Introduction
Primary breast mucinous cystadenocarcinoma (MCA) is
a rare and newly recognized entity according to the 5th edition of
the World Health Organization classification of breast tumors
(1). Since it was first reported by
Koenig and Tavassoli in 1998 (2),
only ~40 cases of primary breast MCA have been reported in the
English language according to the PubMed database (https://pubmed.ncbi.nlm.nih.gov/). Most of these
cases are case reports. Primary breast MCA, similar to
pancreatic-biliary or ovarian variants, features cystic structures
lined with tall columnar cells rich in intracytoplasmic mucin
(1). Before the primary MCA of the
breast is diagnosed, ovarian and pancreatic sources should be
excluded. The entity is typically triple-negative breast cancer
(TNBC), which is characterized by negative expression of HER2,
progesterone receptor (PR) and estrogen receptor (ER) (1), but rare HER2-positive cases have been
reported. To the best of our knowledge, only five cases of
HER2-positive MCA have been reported in the English language
(3–7). Due to the rarity of the disease, the
clinical characteristics, molecular pathogenesis and prognosis of
HER2-positive MCA are poorly understood, and the standard treatment
regimen remains controversial.
In the present study, a HER2-positive primary breast
MCA case is reported. Although the patient did not receive
anti-HER2 targeted therapy after surgery, there was still no
recurrence or distant metastasis after 104 months of follow-up.
This unusual tumor was genetically profiled to provide an improved
understanding of its molecular characteristics.
Case report
A 64-year-old woman was admitted to The Second
Affiliated Hospital of Guangzhou University of Chinese Medicine
(Guangzhou, China) on March 7, 2016, complaining of a left breast
mass 1 week prior. Mammogram revealed a high-density mass measuring
~22×20 mm in the left breast, which had an irregular shape with
some marginal burrs and no malignant calcification (Fig. S1). The mass was classified as
Breast Imaging Reporting and Data System (BI-RADS) 5th Edition
(2013) category 4C (8). The patient
subsequently underwent left breast-conserving surgery and sentinel
lymph node biopsy on March 11, 2016.
On gross examination, the tumor measured 2.2×1.8×1.7
cm and had well-defined boundaries. The cut surface appeared
gray-white and gelatinous. The samples were sent for standard
pathological analysis. The tissues were prepared as
paraffin-embedded tissue blocks after being fixed in 10% neutral
formalin fixative for 24 h at 25°C. Sections that were 4-µm thick
were produced for hematoxylin-eosin, Alcian blue and periodic
acid-Schiff (AB-PAS) and immunohistochemical staining, as well as
HER2 fluorescence in situ hybridization (FISH). The sections
were deparaffinized with xylene and rehydrated with anhydrous
ethanol, 95% ethanol, 80% ethanol, 70% ethanol and distilled water.
Subsequently, the sections were stained with hematoxylin for 10 min
and eosin for 4 min, both at room temperature. AB-PAS staining was
carried out using the AB-PAS Stain Kit (cat. no. G1285; Beijing
Solarbio Science and Technology Co., Ltd.), following the
manufacturer's instructions. All immunohistochemical staining was
performed using the antibodies listed in Table SI and a Ventana BenchMark ULTRA
immunostainer (Roche Tissue Diagnostics) according to the
manufacturer's protocols. Chromogenic detection was performed using
the OptiView DAB IHC Detection Kit (cat. no. 760-700; Roche Tissue
Diagnostics), which contains biotinylated secondary antibody,
streptavidin-HRP conjugate and DAB substrate. Histopathological
features (H&E staining), immunohistochemical profiles and
AB-PAS staining results were analyzed using an Olympus BX46
brightfield microscope. All images of H&E staining,
immunohistochemical staining and AB-PAS staining were captured
using an Olympus DP27 digital camera (Evident Corporation) mounted
on either a BX46 microscope or a NanoZoomer 2.0 HT (Hamamatsu
Photonics K.K.). The HER2 FISH assay was performed using a HER2
gene detection kit (cat. no. F.01359; Guangzhou LBP Medicine
Science & Technology Co., Ltd.) according to the manufacturer's
protocols. HER2 FISH photomicrographs were collected using a
fluorescence microscope (BX51; Olympus Corporation) and a ProgRes
MF cool CCD (Jenoptik).
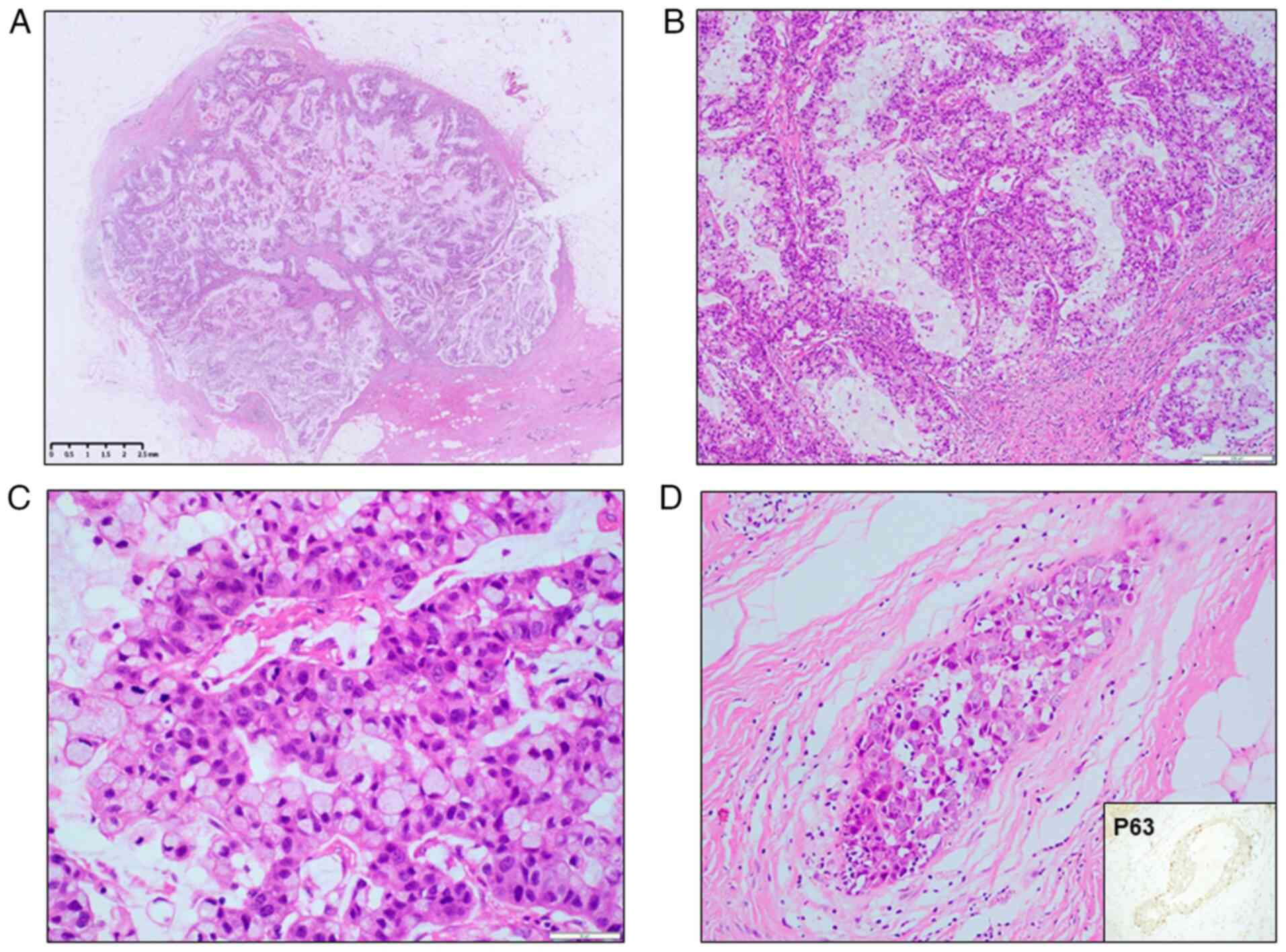
Microscopic examination revealed a cystic solid mass
within the breast tissue, which had relatively clear boundaries but
no capsule (Fig. 1A). The cystic
spaces were lined with tall columnar tumor cells arranged in a
complex pattern, including multilayered, papillary, small-cluster
and scattered configurations (Figs.
1B and S2). The tumor cells
exhibited moderate-to-severe nuclear atypia (grades 2–3; Fig. 1C) according to the Nottingham
grading system (1). A small focus
of high-grade ductal carcinoma in situ (DCIS) was observed
adjacent to the tumor, and the diagnosis was further confirmed by
the presence of intact myoepithelial cells demonstrated through
immunohistochemical staining for p63 (Fig. 1D). Immunohistochemistry revealed
that the tumor cells were positive for CK7, E-cadherin, GATA-3,
mammaglobin, mucin (MUC)1, MUC2 and MUC5AC (Figs. 2A, 2B and S3A-E). p53 protein showed strong diffuse
nuclear immunoreactivity in ~90% of the tumor cells (Fig. S3F). The Ki-67 labeling index was
~50% (Fig. S3G). ER, PR, GCDFP15,
CK5/6, EGFR, CK20, CDX2, SATB2, paired box 8 (PAX8), WT1 and
transcription termination factor 1 expression was negative
(Figs. 2C and S4). Calponin, smooth muscle myosin heavy
chain and p63 staining revealed that myoepithelial cells were
absent both at the periphery and in the inner part of the tumor
(Fig. S5). HER2
immunohistochemical staining was positive (score 3+; Fig. 2D) and HER2 FISH revealed a clustered
amplification pattern (Fig. 2E).
AB-PAS staining confirmed that the tumor had abundant, blue-stained
acidic intracellular and extracellular mucin (Fig. 2F). The surgical margins were
negative and the sentinel lymph node biopsy results were negative
(0/9).
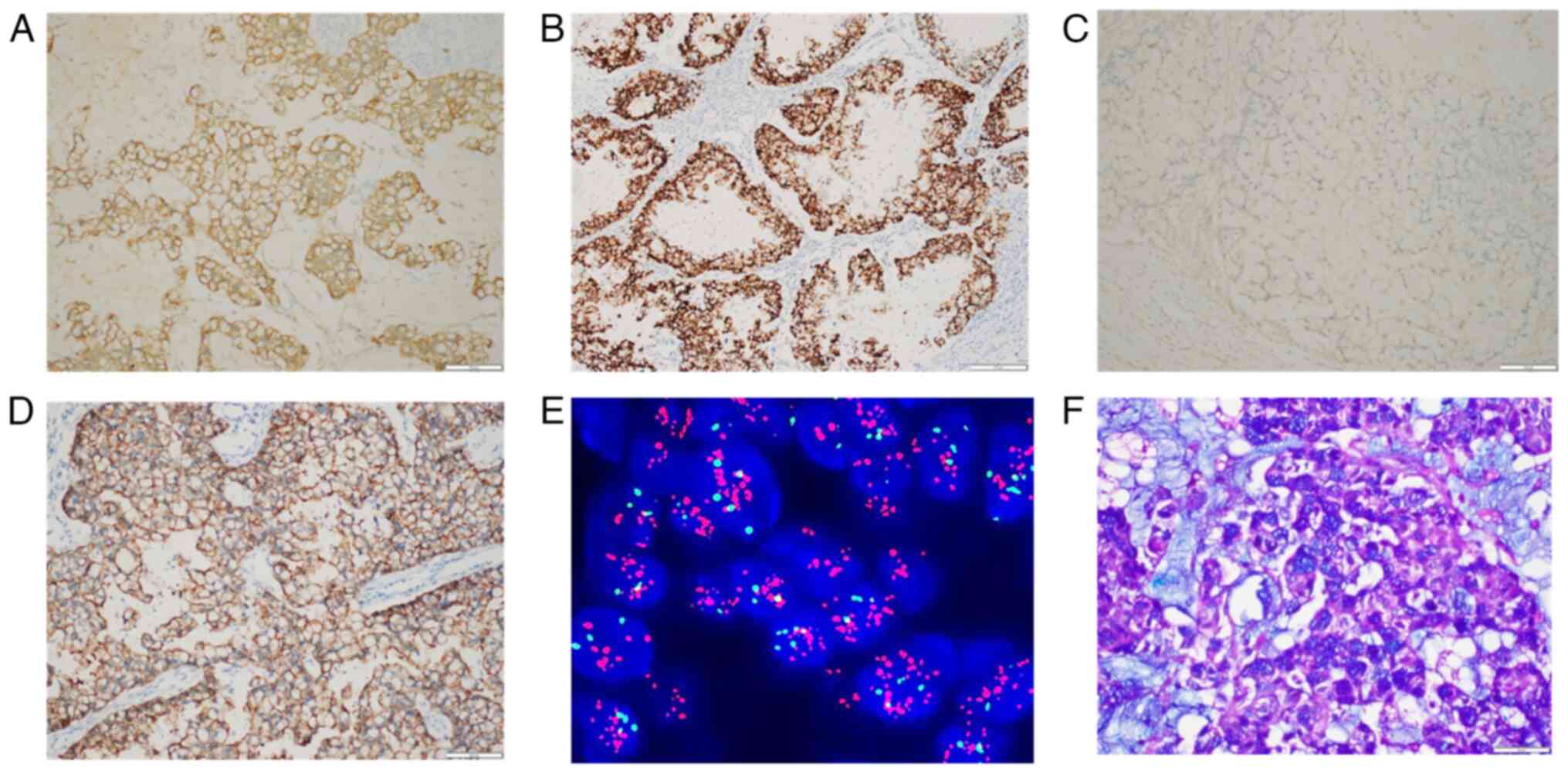 | Figure 2.IHC, FISH and AB-PAS staining images.
(A) Positive staining for CK7 (IHC magnification, ×200). (B)
Positive staining for mammaglobin (IHC magnification, ×200). (C)
Negative staining for CK20 (IHC magnification, ×200). (D) Positive
staining for HER2, score 3+ (IHC magnification, ×200). (E) FISH
showed clustered amplification of the HER2 gene (magnification,
×1,000). (F) AB-PAS staining showed blue-stained intracellular and
cystic mucin (magnification, ×400). IHC, immunohistochemical; FISH,
fluorescence in situ hybridization; AB-PAS, Alcian blue and
periodic acid-Schiff. |
To exclude the possibility of metastatic spread of
MCA from other organs, which are more commonly associated with MCA
occurrence, to the breast, a comprehensive physical examination and
a series of imaging investigations were conducted. The pelvic
ultrasound examination showed no abnormalities in both ovaries, the
abdominal ultrasound did not detect any abnormal changes in the
pancreatic region and chest radiography demonstrated normal
pulmonary findings. In the present case, positive
immunohistochemical staining for GATA-3 and mammaglobin suggested a
primary breast origin, whereas positive CK7 and negative PAX8, CK20
and CDX2 helped to exclude the possibility of the MCA originating
from the ovaries, pancreas and gastrointestinal tract. In addition,
the presence of a small focus of DCIS around the tumor further
suggested a primary breast origin.
Primary MCA of the breast also needs to be
distinguished from other primary breast tumors with mucin
secretion, including mucinous carcinoma of the breast and
encapsulated papillary carcinoma. Primary MCA of the breast
typically displays multilocular cystic architecture lined by
columnar epithelial cells containing abundant intracellular mucin
and often shows ER/PR negativity. By contrast, mucinous carcinoma
features tumor cell clusters suspended in extracellular mucin
pools, demonstrating low-to-moderate nuclear atypia and is strongly
associated with ER/PR positivity and HER2 negativity (1). Encapsulated papillary carcinoma
presents as a well-demarcated lesion with papillary proliferations
surrounded by a fibrous capsule, showing minimal mucin production
and typically expresses ER/PR positivity but lacks HER2
amplification (1). Integrated
evaluation of clinical, radiological and histopathological features
revealed the following diagnostic findings: i) A patient-reported
palpable mass in the left breast; ii) mammographic identification
of a high-density lesion (BI-RADS 4C) with no metastatic evidence
on other imaging tests; iii) histological confirmation of a 2.2-cm
cystic tumor lined by tall columnar cells with abundant
intracytoplasmic mucin, accompanied by adjacent DCIS and negative
sentinel lymph node biopsy (0/9); and iv) immunohistochemical
profiling demonstrating GATA-3 and mammaglobin co-expression with
concurrent negativity for ER, PR, CK20, CDX2 and PAX8. These
cumulative findings conclusively established the diagnosis of
primary breast MCA as pT2pN0cM0 on March 19, 2016, based on the
American Joint Committee on Cancer Staging Manual (7th edition)
(9).
After surgery, considering that the patient had no
lymph node metastasis or distant metastasis and the TNM stage was
pT2pN0cM0, the patient received chemotherapy and radiotherapy
according to the National Comprehensive Cancer Network guidelines
for breast cancer version 2.2015 (10). Chemotherapy was initiated on March
24, 2016 using an EC-T regimen: Four cycles of epirubicin (60
mg/m2 intravenous on day 1) combined with
cyclophosphamide (600 mg/m2 intravenous on day 1)
administered every 21 days, followed by four cycles of docetaxel
(100 mg/m2 intravenous on day 1) every 21 days.
Radiotherapy commenced on August 31, 2016 with breast-conserving
intensity-modulated radiotherapy, delivering 29 fractions
comprising 52.2 Gy to clinical target volume (CTV) 2 (ipsilateral
breast) and 63.8 Gy to CTV1 (tumor bed and 1 cm surrounding
tissue). No chemotherapy- or radiotherapy-related adverse events
were documented. Although anti-HER2 targeted therapy was
recommended as part of the treatment plan, the patient declined
this intervention due to economic limitations.
To further investigate the genetic profile of
HER2-amplified breast MCA, additional next-generation sequencing
(NGS) analysis was performed on May 23, 2022. Genetic profiling was
conducted on formalin-fixed paraffin-embedded (FFPE) tumor samples
using the Solid Tumor Multi-gene Combined Detection Kit (cat. no.
MFG030041; BGI group), which comprehensively analyzes a panel of
688 cancer-associated genes (Table
SII). DNA was extracted from FFPE tissues with a nucleic acid
extraction kit (cat. no. MFG010019; BGI Group). DNA concentration
was measured by a Qubit fluorometer (Invitrogen; Thermo Fisher
Scientific, Inc.) in conjunction with the Qubit dsDNA HS (High
Sensitivity) Assay Kit (cat. no. Q32854; Invitrogen; Thermo Fisher
Scientific, Inc.). Library construction was performed following the
protocols recommended in the aforementioned Solid Tumor Multi-gene
Combined Detection Kit. Final library DNA quantification was
measured by Qubit fluorometer in conjunction with the Qubit dsDNA
HS (High Sensitivity) Assay Kit. The DNA concentration must exceed
16 ng/µl, with a total yield >320 ng. The sequencing reactions
were performed in accordance with the instructions of the General
Sequencing Kit (cat. no. 940-001802-00; Shenzhen MGI Technology
Co., Ltd.) and the gene sequencer (MGISEQ-2000) manufactured by BGI
Group. The sequencing type was 100 bp for length and paired end for
the direction of sequencing. Data analysis was conducted using
Solid Tumor Multi-Gene Detection and Analysis Software
(oseq_T-v1.4.5.0; BGI Group). Table
I summarizes the identified missense mutations (TP53, LATS1 and
NFE2L2) and copy number gains (ERBB2, CDK12, GRB7, RARA, AXIN2,
PRKAR1A, SOX9, ZNF217 and GNAS). The microsatellite status of the
tumor was stable, and no clinically significant germline mutations
were detected.
 | Table I.Somatic variants detected by
next-generation seque-ncing. |
Table I.
Somatic variants detected by
next-generation seque-ncing.
| Gene | Result | Allele frequency or
copy Number |
|---|
| ERBB2 | Copy number gain | 15.14 |
| TP53 | p.F134I | 51.86% |
|
| c.400T>A |
|
| LATS1 | p.H820Y |
|
|
| c.2458C>T | 34.48% |
| NFE2L2 | p.V319A | 17.53% |
|
| c.956T>C |
|
| CDK12 | Copy number gain | 12.60 |
| GRB7 | Copy number gain | 16.01 |
| RARA | Copy number gain | 12.09 |
| AXIN2 | Copy number gain | 9.68 |
| PRKAR1A | Copy number gain | 18.74 |
| SOX9 | Copy number gain | 10.31 |
| ZNF217 | Copy number gain | 9.13 |
| GNAS | Copy number gain | 6.42 |
After the completion of treatment, the patient
underwent 6-monthly assessments for suspicious symptoms, combined
with ultrasonographic examinations of the breasts and axillary
lymph nodes, cervical and supraclavicular lymph nodes, abdominal
region and gynecological system, along with annual chest CT
surveillance. The most recent breast ultrasound and chest CT
(November 22, 2024) show no discernible abnormalities (Fig. S6). The patient achieved a
disease-free survival of >104 months (from March 2016 resection
to November 2024 follow-up), with no evidence of recurrence or
metastasis throughout this period. All critical time points
throughout the clinical course of the patient have been
systematically summarized in Table
SIII.
Discussion
Primary MCA of the breast is rare, with only ~40
cases documented in the English language according to the PubMed
database (https://pubmed.ncbi.nlm.nih.gov/). Notably,
HER2-positive MCAs are considerably rarer and, to the best of our
knowledge, only five individual case reports have been reported
prior to the present case (3–7).
Table II presents the
clinicopathological characteristics of the present patient and the
previously documented patients with HER2-positive breast MCA. All 6
patients with HER2-positive MCA were female, and their ages ranged
from 55–73 years. The tumor size varied from 2 to 18 cm. In total,
4 patients (3 of whom had confirmed HER2 FISH amplification) were
reported to have a HER2 IHC 3+ score, and the other 2 cases had a
HER2 IHC 2+ score with HER2 FISH amplification. All published cases
of HER2-positive MCA were ER- and PR-negative. All patients
underwent surgical treatment: 2 underwent partial mastectomy and
the others underwent radical mastectomy. In total, 5 patients
underwent lymph node evaluation, of whom 2 had lymph node
metastases. Notably, among the 2 patients with lymph node
metastases, 1 case showed discordant histology: The primary tumor
was mixed MCA and invasive lobular carcinoma, whereas the
metastasis was exclusively lobular carcinoma. Some patients
received chemotherapy and radiotherapy: 3 patients received
postoperative radiotherapy and chemotherapy and 1 patient received
only chemotherapy. Notably, although these cases were
HER2-positive, only 2 cases eventually received anti-HER2 targeted
therapy. The present case did not receive anti-HER2 targeted
therapy for economic reasons. The follow-up time ranged from 10 to
104 months, and the present case had the longest follow-up of 104
months. The follow-up data demonstrated that no recurrence or
distant metastasis occurred in any of the 6 patients, regardless of
whether they received anti-HER2 targeted therapy. Data from these
limited cases suggest that HER2 positivity does not appear to alter
the favorable prognosis of MCA (on the basis of the current
understanding of triple-negative MCA). Given the limited number of
cases, the potential benefit of anti-HER2 targeted therapy in this
patient population requires validation through larger prospective
studies.
 | Table II.Summary of the clinical features of
previously reported HER2-positive cases and the present case. |
Table II.
Summary of the clinical features of
previously reported HER2-positive cases and the present case.
| First author,
year | Age, years | Size, cm | Treatment | LNM |
Immunohistochemistry | HER2 FISH | Follow-up data | (Refs.) |
|---|
| Petersson et
al, 2010 | 73 | 4.5 | Mastectomy and
LND | - | +ve: CK7 and HER2
(3+) -ve: ER, PR, CK20 and CDX2 | A | NA | (3) |
| Kucukzeybek et
al, 2014 | 55 | 2 | Partial mastectomy,
LND, chemo trastuzumab and rad | - | +ve: HER2 (2+) CK7
and Ki-67 (30%) -ve: ER, PR and CK20 | A | ANED, 10 months | (4) |
| Seong et al,
2016 | 59 | 2 | Partial
mastectomy | NA | +ve: HER2 (3+) -ve:
ER and PR | NA | NA | (5) |
| Kaur et al,
2022 | 65 | 18 | Mastectomy, LND and
chemo | + | +ve: HER2 (3+), CK7,
GATA3, mamaglobin, MUC1, CK20 (focal) and Ki-67 (90%) -ve: ER, PR,
CDX2, SATB2, TTF1, PAX8, WT1, MUC2 and MUC5AC | A | ANED, 46 months | (6) |
| Guzelis et al,
2024 | 68 | 5 | Mastectomy, LND,
chemo trastuzumab and rad | +a | +ve: HER2 (2+), CK7
and p53 (10%) -ve: ER, PR and CK20 | A | ANED, 72 months | (7) |
| Present case | 64 | 2.2 | Mastectomy, LND,
chemo and rad | - | +ve: HER2 (3+), CK7,
E-cadherin, GATA3, mammaglobin, MUC1, MUC2, MUC5AC, p53 and Ki-67
(50%) -ve: ER, PR, GCDFP15, CK5/6, EGFR, CK20, CDX2, SATB2, PAX8,
WT1 and TTF1 | A | ANED, 104 months |
|
At present, little is known about the genetic
background of MCA. Lin et al (11) discovered mutations in TP53, RB1 and
BAP1 in a 72-year-old woman with breast MCA, indicating
abnormalities in tumor suppressor genes. Lei et al (12) discovered pathogenic mutations in the
PIK3CA, KRAS, MAP2K4, RB1, KDR, PKHD1, TERT and TP53 genes after
reporting a case of breast MCA in a 59-year-old woman. The genetic
profile closely resembled that of typical high-grade TNBC, which
frequently has PIK3CA, TP53, KRAS and RB1 mutations (13). Chen et al (14) discovered a PIK3CA hotspot mutation
in a breast MCA case, but whole-genome NGS revealed no alterations
in KRAS, NRAS, BRAF or AKT. Cao et al (15) used DNA analysis to detect mutations
in BARD1, KDR, MUC6, TP53 and BRIP1 in a 51-year-old woman with
breast MCA. All 4 cases of MCA that underwent genomic analysis were
triple-negative. Based on a literature review and to the best of
our knowledge, there have been no reports of genetic profiling for
HER2-positive breast MCA to date. Although the present case shares
similar clinical and histomorphological features with previously
reported triple-negative MCA cases (15,16),
their genetic profiles show distinct differences. In the present
case, the NGS test revealed that most of the mutated genes were
located on chromosome 17. Notably, ERBB2 exhibited amplification of
up to 15 copies. Additionally, a series of concomitant
amplifications of HER2-associated genes, including CDK12 and GRB7,
were detected. According to existing knowledge, co-amplification of
CDK12 and GRB7, along with ERBB2, is a common molecular event in
HER2-positive breast cancer (17,18).
As a transcriptional regulator, CDK12 is involved primarily in the
maintenance of genomic stability; its amplification and
upregulation can increase the genomic stability of cancer cells,
leading to resistance to chemotherapy or targeted therapy (19). Moreover, the upregulation of GRB7,
which acts as a linking molecule of HER2, strengthens the HER2
signaling process and can also promote the malignant growth of
tumor cells and drug resistance (20). However, the present patient did not
harbor a PIK3CA gene mutation, unlike previously reported patients
with triple-negative MCA (12,14).
Therefore, we consider that the present case represents a
relatively typical type of breast cancer driven by the
amplification of chromosome 17, resulting in the amplification of
HER2 and a series of other oncogenes. A comparison of the results
from the present case with those of previously reported cases
revealed the heterogeneity of genomic alterations among the
different MCA cases. Hence, we hypothesize that, on the basis of
differences in the molecular expression profiles of tumors, it may
be possible to categorize breast MCAs into two distinct subgroups,
namely, the ‘HER2-driven’ and ‘non-HER2-driven’ subgroups. This
hypothesis awaits further research and validation with the
accumulation of more cases.
In addition, the present case harbored LATS1
mutation, NFE2L2 gene mutation and TP53 gene mutation. The
downregulation of LATS1 and NFE2L2 expression serves a vital role
in the carcinogenesis and progression of breast cancer (21,22).
This may be one of the reasons for the favorable prognosis of the
present case. However, the roles of these genes in primary breast
MCA require further study, and the lack of investigations into
these genes is a limitation of the present case report.
In conclusion, the present study reports a rare case
of a primary breast MCA with concomitant HER2 amplification. NGS
testing revealed that the tumor had the typical genomic features of
HER2-driven breast cancer. Although the patient did not receive
anti-HER2 targeted therapy, no recurrence or metastasis was
observed after 104 months of follow-up, suggesting that
HER2-positive breast MCA may have a favorable prognosis, similar to
triple-negative breast MCA.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The NGS data generated in the present study may be
found in the SRA under accession number PRJNA1229825 or at the
following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1229825.
Authors' contributions
LL, HY, GZ contributed to study design. ML, XQ, XZ
contributed to data acquisition. LL wrote the manuscript. LL and HY
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beis-Filho J, Gobbi H and Peed AM: WHO
Classification of Tumours Editorial Board Breast Tumours.
International Agency for Research on Cancer. Metaplastic Carcinoma;
Lyon: pp. 134–138. 2019
|
|
2
|
Koenig C and Tavassoli FA: Mucinous
cystadenocarcinoma of the breast. Am J Surg Pathol. 22:698–703.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petersson F, Pang B, Thamboo TP and Putti
TC: Mucinous cystadenocarcinoma of the breast with amplification of
the HER2-gene confirmed by FISH: The first case reported. Hum
Pathol. 41:910–913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kucukzeybek BB, Yigit S, Sari AA, Rezanko
T, Durak E and Sadullahoglu C: Primary mucinous cystadenocarcinoma
of the breast with amplification of the HER2 gene confirmed by
FISH-case report and review of the literature. Pol J Pathol.
65:70–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seong M, Ko EY, Han BK, Cho SY, Cho EY,
Lee SK and Lee JE: Radiologic findings of primary mucinous
cystadenocarcinoma of the breast: A report of two cases and a
literature review. J Breast Cancer. 19:330–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur K, Shah A, Gandhi J and Trivedi P:
Mucinous cystadenocarcinoma of the breast: A new entity with broad
differentials-A case report. J Egypt NatI Cancer Inst. 34:92022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guzelis I, Kucukzeybek BB, Uyaroglu MA,
Gokova MB, Sezgin G and Kucukzeybek Y: HER2-positive mucinous
cystadenocarcinoma of the breast coexisting with invasive lobular
carcinoma: A case report and review of the literature. Diagn
Cytopathol. 52:E208–E214. 2024. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sickles E, D'Orsi CJ, Bassett LW, Appleton
CM, Berg WA, Burnside ES, Feig SA, Gavenonis SC, Newell MS and
Trinh MM: ACR BI-RADS® Mammography. ACR
BI-RADS® Atlas, Breast Imaging Reporting and Data
System. American College of Radiology; Reston, VA: 2013
|
|
9
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
10
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer version 2.2015. J Natl Comp Canc
Netw. 13:448–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin LH, Hernandez O, Zhu K, Guth A, Cotzia
P and Darvishian F: Genetic profile of primary mucinous
cystadenocarcinoma of the breast-A case report. Breast J.
27:731–734. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lei T, Shi YQ and Chen TB: Mammary
mucinous cystadenocarcinoma with long-term follow-up: Molecular
information and literature review. Diagn Pathol. 18:132023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geyer FC, Pareja F, Weigelt B, Rakha E,
Ellis IO, Schnitt SJ and Reis-Filho JS: The spectrum of
triple-negative breast disease: High- and low-grade lesions. Am J
Pathol. 187:2139–2151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen WY, Hu YH, Tsai YH, Hang JF, Tan PH
and Che CJ: Mucinous cystadenocarcinoma of the breast harbours
TRPS1 expressions and PIK3CA alterations. Histopathology.
84:550–555. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao X, Luo Y, Shen S and Ren X: Primary
mucinous cystadenocarcinoma of the breast: A case report and
literature review. Oncol Lett. 29:602024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budzik MP: Primary breast mucinous
cystadenocarcinoma-histopathological analysis. Transl Cancer Res.
12:230–232. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrari A, Vincent-Salomon A, Pivot X,
Sertier AS, Thomas E, Tonon L, Boyault S, Mulugeta E, Treilleux I,
MacGrogan G, et al: A whole-genome sequence and transcriptome
perspective on HER2-positive breast cancers. Nat Commun.
7:122222016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lesurf R, Griffith OL, Griffith M, Hundal
J, Trani L, Watson MA, Aft R, Ellis MJ, Ota D, Suman VJ, et al:
Genomic characterization of HER2-positive breast cancer and
response to neoadjuvant trastuzumab and chemotherapy-results from
the ACOSOG Z1041 (Alliance) trial. Ann Oncol. 28:1070–1077. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lui GYL, Grandori C and Kemp CJ: CDK12: An
emerging therapeutic target for cancer. J Clin Pathol. 71:957–962.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bivin WW, Yergiyev O, Bunker ML, Silverman
JF and Krishnamurti U: GRB7 expression and correlation with HER2
amplification in invasive breast carcinoma. Appl Immunohistochem
Mol Morphol. 25:553–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng HC, Xiang LW, Cui ZG, Xue H, Ying E
and Zhao MZ: The clinicopathological and prognostic significances
of LATS1 expression in breast cancer. Histol Histopathol.
37:665–677. 2022.PubMed/NCBI
|
|
22
|
Wang R, Liang L, Matsumoto M, Iwata K,
Umemura A and He F: Reactive oxygen species and NRF2 signaling,
friends or foes in cancer? Biomolecules. 13:3532023. View Article : Google Scholar : PubMed/NCBI
|