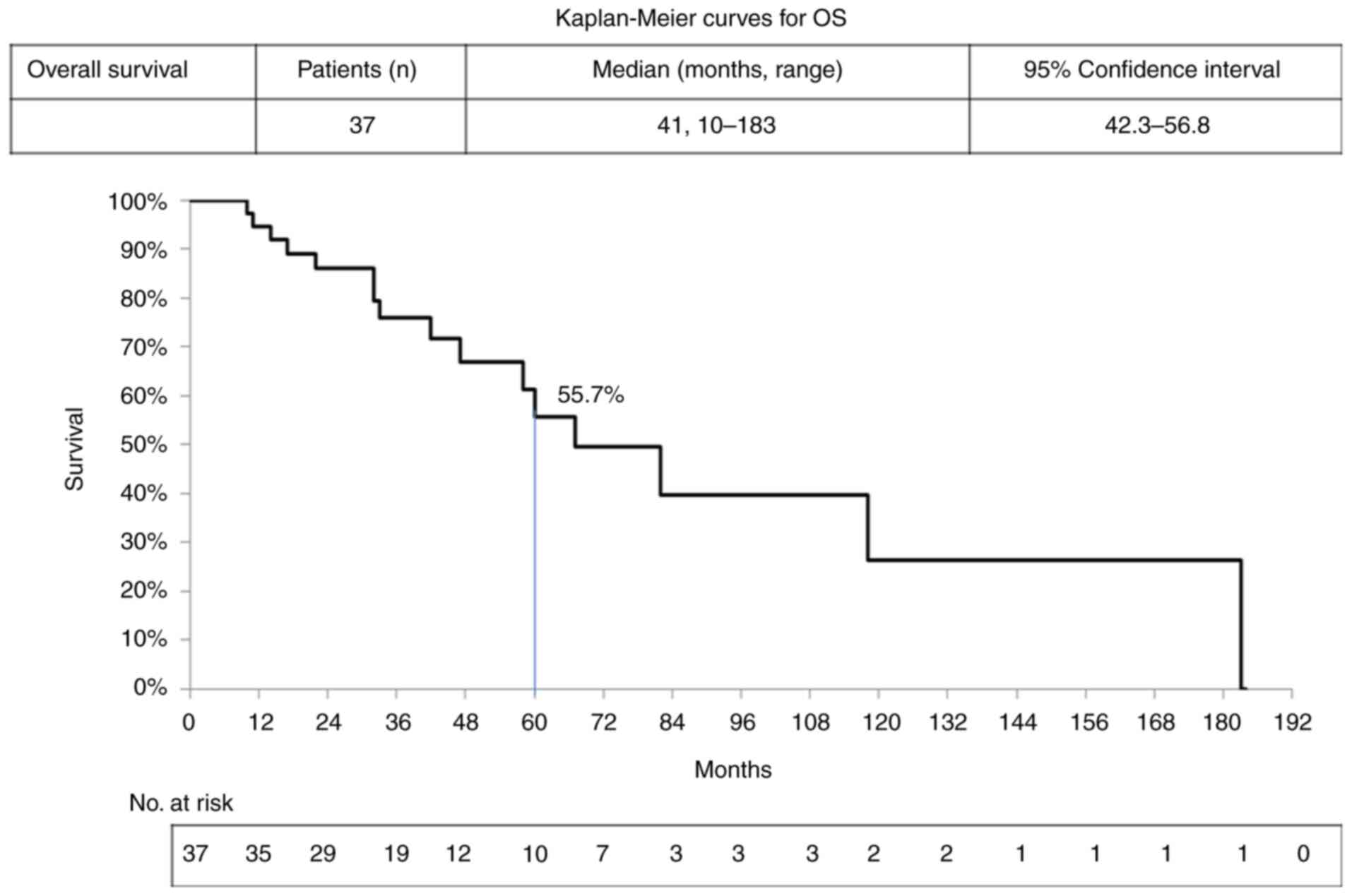
141 months and a 5-year (60 months) PFS rate of 53.6% (Fig. 2). The median OS of the patients was
41 months, with total OS ranging from 10 to 183 months and a 5-year
(60 months) OS rate of 55.7% (Fig.
3). AEs were reported by 78.4% of patients receiving first-line
imatinib therapy, with the most commonly reported AEs being eyelid
edema and nausea; these were experienced by 8 patients (21.6%).
Anemia was experienced by 5 patients (13.5%). In addition, 3
patients (8.1%) had grade 3 anemia (Table IV).
 | Table II.Treatment outcome of first-line
therapy in different gene mutations of 37 patients with locally
advanced gastrointestinal stromal tumor. |
Table II.
Treatment outcome of first-line
therapy in different gene mutations of 37 patients with locally
advanced gastrointestinal stromal tumor.
| Mutation | Patients, n
(%) | Partial response, n
(%) | Stable disease, n
(%) | Progressive
disease, n (%) | Primary tumor
resection, n (%) | No primary tumor
resection, n (%) |
|---|
| KIT exon 9
insertion | 2 (100.0) | 1 (50.0) | 1 (50.0) | 0 | 1 (50.0) | 2 (100.0) |
| KIT exon
11 | 25 (100.0) | 14 (56.0) | 10 (40.0) | 1 (4.0) | 17 (68.0) | 25 (100.0) |
|
Insertion | 5 (20.0) | 2 (40.0) | 3 (60.0) | 0 | 4 (80.0) | 5 (20.0) |
|
Deletion | 14 (56.0) | 9 (64.2) | 4 (28.6) | 1 (7.1) | 8 (57.1) | 14 (56.0) |
| Point
mutation | 5 (20.0) | 3 (60.0) | 2 (40.0) | 0 | 4 (80.0) | 5 (20.0) |
|
Duplication | 1 (4.0) | 0 | 1 (100.0) | 0 | 1 (100.0) | 1 (4.0) |
| PDGFRα exon
10 point mutation | 2 (100.0) | 0 | 2 (100.0) | 0 | 1 (50.0) | 2 (100.0) |
| KIT exon 11
deletion; | 2 (100.0) | 2 (100.0) | 0 | 0 | 1 (50.0) | 2 (100.0) |
| PDGFRα exon
10 point mutation |
|
|
|
|
|
|
| No mutation in both
KIT and PDGFRα | 6 (100.0) | 3 (50.0) | 2 (33.3) | 1 (16.7) | 4 (66.7) | 6 (100.0) |
| Total | 37 (100.0) | 20 (54.1) | 15 (40.5) | 2 (5.4) | 24 (64.9) | 37 (100.0) |
| P-value |
|
| P=0.732 |
| P=0.977 |
 | Table III.Pathologic and treatment evaluation
of 37 patients with locally advanced gastrointestinal stromal
tumor. |
Table III.
Pathologic and treatment evaluation
of 37 patients with locally advanced gastrointestinal stromal
tumor.
| A, Resvponders |
|---|
|
|---|
| Case no. | Sex | Age, years | Tumor location | Duration of
first-line imatinib therapy, months | PFS of first-line
imatinib therapy, months | Duration of
second-line therapy, months | PFS of second-line
therapy, months | Duration of
third-line therapy, months | PFS of third-line
therapy, months | OS, months | Gene analysis
result | Best overall
response/resection | Survival |
|---|
| 1 | M | 62 | Stomach | 8 | 32 | - | - | - | - | 32 | Deletion in
KIT exon 11 (c.1648_1671del 24; p.K550_W557del) | PR/Yes | No |
| 2 | F | 83 | Stomach | 8 | 15 | - | - | - | - | 33 | Insertion in
KIT exon 11 (p.Y578_D579insPY) | PR/Yes | No |
| 3 | F | 74 | Stomach | 17 | 48 | - | - | - | - | 58 | Deletion in
KIT exon11 (c.1674_1676delGGT; p.K558_V559>N); point
mutation in exon 10 of PDGFRα (c.1432T>C) (p.S478P) | PR/No | Lost follow up |
| 4 | M | 74 | Stomach | 16 | 66 | - | - | - | - | 66 | Deletion in
KIT exon 11 (c.1669_1674del; p.Trp557_Lys558del) | PR/Yes | Yes |
| 5 | M | 48 | Stomach | 41 | 41 | 14 | 14 | 5 | 5 | 70 | Deletion in
KIT exon 11 (c.1669_1674del6; p.W557_K558del); point
mutation in PDGFRα exon 10 | PR/Yes | Yes |
| 6 | M | 67 | Stomach | 14 | 48 | - | - | - | - | 48 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | PR/Yes | Yes |
| 7 | M | 54 | Stomach | 14 | 45 | - | - | - | - | 45 | Insertion in
KIT exon 11 (p.H580_581insYDH) | PR/Yes | Yes |
| 8 | M | 46 | Stomach | 11 | 25 | - | - | - | - | 25 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | PR/Yes | Yes |
| 9 | F | 66 | Stomach | 21 | 22 | - | - | - | - | 22 | Point mutation in
KIT exon 11 (c.1676 T>A; p V559D) | PR/Yes | Yes |
| 10 | M | 78 | Stomach | 15 | 15 | - | - | - | - | 15 | Deletion in
KIT exon 11 (c.1661_1675 del; p.E554_558del) | PR/No | Yes |
| 11 | M | 52 | Omentum | 15 | 141 | 4 | 126 | - | - | 141 | Deletion in
KIT exon 11 (c.1671_1676del GAAGGT; p.W557_V559>C) | PR/Yes | Yes |
| 12 | M | 51 | Small
intestine | 16 | 59 | - | - | - | - | 73 | Deletion in
KIT exon 11 | PR/Yes | Yes |
| 13 | F | 57 | Duodenum | 11 | 41 | - | - | - | - | 41 | Deletion across
KIT intron 10 of exon 11 boundary | PR/Yes | Yes |
| 14 | M | 76 | Small
intestine | 29 | 31 | - | - | - | - | 31 | Insertion in
KIT gene exon 9 (p.S501_A502insAY) | PR/No | Yes |
| 15 | M | 50 | Rectum | 15 | 65 | - | - | - | - | 67 | Point mutation in
KIT exon 11 (c.1727T>C; p.L576P) | PR/Yes | No |
| 16 | M | 53 | Rectum | 20 | 20 | - | - | - | - | 22 | Deletion in
KIT exon 11 (c.1669_1674del TGGAAG; p.W557_K558del) | PR/No | Lost follow up |
| 17 | M | 64 | Rectum | 15 | 15 | 22 | 22 | - | - | 60 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | PR/Yes | Lost follow up |
| 18 | M | 81 | Rectum | 6 | 7 | - | - | - | - | 17 | Deletion in
KIT exon 11 (c.1674_1676delGGT; p.K558_V559>N) | PR/No | No |
| 19 | M | 41 | Rectum | 18 | 27 | 9 | 9 | - | - | 27 | Point mutation in
KIT exon 11 (c.1674 G>T; p.K558N) and (c.1676T>C; V.
559) | PR/No | Yes |
| 20 | F | 73 | Rectum | 8 | 18 | - | - | - | - | 18 | Deletion in
KIT exon 11 (c.1669_1674del; p.W557_K558del) | PR/Yes | Yes |
|
| B,
Non-responders |
|
| Case
no. | Sex | Age,
years | Tumor
location | Duration of
first-line imatinib therapy, months | PFS of
first-line imatinib therapy, months | Duration of
second-line therapy, months | PFS of
second-line therapy, months | Duration of
third-line therapy, months | PFS of
third-line therapy, months | OS,
months | Gene analysis
result | Best overall
response/resection |
Survival |
|
| 21 | F | 60 | Stomach | 13 | 47 | 5 | 6 | 24 | 26 | 118 | Point mutation in
KIT exon 11 (c.1669T>A; p.W557R) | SD/Yes | No |
| 22 | M | 70 | Stomach | 9 | 32 | - | - | - | - | 32 | Point mutation in
KIT exon 11 (c.1679_1680TT>AG; p.V560E) | SD/Yes | Lost follow up |
| 23 | M | 80 | Stomach | 36 | 47 | - | - | - | - | 47 | Duplication
mutation at 12bp in KIT exon11 | SD/Yes | Lost follow up |
| 24 | F | 38 | Stomach | 41 | 70 | 8 | 10 | - | - | 82 | Deletion in exon 11
of KIT gene (delK550_Q556insM) | SD/No | No |
| 25 | F | 72 | Stomach | 11 | 48 | - | - | - | - | 48 | Insertion in
KIT exon 11 (p.K558dup); mutation was not found in
PDGFRα exon 10,12,14,18 | SD/Yes | Yes |
| 26 | M | 65 | Stomach | 19 | 36 | - | - | - | - | 36 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | SD/Yes | Yes |
| 27 | F | 33 | Stomach | 16 | 43 | - | - | - | - | 43 | Deletion in
KIT exon 11 (c.1674_1676 del; p.K558_V559 delinsN) | SD/Yes | Yes |
| 28 | M | 44 | Stomach | 10 | 19 | - | - | - | - | 19 | Point mutation in
PDGFRα exon 10 (c.1436G>A; p.R479Q) | SD/Yes | Yes |
| 29 | M | 87 | Stomach | 3 | 11 | - | - | - | - | 11 | Deletion in
KIT exon 11 (c.1735_1737 del; p.D579del) | SD/Yes | Lost follow up |
| 30 | M | 72 | Stomach | 9 | 9 | 4 | 4 | - | - | 14 | Deletion in
KIT exon 11 (c.1671_1676del GAAGGT; p.W557_V559>C) | PD/No | Lost follow up |
| 31 | F | 35 | Stomach | 4 | 4 | 5 | 5 | - | - | 10 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | PD/No | Lost follow up |
| 32 | F | 70 | Pancreas | 11 | 83 | - | - | - | - | 83 | Insertion in
KIT exon 11 of KIT gene (p.K558>NP) | SD/Yes | Yes |
| 33 | F | 41 | Duodenum | 29 | 29 | - | - | - | - | 29 | Mutation was not
found in KIT exon 9,11,13,17,18; mutation was not found in
PDGFRα exon 10,12,14,18 | SD/No | Yes |
| 34 | M | 67 | Duodenum | 36 | 36 | - | - | - | - | 36 | Insertion in
KIT exon 11 (p.P573_T574insDP) | SD/No | Yes |
| 35 | M | 74 | Mesocolon | 56 | 131 | 52 | 52 | - | - | 183 | Point mutation in
exon 10 of PDGFRα gene (c.1432T>C; p.S478P) | SD/No | No |
| 36 | M | 67 | Pelvic area | 21 | 41 | 12 | 12 | - | - | 42 | Deletion in
KIT exon 11(delK558_V559insN) | SD/No | Lost follow up |
| 37 | M | 58 | Rectum | 29 | 77 | 17 | 48 | - | - | 77 | Insertion in
KIT exon 9 (p.S501_A502insAT) | SD/Yes | Yes |
 | Table IV.Adverse events of first-line therapy
in different gene mutations in 37 patients with locally advanced
gastrointestinal stromal tumor. |
Table IV.
Adverse events of first-line therapy
in different gene mutations in 37 patients with locally advanced
gastrointestinal stromal tumor.
| Adverse events | KIT exon 9
insertion (n=2), n (%) | KIT exon 11
insertion (n=5), n (%) | KIT exon 11
deletion (n=14), n (%) | KIT exon 11
point mutation (n=5), n (%) | KIT exon 11
duplication (n=1), n (%) | PDGFRα exon
10 point mutation (n=2), n (%) | KIT exon 11
deletion; PDGFRα exon 10 point mutation (n=2), n (%) | No mutation in both
KIT and PDGFRα (n=6), n (%) |
|---|
| All grade |
|
|
|
|
|
|
|
|
|
Nausea | - | 2 (40.0) | 4 (28.6) | 1 (20.0) | - | - | - | 1 (16.7) |
|
Vomiting | - | - | 2 (14.3) | 1 (20.0) | - | - | - | 1 (16.7) |
|
Gastritis | - | - | 1 (7.1) | 1 (20.0) | - | - | - | - |
|
Edema | 1 (50.0) | 1 (20.0) | 2 (14.3) | 2 (40.0) | - | - | - | - |
| Eyelid
edema | 1 (50.0) | 2 (40.0) | 1 (7.1) | 1 (20.0) | - | 1 (50.0) | - | 2 (33.3) |
| General
malaise | - | 1 (20.0) | - | - | - | - | - | - |
| Skin
rash | - | 2 (40.0) | 1 (7.1) | 1 (20.0) | - | - | - | - |
|
Diarrhea | - | - | - | 1 (20.0) | - | 1 (50.0) | - | - |
|
Fatigue | - | - | 1 (7.1) | 1 (20.0) | - | - | - | - |
| Renal
function impairment | - | - | - | - | - | - | - | 1 (16.7) |
| Liver
function impairment | - | - | 1 (7.1) | - | - | - | - | - |
|
Hand-foot skin reaction | 1 (50.0) | - | 1 (7.1) | - | - | - | - | - |
|
Anemia | - | - | 3 (21.4) | - | - | 1 (50.0) | - | 1 (16.7) |
|
Leukopenia | - | - | 1 (7.1) | - | - | - | - | 1 (16.7) |
| P-value | P=0.967 |
|
|
|
|
|
|
|
| Grade ≥3 |
|
|
|
|
|
|
|
|
|
Anemia | - | - | 2 (14.3) | - | - | - | - | 1 (16.7) |
Discussion
Curative resection is generally recommended for
patients with GISTs, but such resection is not considered for
patients with LAGISTs due to factors such as tumor location, size
and increased risk of tumor rupture or metastasis (2–5). The
present study reported on the experience of treating 37 patients
with LAGIST at a single institution and the outcomes of first-line
therapy in relation to various gene mutations. Although a higher
proportion of men (67.6%) participated in the present study
compared with other studies (55.0–66.7%), the median age (65 years)
of the participants was consistent with that of those in other
studies (57.4–63 years) (2,8,12).
The stomach was the most commonly affected region in
the present study, consistent with findings reported in at least
one other study (6). Most patients
exhibited favorable clinical responses to first-line imatinib
therapy; 20 (54.1%) experienced PR, 15 (40.5%) maintained SD and 2
(5.4%) exhibited disease progression. The data were consistent with
the findings of other studies that demonstrated a 45–92% response
rate (17–24), although studies have indicated a
marked increase in primary resistance to imatinib, particularly in
GISTs with PDGFRα mutations and those with mutations in exon
9 of the KIT gene (9,25).
However, the mutation status of the KIT and PDGFRα
genes was not helpful in predicting treatment response (P=0.602) or
surgical resectability (P=0.952). Due to the relatively small
patient number for each genetic mutation, it would not have been
suitable from the perspective of statistics to create Kaplan-Meier
plots or waterfall plots by grouping the patients by gene. The most
common AEs of all grades were nausea, vomiting, gastritis, edema,
eye lid edema, general malaise, skin rash, diarrhea, fatigue, renal
function impairment, liver function impairment, hand-foot skin
reaction, anemia and leukopenia and that of grade ≥3 was anemia
(Table IV). No significant
differences were observed in the AEs experienced during first-line
therapy by patients whose tumors exhibited any mutations;
comparisons were made among KIT exon 9 insertion, KIT
exon 11 insertion, KIT exon 11 deletion, KIT exon 11
point mutation, KIT exon 11 duplication, PDGFRα exon
10 point mutation, KIT exon 11 deletion, PDGFRα exon
10 point mutation and no mutation in both KIT and
PDGFRα (Table IV). The AEs
noted in the present study were generally mild, well-tolerated and
manageable with no marked hematologic toxicity at standard dosages
in patients treated with first-line imatinib therapy in another
multicenter cohort study (26). In
the present study, 24 (64.9%) patients with initially unresectable
LAGISTs underwent primary tumor resection. In addition, 21 patients
who received treatment with first-line imatinib and 3 who achieved
PR following treatment with first-line imatinib but then SD
switched to second-line sunitinib, which achieved a superior
response. Second-line sunitinib treatment was effective for these
patients with LAGISTs, increasing the likelihood of achieving
complete resection and minimizing the risk of tumor spillage. The
present study demonstrated that omental LAGIST had the best
response rate (100%) when compared with rectal LAGIST (85.7%),
small intestinal LAGIST (60%) and gastric LAGIST (47.6%),
indicating omental and rectal LAGIST show a better response to
imatinib compared with other organs. In addition, the responders
group had a higher resection rate (70%) compared with the
non-responders group (58.8%).
Overall, the median PFS was 36 months, longer than
the 18–20 months reported in a previous study (20). Additionally, at the conclusion of
follow-up, 21 patients (56.8%) were still alive. The different
response rate and survival outcomes may arise from the different
definitions of LAGIST, different gene mutation patterns, different
tumor sites, different ethnicity and different treatment doses. For
instance, a previous study enrolled 746 patients with a median PFS
of 18 months in the standard-dose arm and 20 months for those
receiving high-dose imatinib (20).
Although the ideal length of imatinib therapy
remains debatable, imatinib should be administered in clinical
settings until a maximal response is achieved. According to the
National Comprehensive Cancer Network guidelines, achieving a
maximal response may require treatment for >6 months (27). A previous study that evaluated
treatment responses discovered that a maximal response is typically
achieved after ~12 months of treatment (26). In the 24 patients in the present
study who subsequently underwent resection, the median time to
primary tumor resection was 16 months. Determining the ideal
duration of first-line treatment should involve regular response
assessments to determine the optimal timing for surgical
intervention (22,26–28).
Combined CT-measured tumor density and RECIST evaluations may aid
in determining an appropriate timing for LAGIST surgical resection
(3).
Clinical research suggests the benefits of imatinib
therapy in patients with GISTs. If it is technically resectable,
neoadjuvant therapy can preserve organ function, avoid tumor
rupture, reduce postoperative complications and increase the R0
resection rate to up to 91% compared with 85% (29). For unresectable GISTs, the response
rate of first-line imatinib therapy is 45–69% (30). A previous study revealed that 15.7%
of patients with initially unresectable GIST became resectable
under first-line imatinib therapy (31). In the present study, the patients
were all initially unresectable and imatinib was used as the
first-line therapy instead of neoadjuvant therapy.
A higher occurrence of primary imatinib resistance
was observed in GISTs with KIT exon 9 mutations, GISTs with
PDGFRα D842V mutations and GISTs with wild-type KIT
and PDGFRα. Hence, evaluation is needed of the potential
differences in treatment responses based on different mutation
types in a prospective, multicenter clinical trial. The present
study has certain limitations. First, a retrospective design was
employed with a non-randomized controlled trial and a relatively
small sample size from a single institution for the mutation
association analysis. Therefore, the findings require verification
in a prospective, multicenter clinical trial required to associate
gene mutations with other TKIs. Second, the present study lacked
comprehensive information on the GIST-associated gene mutation
status of the patients, which may influence the risk of recurrence
and survival outcomes for GISTs. Third, the relatively brief
follow-up duration may have resulted in an underestimation of the
effects of first-line imatinib therapy on PFS and OS. A total of 1
patient progressed fast (first-line failure, 4 months; second-line,
5 months) and was lost to follow-up after 10 months (case no. 31),
and at the time of the study's conclusion, 5 patients are still
under treatment, 21 patients are still alive and 9 patients have a
short follow-up time of <24 months. In spite of the limitations
of the present study, it is evident that first-line imatinib
therapy is effective and safe in reducing tumor size in patients
with LAGISTs, yielding comparable rates of complete resection.
In conclusion, the gene mutation status was
demonstrated to have limited value as an indicator for assessing
treatment response and surgical resectability. Additionally, no
significant variations were observed in complication rates.
Although most patients clinically benefited from first-line
imatinib therapy with manageable side effects, future studies with
long-term follow-ups are required to verify the results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the National
Science and Technology Council of Taiwan (grant nos. MOST
111-2314-B-037-070-MY3, NSTC 112-2314-B-037-050-MY3, NSTC
113-2321-B-037-006 and NSTC 113-2314-B-037-057) and Taiwan's
Ministry of Health and Welfare (grant no.
MOHW113-TDU-B-222-134014). Additional funding was provided from
Taiwan's National Health and Welfare Surcharge on Tobacco Products,
by Kaohsiung Medical University Hospital (grant nos. KMUH111-2M29,
KMUH112-2R37, KMUH112-2R38, KMUH112-2R39, KMUH112-2M27,
KMUH112-2M28, KMUH112-2M29, KMUH-S11303 and KMUH-SH11309) and by a
Kaohsiung Medical University Research Center Grant (grant no.
KMU-TC113A04). The present study was also supported by a grant from
the Taiwan Precision Medicine Initiative and by the Taiwan Biobank
of Academia Sinica.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WCS and JYW were involved in the conception and
design of this study. WCS wrote the manuscript. WCS, CWH, HLT, YCC
and TKC performed data acquisition. WCS, PJC, YSY and TCY
contributed to the analysis and interpretation of data. JYW
reviewed and edited the manuscript and supervised the study. WCS
and JYW confirmed the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study fully complied with the
Declaration of Helsinki and was approved by the Institutional
Review Board of Kaohsiung Medical University Hospital [approval no.
KMUHIRB-E(I)-20240084]. According to regulations at our
institution, patient consent was waived for the present study due
to its retrospective nature.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirota S: Differential diagnosis of
gastrointestinal stromal tumor by histopathology and
immunohistochemistry. Transl Gastroenterol Hepatol. 3:272018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coffey RJ, Washington MK, Corless CL and
Heinrich MC: Ménétrier disease and gastrointestinal stromal tumors:
Hyperproliferative disorders of the stomach. J Clin Invest.
117:70–80. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su WC, Tsai HL, Yeh YS, Huang CW, Ma CJ,
Chen CY, Chang TK and Wang JY: Combined computed
tomography-measured tumor density and RECIST for evaluating
neoadjuvant therapy in locally advanced gastrointestinal stromal
tumors. Transl Cancer Res. 7:634–644. 2018. View Article : Google Scholar
|
|
4
|
Rodrigues J, Campanati RG, Nolasco F,
Bernardes AM, Sanches SRA and Savassi-Rocha PR: Pre-operative
gastric gist downsizing: The importance of neoadjuvant therapy. Arq
Bras Cir Dig. 32:e14272019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Mehren M and Joensuu H:
Gastrointestinal stromal tumors. J Clin Oncol. 36:136–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Zhang Z, Sun J, Li J, Zeng Z, Ma M,
Ye X, Feng F and Kang W: Comparison of prognosis between
neoadjuvant imatinib and upfront surgery for GIST: A systematic
review and meta-analysis. Front Pharmacol. 13:9664862022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casali PG, Jost L, Reichardt P, Schlemmer
M and Blay JY: Gastrointestinal stromal tumours: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl):S64–S67. 2009. View Article : Google Scholar
|
|
8
|
Wang D, Zhang Q, Blanke CD, Demetri GD,
Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M
and Eisenberg BL: Phase II trial of neoadjuvant/adjuvant imatinib
mesylate for advanced primary and metastatic/recurrent operable
gastrointestinal stromal tumors: Long-term follow-up results of
radiation therapy oncology group 0132. Ann Surg Oncol.
19:1074–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joensuu H: Gastrointestinal stromal tumor
(GIST). Ann Oncol. 17 (Suppl 10):x280–x286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel SR and Reichardt P: An updated
review of the treatment landscape for advanced gastrointestinal
stromal tumors. Cancer. 127:2187–2195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer S, George S, von Mehren M and
Heinrich MC: Early and next-generation KIT/PDGFRA kinase inhibitors
and the future of treatment for advanced gastrointestinal stromal
tumor. Front Oncol. 11:6725002021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding P, Wu J, Wu H, Sun C, Guo H, Lowe S,
Yang P, Tian Y, Liu Y, Meng L and Zhao Q: Inflammation and
nutritional status indicators as prognostic indicators for patients
with locally advanced gastrointestinal stromal tumors treated with
neoadjuvant imatinib. BMC Gastroenterol. 23:232023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Yin Y, Shen C, Yin X, Cai Z, Pu L,
Fu W, Wang Y and Zhang B: Preoperative imatinib treatment in
patients with locally advanced and metastatic/recurrent
gastrointestinal stromal tumors: A single-center analysis. Medicine
(Baltimore). 99:e192752020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vassos N, Jakob J, Kähler G, Reichardt P,
Marx A, Dimitrakopoulou-Strauss A, Rathmann N, Wardelmann E and
Hohenberger P: Preservation of organ function in locally advanced
non-metastatic gastrointestinal stromal tumors (GIST) of the
stomach by neoadjuvant imatinib therapy. Cancers (Basel).
13:5862021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai HL, Shi HY, Chen YC, Huang CW, Su WC,
Chang TK, Li CC, Chen PJ, Yeh YS, Yin TC and Wang JY: Clinical and
cost-effectiveness analysis of mFOLOFX6 with or without a targeted
drug among patients with metastatic colorectal cancer: Inverse
probability of treatment weighting. Am J Cancer Res. 13:4039–4056.
2023.PubMed/NCBI
|
|
17
|
Hsiao HH, Liu YC, Tsai HJ, Chen LT, Lee
CP, Chuan CH, Wang JY, Yang SF, Tseng YT and Lin SF: Imatinib
mesylate therapy in advanced gastrointestinal stromal tumors:
Experience from a single institute. Kaohsiung J Med Sci.
22:599–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Oosterom AT, Judson I, Verweij J,
Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A,
Sciot R, Van Glabbeke M, et al: Safety and efficacy of imatinib
(STI571) in metastatic gastrointestinal stromal tumours: A phase I
study. Lancet. 358:1421–1423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blanke CD, Rankin C, Demetri GD, Ryan CW,
von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki
RG, et al: Phase III randomized, intergroup trial assessing
imatinib mesylate at two dose levels in patients with unresectable
or metastatic gastrointestinal stromal tumors expressing the kit
receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seshadri RA and Rajendranath R:
Neoadjuvant imatinib in locally advanced gastrointestinal stromal
tumors. J Cancer Res Ther. 5:267–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Burg SJC, van de Wal D, Roets E,
Steeghs N, van Sandick JW, Kerst M, van Coevorden F, Hartemink KJ,
Veenhof XAAFA, Koenen AM, et al: Neoadjuvant imatinib in locally
advanced gastrointestinal stromal tumors (GISTs) is effective and
safe: Results from a prospective single-center study with 108
Patients. Ann Surg Oncol. 30:8660–8668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lam TJR, Udonwa SA, Masuda Y, Yeo MHX,
Farid Bin Harunal Ras M and Goh BKP: A systematic review and
meta-analysis of neoadjuvant imatinib use in locally advanced and
metastatic gastrointestinal stromal tumors. World J Surg.
48:1681–1691. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khosroyani HM, Klug LR and Heinrich MC:
TKI treatment sequencing in advanced gastrointestinal stromal
tumors. Drugs. 83:55–73. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH, Kim Y, Choi JW and Kim YS:
Correlation of imatinib resistance with the mutational status of
KIT and PDGFRA genes in gastrointestinal stromal tumors: A
meta-analysis. J Gastrointestin Liver Dis. 22:413–418.
2013.PubMed/NCBI
|
|
26
|
Li W, Li X, Yu K, Xiao B, Peng J, Zhang R,
Zhang L, Wang K, Pan Z, Li C and Wu X: Efficacy and safety of
neoadjuvant imatinib therapy for patients with locally advanced
rectal gastrointestinal stromal tumors: A multi-center cohort
study. Front Pharmacol. 13:9501012022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane
JM, et al: Soft tissue sarcoma, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:536–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang SY, Wu CE, Lai CC, Chen JS, Tsai CY,
Cheng CT, Yeh TS and Yeh CN: Prospective evaluation of neoadjuvant
imatinib use in locally advanced gastrointestinal stromal tumors:
Emphasis on the optimal duration of neoadjuvant imatinib use,
safety, and oncological outcome. Cancers (Basel). 11:4242019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iwatsuki M, Harada K, Iwagami S, Eto K,
Ishimoto T, Baba Y, Yoshida N, Ajani JA and Baba H: Neoadjuvant and
adjuvant therapy for gastrointestinal stromal tumors. Ann
Gastroenterol Surg. 3:43–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohammadi M, NS IJ, Hollander DD, Bleckman
RF, Oosten AW, Desar IME, Reyners AKL, Steeghs N and Gelderblom H:
Improved efficacy of first-line imatinib in advanced
gastrointestinal stromal tumors (GIST): The Dutch gist registry
data. Target Oncol. 18:415–423. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medrano Guzman R, Lopez Lara X, Arias
Rivera AS, Garcia Rios LE and Brener Chaoul M: Neoadjuvant imatinib
in gastrointestinal stromal tumors (GIST): The first analysis of a
Mexican population. Cureus. 16:e650012024.PubMed/NCBI
|