Introduction
Breast cancer is one of the malignancies that is
occasionally associated with paraneoplastic dermatomyositis (DM)
(1). Triple-negative breast cancer
(TNBC) is a type of breast cancer that lacks expression of the
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2), and accounts for 10–24%
of all breast cancer cases (1).
Locally advanced breast cancer (LABC) is rare and defined as
patients with stage III breast cancer according to the system
defined by the American Joint Committee on Cancer, the 7th edition.
Biologically aggressive phenotypes of LABC include inflammatory
breast cancer and other types of rapidly proliferating breast
cancer, such as TNBC (2).
Neoadjuvant chemotherapy is the standard treatment for locally
advanced TNBC and has been shown to notably improve patient
outcomes (3).
DM is a debilitating autoimmune disease that
primarily affects muscle tissue and skin. It exhibits several
characteristic symptoms, including progressive muscle weakness,
which is particularly evident in proximal muscles such as the
shoulders and buttocks. Patients often present with distinctive
rashes, including heliotrope (purple discoloration on the eyelids)
and Gottron's papules (raised, scaly papules on the knuckles)
(4). The precise etiology of DM
remains elusive; however, it is classified as an autoimmune disease
in which the immune system targets healthy tissue (5).
Paraneoplastic syndrome has been observed to link
seemingly disparate conditions, such as DM, to occult cancer. These
syndromes arise when a tumor prompts the immune system to generate
aberrant antibodies or other immune responses that affect distant
organs (6). The main symptoms
include progressive proximal muscle weakness, extra-muscular
manifestations such as dyspnea or dysphagia, and skin changes
(7). Although oncological
treatments can provide significant relief from DM, it is not clear
whether the severity of DM is associated with the severity of the
tumors. Furthermore, there are no standard treatment protocols
regarding DM combined with breast cancer (8).
It is estimated that 15–30% of adult DM cases are
paraneoplastic DM, with breast cancer being the primary factor
(9,10). Despite its relatively low incidence,
early diagnosis and management of DM are crucial to improving
patient prognosis. As symptoms of DM can occur before, during and
even after the onset of breast cancer, it is challenging to
recognize the potential link between the two diseases (11). The present study aimed to report the
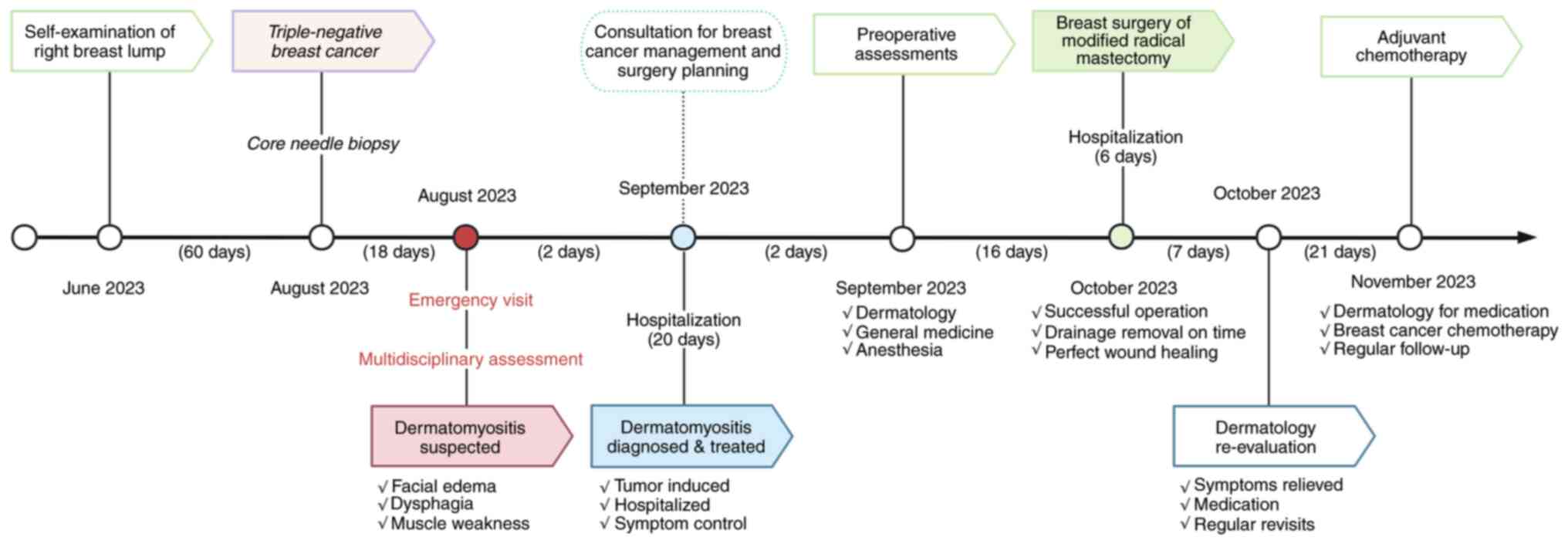
management of a case of locally advanced TNBC with DM (Fig. 1) that achieved significant remission
while reviewing the literature to elucidate the current
understanding of this paraneoplastic syndrome, and to explore its
pathogenesis, clinical manifestations, diagnosis and treatment
strategies.
Case report
Clinical findings
In August 2023, a 43-year-old woman with no history
of underlying medical or genetic conditions presented to the
emergency department of Peking Union Medical College Hospital
(Beijing, China) with a 1-month history of progressive limb
weakness, dysphagia and a 1-week history of dyspnea. The patient
presented with recurrent purplish facial erythema, marked edema
with pruritus, and recent progression involving the trunk and
limbs, with suspected involvement of the muscles used for
swallowing. The preliminary diagnosis from the dermatologist was
DM. Due to the potential association with carcinoma, the
dermatologist recommended further comprehensive examination.
A physical examination revealed an irregular mass
that was ~5.5 cm from the nipple in the right breast, with a hard
texture, unclear boundary and poor mobility. Upon initial
evaluation, the manual muscle test score was recorded as 4/5 in
proximal muscles, and symmetrical sensation was observed (12). Muscle tone and limb strength were
normal on second examination. Physiological reflexes were present,
and no pathological reflexes were elicited. Doppler ultrasound
demonstrated a low echogenic and irregular mass in the right
breast, with weak echogenic deposition behind and a rich blood flow
signal within the mass (Fig. 2A).
Ultrasound also revealed multiple lymph nodes in the right axilla.
Mammography showed a dense mass in the right breast with irregular
margins and indistinct borders, and traces of calcification within
the mass (Fig. 2B). The mass was
classified as category 4c using the breast imaging reporting and
data system standards (13).
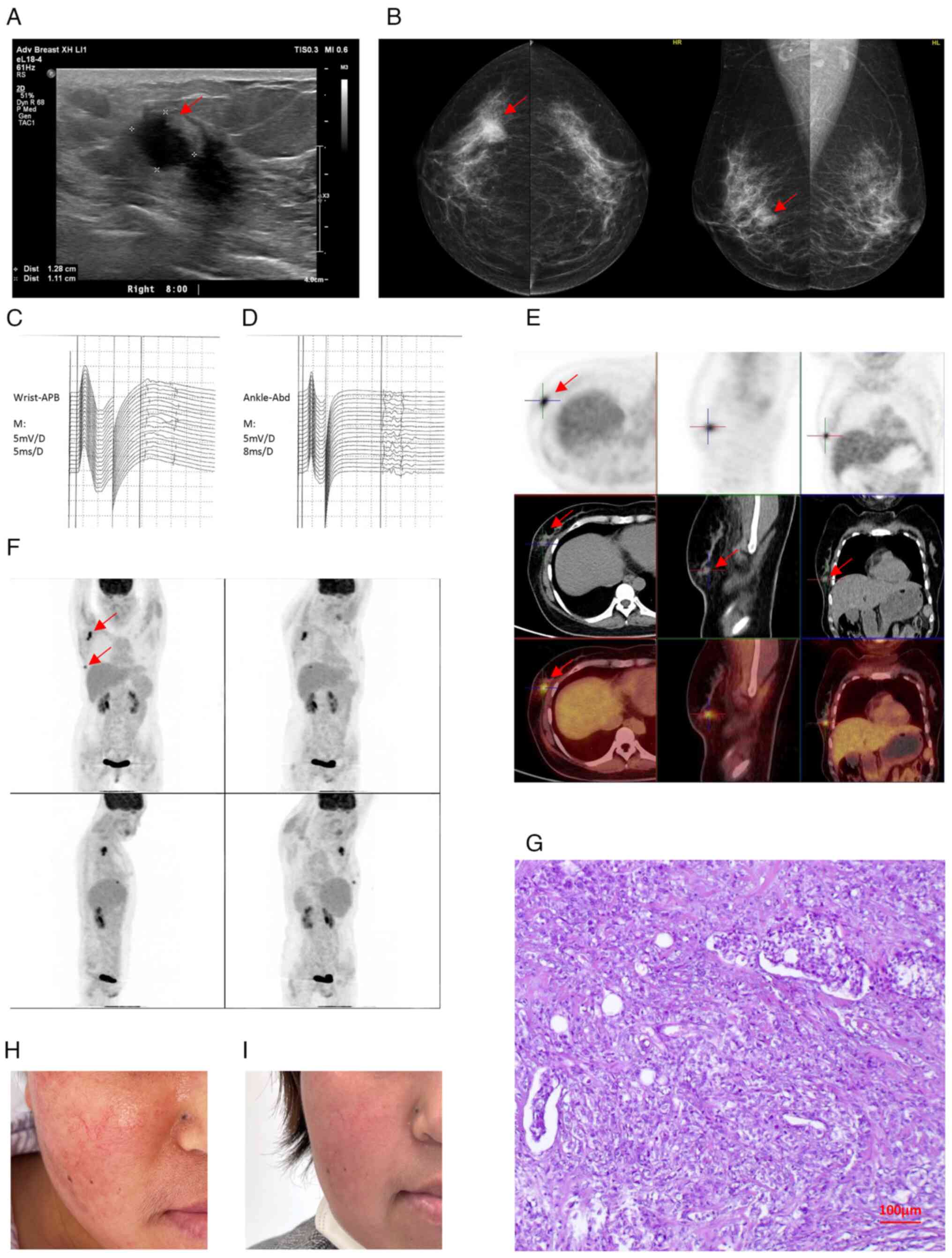 | Figure 2.Clinical imaging and pathological
findings of triple-negative breast cancer presenting with
dermatomyositis. (A) Ultrasound imaging of the breast mass. The
mass appears as a well-defined, hypoechoic lesion with irregular
margins (red arrow indicating the mass). (B) Mammogram of the
breast mass. The mass appears as a dense, spiculated lesion (red
arrow indicating the mass). (C) Upper extremity EMG waveform.
Denervation potentials are present, indicative of muscle
involvement. (D) Lower extremity EMG waveform. Denervation
potentials are present, indicative of muscle involvement. (E)
Whole-body PET-CT scan results. The mass demonstrates increased
fluorodeoxyglucose uptake, indicative of malignancy (red arrow
indicating the mass). (F) Whole-body PET-CT axial scans. The mass
exhibits intense fluorodeoxyglucose uptake (arrow), consistent with
the pathological result. (G) Hematoxylin and eosin staining of the
tumor (magnification, ×100). (H) Preoperative facial rash and
edema. The patient exhibited erythema, swelling and heliotrope rash
characteristic of dermatomyositis. (I) Postoperative facial
appearance. The rash and edema resolved following tumor resection.
EMG, electromyography; PET-CT, positron emission
tomography-computed tomography; APB, Abductor Pollicis Brevis
muscle activity; ABD, abduction muscle activity. |
Laboratory tests indicated that the complete blood
count was close to normal, while blood biochemistry revealed the
following: Alanine transaminase, 29 U/l (normal range, 0–40 U/l);
lactate dehydrogenase level of 256 U/l (normal range, 109–245 U/l);
creatinine, 41 µmol/l (normal range for adults, 44–97 µmol/l);
urea, 2.00 mmol/l (normal range, 3.2–7.1 mmol/l); creatine kinase
(CK), 1,010 U/l (normal range, 18–198 U/l); CK-MB-mass, 9.9 µg/l
(normal range, 0–5 µg/l); myoglobin, 252 µg/l (normal range, 0–90
ng/ml); and high-sensitivity C-reactive protein, 4 mg/l (normal
range, 0.068–8.2 mg/l). The coagulation profile demonstrated a
fibrinogen level of 4.24 g/l (normal range, 2–4 g/l), a D-dimer
concentration of 2.38 mg/l (normal range, 0–5 mg/l). Additionally,
the rheumatoid factor, anti-streptococcal hemolysin ‘O’ test,
antinuclear antibody profile, immunoglobulin and complement levels
were all revealed to be clinically negative, and so were the tumor
markers. Cardiac ultrasound suggested that the left ventricular
ejection fraction was 66%, and there were no obvious abnormalities
in the intracardiac structure and blood flow. A blood sample was
sent to the laboratory (KingMed Diagnostics Group Co., Ltd.), where
the antibody spectrum of myocarditis was assessed using a
commercially available kit (KingMed Diagnostics Group Co., Ltd.),
to investigate the potential paraneoplastic nature of DM. Testing
revealed that anti-transcription intermediary factor 1-γ
autoantibodies were positive, providing evidence for the
association of DM with malignancy. Other antibodies of the
myocarditis spectrum (including ribonucleoprotein, anti-Ku,
anti-PM-Scl, anti-Jo-1, anti-transcription intermediary factor 1-γ,
and anti-PL-7 antibodies, etc.) were tested but found to be
negative, ruling out other possible subtypes of DM linked to these
antibodies. Electromyography suggested myogenic damage to the upper
and lower extremities (Fig. 2C and
D), and a CT scan of the chest, abdomen and pelvis suggested
that there were no specific abnormalities except for mild
subcutaneous soft tissue edema of the chest wall.
Diagnostic assessment
Invasive breast cancer was diagnosed by pathology
from a core needle biopsy of the breast in August, 2023, two months
after the patient first discovered the lump and 18 days prior to
her emergency department visit for suspected DM. TNBC was
classified based on the biopsy pathology report of negative
expression of ER, PR and HER2 (score 1+) by immunohistochemistry
(according to the system defined by the American Joint Committee on
Cancer, the 7th edition) (data not shown). The presence of distant
metastases to internal organs or bone was ruled out by positron
emission tomography-computed tomography examination (Fig. 2E and F) and emission computed
tomography of the bones. Consequently, the patient was diagnosed
with locally advanced TNBC. According to the medical history of the
patient, tumor-related DM was diagnosed by an oncologist and
dermatologist.
Treatment
The patient underwent a preliminary
multidisciplinary assessment in the emergency department, which
included anesthesiology, medical oncology, breast surgery and
dermatology. General anesthesia of the patient was not appropriate
for surgical treatment due to progressive muscle weakness and the
high risk of respiratory distress, and inability to extubate after
anesthesia. Neoadjuvant chemotherapy was also not feasible due to
the poor general condition of the patient and high risk of
chemotherapy side effects.
Outpatient phase
Initial treatment began in September 2023, with
methylprednisolone sodium succinate prescribed at 40 mg/day
(intravenous infusion). Despite 1 week of therapy, the symptoms of
the patient, including limb weakness and dyspnea, were not
sufficiently controlled.
Inpatient phase (high-dose
corticosteroid pulse therapy)
To rapidly alleviate the paraneoplastic syndrome,
which was mainly characterized by severe dyspnea and muscle
weakness, the patient was admitted to the Department of Dermatology
(Peking Union Medical College Hospital, Chinese Academy of Medical
Science and Peking Union Medical College, Beijing, China) in
September 2023 and received high-dose corticosteroid pulse therapy
(defined as the use of high doses of corticosteroids over a short
period to achieve rapid control of symptoms). Upon admission, the
treatment regimen was escalated to a prednisone-equivalent dose of
60 mg/day, consisting of methylprednisolone 40 mg daily
(intravenous infusion) and an additional 8 mg oral
methylprednisolone daily, aiming to achieve rapid symptom control.
Corticosteroid therapy was initiated to rapidly alleviate the
symptoms of paraneoplastic DM, including dyspnea and limb weakness.
The treatment aimed to stabilize the condition of the patient prior
to surgical intervention and improve quality of life. Meanwhile a
multidisciplinary consultation was held to assess the condition of
the patient and determine timely surgical conditions. Following
corticosteroid therapy, the patient demonstrated partial clinical
improvement with dyspnea, muscle weakness and cutaneous lesions.
Laboratory tests showed a decline in CK levels (40 U/l; normal
range, 18–198 U/l), confirming the effectiveness of the treatment.
After 4 weeks of adequate corticosteroid treatment, dose tapering
was initiated and the preparations for surgery continued as
planned.
In October 2023, the patient was admitted to the
Department of Breast Surgery (Peking Union Medical College
Hospital, Chinese Academy of Medical Science and Peking Union
Medical College, Beijing, China), and a modified radical mastectomy
was performed after consultive preparation and joint management by
the oncology, dermatology, general medicine and anesthesia
departments. During this perioperative period, the patient received
intravenous immunoglobulin therapy (human immunoglobulin) at a
daily dose of 30 g via intravenous infusion for a total of 5 days.
This therapy was initiated to manage DM-related symptoms and to
ensure the patient was in optimal condition for the planned
surgical procedure. The procedure went well, and TNBC was confirmed
(Fig. 2G), with pathological
analysis of paraffin-embedded tissue reporting invasive carcinoma
of the right breast (non-specific, moderately poorly
differentiated, histological grade 3 (American Joint Committee on
Cancer, the 7th edition). The tumor was 5×1 cm with multiple
intratumorally emboli, no significant nerve invasion and a small
amount of intermediate-grade ductal carcinoma seen in the
periphery. Mammary adenopathy and fibroadenoma were also observed.
No lesions were observed in the nipple and areola, and lymph node
metastatic carcinoma was revealed (right axilla 7/12).
Immunohistochemical analysis (14)
results of the final pathology report were as follows: ER (−), PR
(−), androgen receptor (−), HER2 (score 0), Ki-67 proliferation
index (80%), p53 tumor suppressor protein (−), CD10 (−),
cytokeratin 14 (partial +), cytokeratin 5/6 (−), epidermal growth
factor receptor (−), p63 (−), E-cadherin (−) and p120 (−) (data not
shown).
On the second postoperative day, the orbital edema,
hoarseness, dyspnea and limb weakness of the patient were markedly
reduced, and the erythema subsided. The patient recovered from
surgery without adverse incidents. A total of 3 weeks after
surgery, the patient was able to walk independently, and most of
the skin symptoms were relieved, particularly the facial rashes
(Fig. 2H and I). A total of 4 weeks
after the operation, the patient gained a full recovery and started
postoperative adjuvant chemotherapy as scheduled. The patient was
administered chemotherapy of epirubicin (150 mg) and
cyclophosphamide (900 mg) in a 2-week regimen four times, followed
by a docetaxel regimen (150 mg) in a 3-week regimen four times.
The patient is under a regular follow-up of every 6
months, and at the latest checkup in November 2024 was in good
general condition, with muscle strength grade score of 5/5, skin
rash improved from the previous state, no dysphagia, and no local
recurrence or distant metastasis.
Literature review
Epidemiology and risk factors of breast
cancer-induced DM
Breast cancer is a well-documented risk factor for
DM, a debilitating autoimmune myopathy. Notably, for DM and breast
cancer, the risk appears to be bidirectional. Patients with DM have
a higher risk of developing cancer, particularly breast cancer,
within 5 years of diagnosis (15).
This highlights the importance of thorough cancer screening in
patients with new-onset DM.
While the exact reasons behind breast cancer
specifically triggering DM are yet to be fully elucidated, some
potential risk factors are emerging. Research suggests a possible
link with specific breast cancer subtypes, particularly aggressive
tumors such as TNBC. Additionally, age might play a role, with some
studies suggesting a higher prevalence of breast cancer-induced DM
in younger women compared with the typical demographic for both
conditions. The average age of breast cancer patients with DM is
reported to be ~45 years, which is younger than the typical age for
breast cancer and DM (16). Further
research is required to confirm these associations, and to identify
additional risk factors for targeted diagnosis and management.
Association between breast cancer and
DM: Unveiling pathogenic mechanisms
The precise mechanisms by which breast cancer
triggers DM remain elusive. Current hypotheses suggest a complex
interplay between the immune system and the tumor microenvironment.
A potential explanation involves a phenomenon known as molecular
mimicry. Breast cancer cells may express antigens structurally
similar to those found in healthy muscle and skin tissues. This
resemblance may misdirect cytotoxic T lymphocytes and autoantibody
production, targeting healthy tissues and causing DM symptoms
(17).
Another compelling theory suggests a role for
anti-melanoma differentiation-associated gene 5 (anti-MDA5)
autoantibodies. These antibodies, often elevated in patients with
DM, have been linked to specific oncological conditions, including
breast cancer (18). Their presence
might contribute to tissue damage through various pathways,
including disruption of cellular protein synthesis and induction of
inflammatory responses (19).
A previous study suggested a potential role for the
tumor microenvironment itself in promoting DM. Breast cancer cells
may release cytokines and other inflammatory mediators that
activate autoreactive immune cells, further contributing to the
autoimmune attack on healthy tissues (20). Elucidating the specific contribution
of these factors and their interactions is crucial for developing
targeted therapeutic strategies for this paraneoplastic syndrome.
Ongoing research in this area explores the role of specific immune
cell subsets, cytokine profiles and the identification of novel
autoantibodies associated with breast cancer-induced DM (21). Investigating these intricate
mechanisms may hold promise to not only improve diagnosis but also
develop potential novel immunomodulatory therapies.
Challenges and tools in diagnosing
breast cancer-associated DM
DM is characterized by distinctive clinical
features. Proximal muscle weakness, predominantly affecting the
shoulders, hips and thighs, is a hallmark symptom. Patients often
have trouble climbing stairs, rising from a seated position or
raising their arms overhead. Additionally, characteristic rashes
can offer valuable diagnostic clues. The heliotrope rash, a
violaceous discoloration on the eyelids, and Gottron's papules,
raised, scaly bumps on the knuckles and extensor surfaces of the
knees and elbows, are frequently observed (22).
However, diagnosing breast cancer-induced DM
presents a unique challenge. The timing of the two conditions can
vary. DM can manifest before, concurrently with, or even years
after the diagnosis of breast cancer (23). This variability requires heightened
awareness and a multi-pronged diagnostic approach.
Diagnosis relies on clinical evaluation, including a
detailed history and examination of muscle weakness and
characteristic rashes. Laboratory investigations often reveal
elevated muscle enzymes such as CK. Skin biopsies from affected
areas can demonstrate specific histological features supportive of
DM. Additionally, the presence of specific autoantibodies, such as
anti-MDA5 autoantibodies, can strengthen the diagnosis and
potentially link it to an underlying malignancy (18). While these tools offer valuable
insights, further research is needed to identify more specific
biomarkers for a definitive diagnosis of breast cancer-induced DM,
particularly in cases with atypical presentations.
Managing DM and eradicating breast
cancer using a two-pronged approach
The management of breast cancer-induced DM
necessitates a dual therapeutic strategy. First, addressing the
underlying inflammatory processes driving DM is crucial.
Corticosteroids, particularly prednisone, are the primary
treatment, and they suppress the immune system and reduce muscle
inflammation and weakness (24). In
severe cases or those unresponsive to corticosteroids alone,
immunosuppressant medications such as azathioprine, methotrexate or
mycophenolate mofetil can be used in conjunction to achieve optimal
disease control (25).
Concurrently, effective treatment of the underlying
breast cancer is paramount for sustained improvement in DM
symptoms. Breast cancer treatment depends on tumor stage, hormone
receptor status and HER2 expression. Surgery remains a cornerstone
of treatment, with the extent ranging from lumpectomy for
early-stage tumors to mastectomy for more advanced presentations
(26). Adjuvant therapy with
chemotherapy and/or radiation therapy may be recommended based on
individual risk factors and tumor characteristics.
Previous research has highlighted the potential
benefits of early and aggressive breast cancer treatment in
improving DM outcomes. Prompt diagnosis and initiation of
appropriate therapy for the malignancy can significantly reduce
disease activity and improve muscle strength in patients with DM
(11). This emphasizes the
importance of a collaborative approach between rheumatologists and
oncologists to ensure optimal management of both conditions.
Prognostic considerations and
management landscape
The prognosis for patients with breast
cancer-induced DM depends on two crucial factors: Early diagnosis
and successful treatment of the underlying malignancy. Prompt
identification and aggressive breast cancer management markedly
improve DM outcomes. Early intervention with appropriate breast
cancer therapy has been shown to lead to a marked reduction in DM
disease activity and a notable improvement in muscle strength
(27). This highlights the need for
heightened awareness of DM in patients with breast cancer,
especially those with unexplained muscle weakness or characteristic
rashes.
Discussion
LABC is a subset of breast cancer that is
characterized by advanced breast tumors in the absence of distant
metastasis. The definition of LABC encompasses tumors >5 cm in
size with regional lymphadenopathy (N1-3), as well as tumors of any
size with direct extension to the chest wall or skin (including
ulcer or satellite nodules). Patients with LABC generally have a
poor prognosis, with a high risk of local recurrence and distant
metastasis. LABC is currently estimated to account for 10% of
breast cancer cases in women (28).
It can be classified as an operable or inoperable disease,
depending on the stage of the tumor (T4, N2 or N3). Historically,
the preferred treatment option for inoperable LABC was neoadjuvant
chemotherapy; however, in selected patients with limited local
disease (axillary lymph node metastases, but not fixed, moderate
skin involvement), radical mastectomy may be considered as the
primary treatment option.
For women with locally advanced TNBC, neoadjuvant
chemotherapy is a viable systemic treatment. It has been
demonstrated to induce pathological complete remission, increase
surgical success, and reduce the extent of surgical intervention,
thereby minimizing surgical morbidity. A number of clinical studies
have demonstrated that 30–40% of patients achieve a complete
clinical response, while 50–60% achieve a partial response
(29). Following the completion of
neoadjuvant chemotherapy, patients may proceed with definitive
local therapy (30). The objective
of surgical intervention is the complete excision of the primary
tumor, including any loco-regional disease and involved skin or
muscles. It is recommended that patients who have undergone surgery
receive postoperative radiation therapy to minimize the risk of
local recurrence (31).
Breast cancer in the context of DM is a rare and
complex disease. The primary symptoms of DM manifest primarily as
dermatological and musculoskeletal conditions, with the potential
to evolve into more severe presentations, such as severe dyspnea.
The patient in the present study presented with this condition. The
association between breast cancer and DM has been confirmed through
epidemiological studies (3);
however, the precise mechanism remains unclear. When a tumor
complicates DM, excision of the tumor lesion is a more effective
treatment to alleviate the symptoms of DM compared to conventional
treatments, such as immunosuppressive therapies or corticosteroids
(32,33). The treatment of DM is primarily
based on systemic corticosteroids (34).
The notable improvement of DM symptoms following
tumor resection in the current case may provide insights into the
pathophysiological links between the two conditions. A potential
explanation is that tumor antigens, which may share structural
similarity with autoantigens in muscle and skin tissues, are
eliminated, thereby halting the autoimmune response (35). Another possibility is the cessation
of autoantibody production that had been stimulated by the presence
of the tumor (36). Additionally,
tumors may secrete factors that modulate the immune response,
exacerbating autoimmune symptoms, which subside once the tumor is
removed (37). While these
mechanisms remain hypothetical, they highlight the importance of
tumor removal as a potential therapeutic strategy for
paraneoplastic syndromes. Future research should focus on
elucidating these complex interactions to develop targeted
treatments.
There is no standardized treatment for breast cancer
associated with DM, and individualized treatments must be based on
the condition of the patient. For refractory LABC with DM, the
timing of treatment for DM and breast cancer is also crucial. Dias
et al (11) reported that
severe muscle symptoms should be controlled with steroids before
radical excision of breast cancer. However, not all steroid
treatments can achieve DM remission. In the present case, potential
risks associated with corticosteroid use, such as delayed wound
healing and surgical site infections, were carefully managed
through close monitoring of the condition of the patient.
Additionally, topical treatments, including Elosone and boric acid
wet compresses, were employed to control skin lesions effectively.
No antibiotics were required, as no signs of infection were
observed throughout the treatment.
Furthermore, in the present study, the
administration of steroids prior to surgical intervention resulted
in a longer overall period of disease control. The patient
exhibited only a negligible response to steroid treatment,
concomitant with a substantial and unremitting deterioration in
their general condition (severe and persistent dyspnea). In light
of this critical condition, both surgical intervention and
chemotherapy were deemed to be highly risky for the patient.
Following a comprehensive risk assessment and thorough
communication between the patient and their physician, a mastectomy
was performed, and the patient made a full recovery. This case may
be indicative of DM as a paraneoplastic syndrome manifestation. A
limitation of this study is the short follow-up period (latest
follow-up was 18 months post-surgery). The results of therapy and
any changes to the health of the patient will continue to be
monitored.
In conclusion, in adult patients presenting with DM,
doctors must prioritize cancer screening to ensure timely diagnosis
of potential malignancies, and the necessity for multidisciplinary
collaboration in the management of the condition of the patient.
When patients present with relatively severe paraneoplastic muscle
symptoms, the upfront resection of the tumor may provide rapid
relief of symptoms, thereby allowing the subsequent treatment to be
initiated, which could improve the prognosis of the patient.
Acknowledgements
Not applicable.
Funding
This study was supported by the National High Level Hospital
Clinical Research Funding (grant no. 2022-PUMCH-C-066).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS contributed to data collection and manuscript
writing. YX and XH contributed to data acquisition and
interpretation. QS and YL supervised the study, designed the study
and critically revised the manuscript. YS, YX, QS and YL confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in this study involving
human participants were in accordance with the ethical standards of
the institutional and national research committee and with the
Declaration of Helsinki and its later amendments.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leon-Ferre RA and Goetz MP: Advances in
systemic therapies for triple negative breast cancer. BMJ.
381:e0716742023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teichgraeber DC, Guirguis MS and Whitman
GJ: Breast cancer staging: Updates in the AJCC cancer staging
manual, 8th edition, and current challenges for radiologists, from
the AJR special series on cancer staging. AJR Am J Roentgenol.
217:278–290. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Chen A, Liu C and Zhang B:
Triple-negative breast cancer with dermatomyositis: A case report
and literature review. Cancer Manag Res. 14:569–576. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aebi S, Karlsson P and Wapnir IL: Locally
advanced breast cancer. Breast. 62 (Suppl 1):S58–S62. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Connell KA and LaChance AH:
Dermatomyositis. N Engl J Med. 384:24372021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang S, Anderson HJ and Lee JB:
Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan
syndrome: Part II. Diagnosis and management. J Am Acad Dermatol.
91:13–22. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sardiña González C, Martínez Vivero C and
López Castro J: Paraneoplastic syndromes review: The great
forgotten ones. Crit Rev Oncol Hematol. 174:1036762022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SH, Chang C and Lian ZX: Polymyositis
and dermatomyositis-challenges in diagnosis and management. J
Transl Autoimmun. 2:1000182019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inaguma G, Shimada A, Tsunoda J, Matsuzaki
T, Nishi T, Seki H and Matsumoto H: Inflammatory breast cancer
associated with amyopathic dermatomyositis: A case report. Surg
Case Rep. 6:2842020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piras M, Panebianco M, Garibaldi M,
Roberto M, Merlonghi G, Pellegrini P and Marchetti P: A Case of
pathological complete response and resolution of dermatomyositis
following neoadjuvant chemotherapy in HER2-positive early breast
cancer. Curr Oncol. 28:1957–1961. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dias LPN, Faria ALA, Scandiuzzi MM, Inhaia
CLCS, Shida JY and Gebrim LH: A rare case of severe myositis as
paraneoplastic syndrome on breast cancer. World J Surg Oncol.
13:1342015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Werner JM, Wlodarczyk J and Seruya M:
Diagnostic accuracy of manual muscle testing to identify nerve
transfer candidates in children with acute flaccid myelitis. Plast
Reconstr Surg. 152:1057–1067. 2023.PubMed/NCBI
|
|
13
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS® fifth edition: A summary of
changes. Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren X, Song Y, Zhang Y, Wu H, Chen L, Pang
J, Zhou L, Shen S and Liang Z: Prognostic significance of different
molecular typing methods and immune status based on RNA sequencing
in HR-positive and HER2-negative early-stage breast cancer. BMC
Cancer. 22:5482022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khanna U, Galimberti F, Li Y and Fernandez
AP: Dermatomyositis and malignancy: Should all patients with
dermatomyositis undergo malignancy screening? Ann Transl Med.
9:4322021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daryanani S: Dermatomyositis and breast
cancer. J Clin Oncol. 16:2890–2891. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azar L and Khasnis A: Paraneoplastic
rheumatologic syndromes. Curr Opin Rheumatol. 25:44–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Q, Lyu K, Li J, Zhang P, Guan W,
Zhang L, Han L, Liu S and Li T: Anti-melanoma
differentiation-associated 5 gene antibody-positive dermatomyositis
exhibit three clinical phenotypes with different prognoses. Clin
Exp Rheumatol. 40:304–308. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nombel A, Fabien N and Coutant F:
Dermatomyositis with anti-MDA5 antibodies: Bioclinical features,
pathogenesis and emerging therapies. Front Immunol. 12:7733522021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blaes F: Pathogenesis, diagnosis and
treatment of paraneoplastic neurologic syndromes. Expert Rev
Neurother. 21:675–686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen KL, Zeidi M and Werth VP: Recent
advances in pharmacological treatments of adult dermatomyositis.
Curr Rheumatol Rep. 21:532019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagaraju K and Lundberg IE: Polymyositis
and dermatomyositis: Pathophysiology. Rheum Dis Clin North Am.
37159–171. (v)2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akagi H and Wada T: A case in which breast
cancer developed at the same time as dermatomyositis, and the onset
of new cancer was able to be predicted by the exacerbating skin
symptoms and parallel increase in the anti-TIF1-γ antibody levels.
Intern Med. 62:3057–3062. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cobos GA, Femia A and Vleugels RA:
Dermatomyositis: An update on diagnosis and treatment. Am J Clin
Dermatol. 21:339–353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon PA, Winer JB, Hoogendijk JE and
Choy EH: Immunosuppressant and immunomodulatory treatment for
dermatomyositis and polymyositis. Cochrane Database Syst Rev.
2012:CD0036432012.PubMed/NCBI
|
|
26
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ,
Chew H, et al: NCCN guidelines® insights: Breast cancer,
version 4.2023. J Natl Compr Canc Netw. 21:594–608. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leatham H, Schadt C, Chisolm S, Fretwell
D, Chung L, Callen JP and Fiorentino D: Evidence supports blind
screening for internal malignancy in dermatomyositis: Data from 2
large US dermatology cohorts. Medicine (Baltimore). 97:e96392018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tryfonidis K, Senkus E, Cardoso MJ and
Cardoso F: Management of locally advanced breast
cancer-perspectives and future directions. Nat Rev Clin Oncol.
12:147–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu J, Tong T, Xu D, Cheng F, Fang C, He C,
Wang J, Wang B, Yang X, Wang K, et al: Deep learning radiomics of
ultrasonography for comprehensively predicting tumor and axillary
lymph node status after neoadjuvant chemotherapy in breast cancer
patients: A multicenter study. Cancer. 129:356–366. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maire M, Debled M, Petit A, Fournier M,
Macgrogan G, Quenel-Thueux N, Charitansky H, Mathoulin-Pelissier S,
Bonnefoi H and Tunon de Lara C: Neoadjuvant chemotherapy and
radiotherapy for locally advanced breast cancer: Safety and
efficacy of reverse sequence compared to standard technique? Eur J
Surg Oncol. 48:1699–1705. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sousa C, Cruz M, Neto A, Pereira K,
Peixoto M, Bastos J, Henriques M, Roda D, Marques R, Miranda C, et
al: Neoadjuvant radiotherapy in the approach of locally advanced
breast cancer. ESMO Open. 4 (Suppl 2):e0006402020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Primiano G, Plantone D, Sauchelli D,
Cuccagna C, Renna R, Iorio R and Servidei S: Resolution of muscle
inflammation after tumor removal in a woman with paraneoplastic
dermatomyositis. J Rheumatol. 39:2359–2360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Racanelli V, Prete M, Minoia C, Favoino E
and Perosa F: Rheumatic disorders as paraneoplastic syndromes.
Autoimmun Rev. 7:352–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bogdanov I, Kazandjieva J, Darlenski R and
Tsankov N: Dermatomyositis: Current concepts. Clin Dermatol.
36:450–458. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fiorentino DF and Casciola-Rosen L:
Autoantibodies and cancer association: The case of systemic
sclerosis and dermatomyositis. Clin Rev Allergy Immunol.
63:330–341. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Firmat F and Lipsett MB: Cancer and
dermatomyositis. Cancer. 11:63–66. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogawa M, Sugiura K, Yokota K, Muro Y and
Akiyama M: Anti-transcription intermediary factor 1-γ
antibody-positive clinically amyopathic dermatomyositis complicated
by interstitial lung disease and breast cancer. J Eur Acad Dermatol
Venereol. 30:373–375. 2016. View Article : Google Scholar : PubMed/NCBI
|