Introduction
Primary liver cancer has emerged as a substantial
economic burden in global public health, characterized by its high
incidence and mortality rates (1).
In these cases, hepatocellular carcinoma (HCC) is the predominant
form, accounting for 75–80% (2).
Notably, China bears a disproportionately high incidence of HCC,
contributing to >50% of cases worldwide (3). Currently, there are various treatment
options for HCC, including surgical resection, ablation,
transarterial chemoembolization (TACE), liver transplantation,
systemic therapy and combination therapy (4–6). Among
these options, liver resection is the preferred choice for patients
with well-functioning livers and localized tumors, offering the
potential for cure (7). However, in
most Asian centers, overall survival (OS) rates for patients remain
unsatisfactory (8). Approximately
half of all HCC patients experience recurrence or metastasis within
5 years after curative resection (9,10).
Therefore, it is imperative to identify risk factors that impact OS
in HCC patients after liver resection.
α-fetoprotein (AFP), as a pivotal biomarker in the
detection of HCC, plays an essential role in accurate diagnosis,
early identification, evaluation of treatment effectiveness,
recurrence monitoring and prognosis prediction for HCC (11). Nonetheless, it is noteworthy that
~30% of HCC patients do not exhibit elevated serum AFP levels
(12). The delayed diagnosis of
AFP-negative HCC (AFP-NHCC) often results in treatment
postponements, affecting patient prognosis (13). Even during postoperative follow-up,
patients with AFP-NHCC continue to undergo regularly AFP level
monitoring, given the close correlation between AFP and tumor
prognosis (14,15). The findings of the present study
indicated that certain patients with AFP-NHCC experience dynamic
changes in AFP levels after resection. While current studies have
focused on identifying prognostic factors in patients with AFP-NHCC
after hepatectomy. A consensus on the prognostic factors for
patients experiencing postoperative AFP level elevation remains
elusive and research on the risk factors related to OS for this
patients cohort is lacking.
The Random Survival Forest (RSF) is a powerful
machine-learning algorithm composed of multiple decision trees,
demonstrating relatively high accuracy, robustness and strong
resistance to over-fitting. Therefore, by combining the RSF with
traditional multivariate Cox regression, more reliable prognostic
factors related to OS can be identified and a nomogram can be
constructed using the aforementioned variables. A nomogram is a
common visual representation of clinical prediction models.
Constructed from a comprehensive combination of multiple clinical
indicators, the nomogram enables physicians to deliver more direct
and accurate prognoses for specific patients. This assists in
adjusting treatments accordingly, aiming for improved clinical
outcomes. Consequently, the present study aimed to predict the OS
of HCC patients who initially tested negative for AFP at baseline
but exhibited a subsequent change to AFP positivity during
follow-up after curative resection. This prediction was based on
clinical data and provides improved guidance for patient
management.
Materials and methods
Patients
The present study retrospectively analyzed 870 HCC
patients who underwent resection at Beijing You'an Hospital,
Capital Medical University, China between January 2013 and January
2021. The age of all patients ranged from 22 to 78 years, with a
mean age of 56.61±9.08 years, and the male-to-female ratio was
3.58:1. The cohort was randomly divided into a training set and a
validation set at a ratio of 7:3. The training set was used for
variable selection and model construction, while the validation set
was used to confirm the performance of the developed model.
Baseline characteristic in the training set and verify set are
given in Table I.
 | Table I.Baseline characteristic in the
training set and validation set. |
Table I.
Baseline characteristic in the
training set and validation set.
| Variable | Group | Training set
(n=600) | Validation set
(n=270) | P-value |
|---|
| Age, n (%) | ≤60 years | 373 (62.17) | 168 (62.22) | 0.988 |
|
| >60 years | 227 (37.83) | 102 (37.78) |
|
| Sex, n (%) | Male | 469 (78.17) | 211 (78.15) | 0.995 |
|
| Female | 131(21.83) | 59 (21.85) |
|
| Hypertension, n
(%) | Yes | 162 (27.00) | 72 (26.67) | 0.918 |
|
| No | 438 (73.00) | 198 (73.33) |
|
| Diabetes, n
(%) | Yes | 140 (23.33) | 52 (19.26) | 0.180 |
|
| No | 460 (76.67) | 218 (80.74) |
|
| Smoking, n (%) | Yes | 251 (41.82) | 119 (44.07) | 0.536 |
|
| No | 349 (58.17) | 151 (55.93) |
|
| Drinking, n
(%) | Yes | 189 (31.50) | 94 (34.81) | 0.334 |
|
| No | 411 (68.50) | 176 (65.19) |
|
| Cirrhosis, n
(%) | Yes | 514 (85.67) | 238 (88.15) | 0.323 |
|
| No | 86 (14.33) | 32 (11.85) |
|
| Child-Pugh, n
(%) | A | 462 (77.00) | 196 (72.59) | 0.161 |
|
| B | 138 (23.00) | 74 (27.41) |
|
| BCLC, n (%) | 0 | 233 (38.83) | 95 (35.19) | 0.304 |
|
| A | 367 (61.17) | 175 (64.81) |
|
| Tumor number, n
(%) | Single | 498 (83.00) | 210 (77.78) | 0.067 |
|
| Multiple | 102 (17%) | 60 (22.22) |
|
| Tumor size, n
(%) | ≤3 cm | 443 (73.83) | 198 (73.33) | 0.877 |
|
| >3 cm | 157 (26.17) | 72 (26.67) |
|
| RBC (mean ± SD),
1012/l | - | 4.18±0.63 | 4.14±0.60 | 0.376 |
| HB (mean ± SD),
g/l | - | 131.51±19.73 | 129.97±18.77 | 0.280 |
| WBC (mean ± SD),
109/l | - | 5.12±2.16 | 4.88±1.98 | 0.113 |
| AST (mean ± SD),
IU/l | - | 31.38±15.00 | 33.07±16.54 | 0.136 |
| ALT (mean ± SD),
IU/l | - | 30.57±18.82 | 33.29±22.17 | 0.063 |
| TBIL (mean ± SD),
µmol/l | - | 19.45±10.06 | 19.42±10.28 | 0.975 |
| DBIL (mean ± SD),
µmol/l | - | 6.66±4.66 | 6.69±4.97 | 0.910 |
| Total Protein (mean
± SD), g/l | - | 65.37±6.80 | 65.21±8.23 | 0.774 |
| GGT (mean ± SD),
U/l | - | 63.97±57.50 | 68.64±54.33 | 0.260 |
| ALP (mean ± SD),
IU/l | - | 86.98±35.86 | 87.95±34.59 | 0.711 |
| Glob (mean ± SD),
g/l | - | 28.08±5.37 | 28.25±5.05 | 0.657 |
| PT (mean ± SD),
s | - | 12.60±1.57 | 12.53±1.44 | 0.526 |
| PTA (mean ± SD),
% | - | 85.94±15.12 | 86.68±14.60 | 0.502 |
| INR (mean ±
SD) | - | 1.12±0.14 | 1.11±0.13 | 0.359 |
| Fibrinogen (mean ±
SD), mg/dl | - | 2.78±0.92 | 2.76±0.91 | 0.827 |
The present study was conducted according to the
Declaration of Helsinki and approved by the Ethics Committee of
Beijing You'an Hospital, Capital Medical University, China
(approval no. LL-2021-152-K) and followed the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards.
The inclusion criteria for the present study were as
follows: i) Pathological diagnosis of HCC, ii) Surgical resection
of single or multiple isolated HCC lesions in one liver lobe, iii)
Preoperative serum AFP level <20 ng/ml. During follow-up (>2
months after resection), patients exhibited elevation of AFP level
and iv) Early-stage HCC patients. Exclusion criteria included: i)
Previous receipt of other treatments such as ablation and TACE ii)
concomitant other malignancies and iii) missing information.
Data collection
Prior to patients undergoing HCC resection, baseline
data and tumor characteristics of all patients were obtained from
the medical records system, including age, sex, medical history,
family history, presence of cirrhosis, Barcelona Clinic Liver
Cancer (BCLC) staging, Child-Pugh classification (16), tumor number and size, AFP levels, as
well as routine blood test parameters, liver function indices and
coagulation indicators. The outcome of resection was primarily
obtained through telephone follow-up. The postoperative AFP levels
were mainly retrieved from the electronic medical records when
patients are readmitted to the hospital.
Follow-up and endpoint
All patients underwent curative liver resection
after completing preoperative evaluations. Postoperatively,
patients were regularly followed up with various assessments,
including physical examinations and monitoring of serum AFP levels.
The clinical endpoint was OS, with follow-up data collected until
January 2023. OS was defined as the time between the first liver
cancer resection and either death or the last follow-up date.
Statistical analysis
Data analysis used IBM SPSS Statistics 27 (IBM
Corp.) and R version 4.1.2 (17).
Normally distributed data were presented as mean ± standard
deviation, while skewed data was represented using quartiles. The
Mann-Whitney U test or Student's t-test was employed to compare
numerical variables between the derivation set and internal
validation set, while Fisher's exact test or χ2 test was
used for assessing categorical variables. Significant predictive
factors were identified using RSF and multivariate Cox regression.
These variables were subsequently incorporated into a nomogram
model. Based on a total score of 130, patients were assigned to the
low, medium and high-risk groups using cutoff values of 43 and 86.
Kaplan-Meier (KM) analysis and log-rank tests were used to compare
differences in OS among the three different risk groups. The areas
under the curves (AUCs) of the time-dependent receiver operating
characteristic (ROCs) curves were employed to evaluate model
accuracy. Additionally, the concordance index (C-index) was used to
assess model discrimination, while calibration curves were employed
to evaluate consistency between training and validation sets.
Clinical utility assessment was conducted through decision curve
analysis (DCA), quantifying net benefits at different probability
thresholds.
Results
Baseline characteristics and survival
outcomes of patients
The final analysis involved 870 eligible patients
with AFP-NHCC, divided into the model development group (n=600) and
the validation group (n=270). Baseline data comprised 26 items. In
the entire cohort, 62.18% of patients were <60 years old and
78.16% were male. Additionally, 86.44% of patients had a background
of cirrhosis, with the majority falling into Child-Pugh A (75.63%)
and 62.30% of patients were in BCLC stage A. No statistical
differences were observed between the model development and
validation groups (all P>0.05), indicating consistency between
the two groups. Detailed demographic and clinical characteristics
between the model development group and the validation group are
presented in Table I.
The 3-, 5- and 8-year overall survival rates for all
patients were 90.3, 76.7 and 60.5%, respectively. The KM curve of
overall survival for the patients is shown in Fig. S1.
Identification of predictors and
development of the nomogram
The variables in the model-building group were
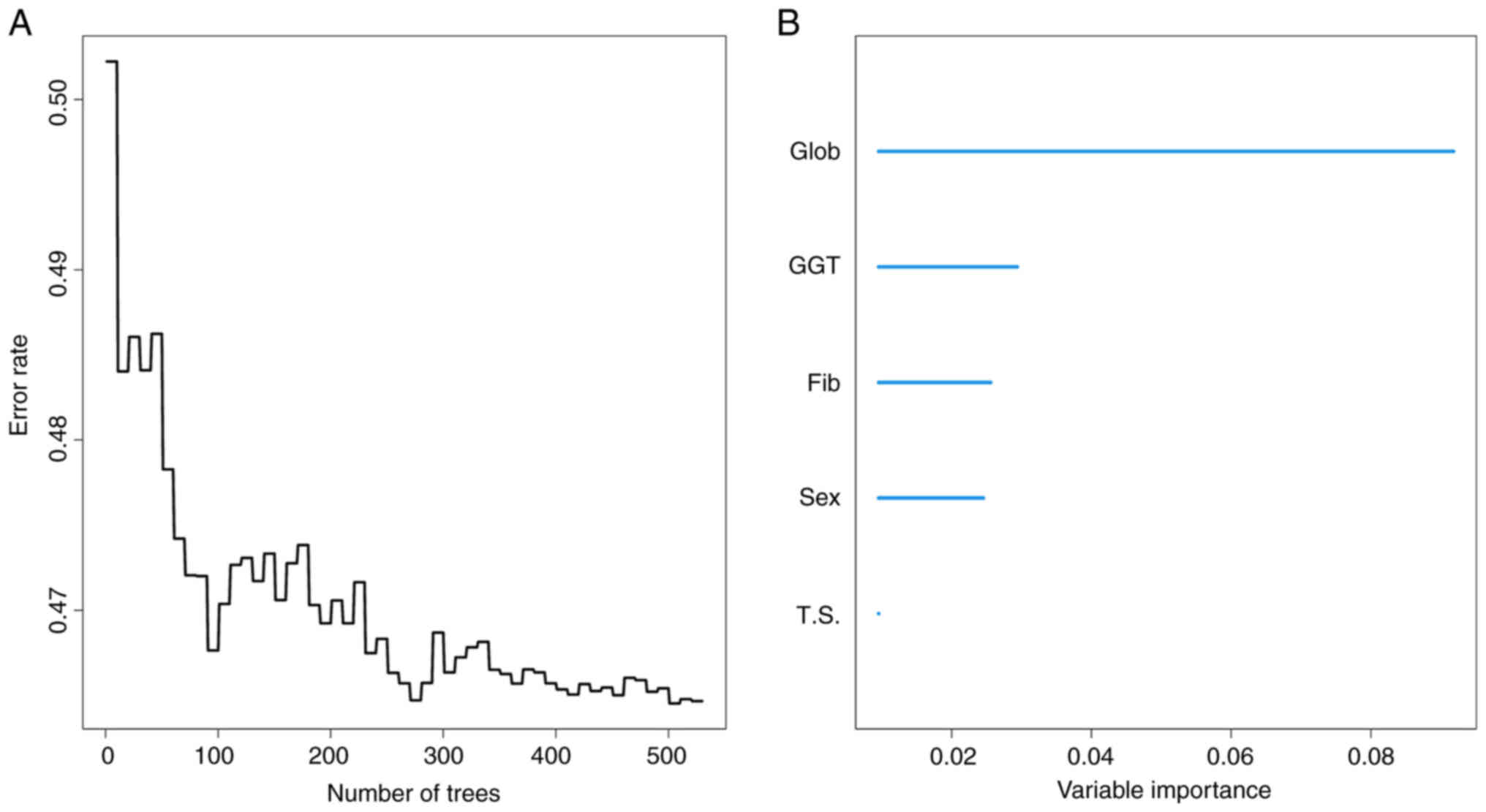
analyzed, tested and adjusted. Finally, RSF identified the top five
prognostic factors, including sex, tumor size, globulin,
gamma-glutamyl transferase (GGT) and fibrinogen (Fig. 1). The reliability of the
aforementioned variables was also confirmed by the multifactorial
COX regression (Table II). The
present study incorporated the aforementioned parameters to combine
into a new model and visualized the model with nomogram (Fig. 2). By calculating the total scores,
surgeons can easily obtain the probability of OS as predicted by
the nomogram.
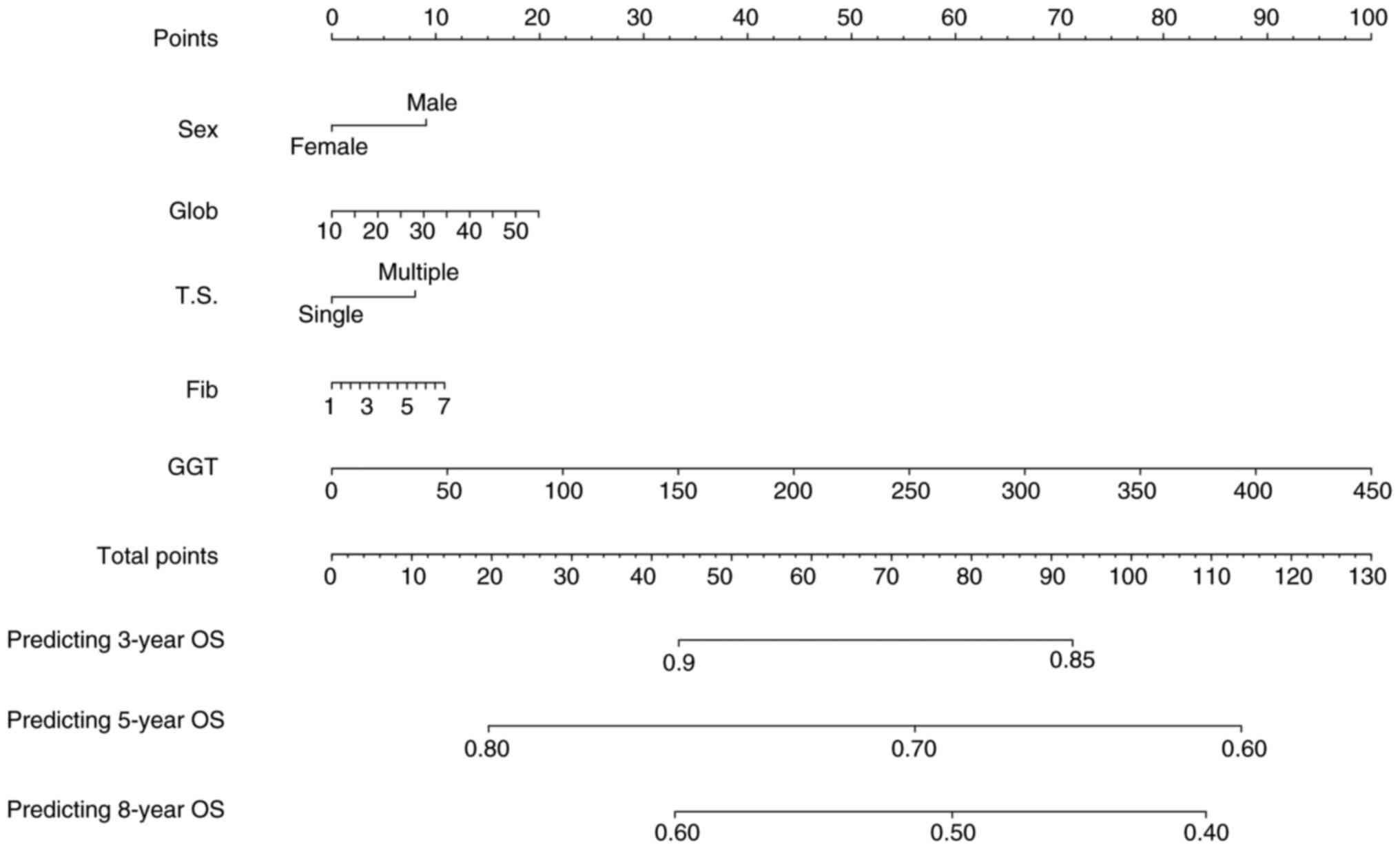 | Figure 2.Nomogram, including sex, Glob, T.S.,
Fib and GGT for 3-, 5- and 8-years OS in patients with AFP-NHCC
with dynamic AFP level changes. Glob, globulin; GGT, gamma-glutamyl
transferase; AFPN-HCC, α-fetoprotein-negative hepatocellular
carcinoma; Fib, fibrinogen; T.S., tumor size; OS, overall
survival. |
 | Table II.Multivariate Cox regression analysis
of risk factors for OS in the training cohort. |
Table II.
Multivariate Cox regression analysis
of risk factors for OS in the training cohort.
| Variable | P-value | HR | 95%CI |
|---|
| Sex | 0.004 | 0.653 | 0.491–0.869 |
| Tumor size | 0.018 | 1.919 | 1.659–3.282 |
| Glob | 0.015 | 1.431 | 1.073–1.729 |
| GGT | 0.032 | 1.671 | 1.098–1.983 |
| Fibrinogen | 0.035 | 1.114 | 1.095–1.306 |
Evaluation of the nomogram
The predictive performance of the nomogram was
assessed using the C-index, revealing a C-index of 0.72 (95% CI:
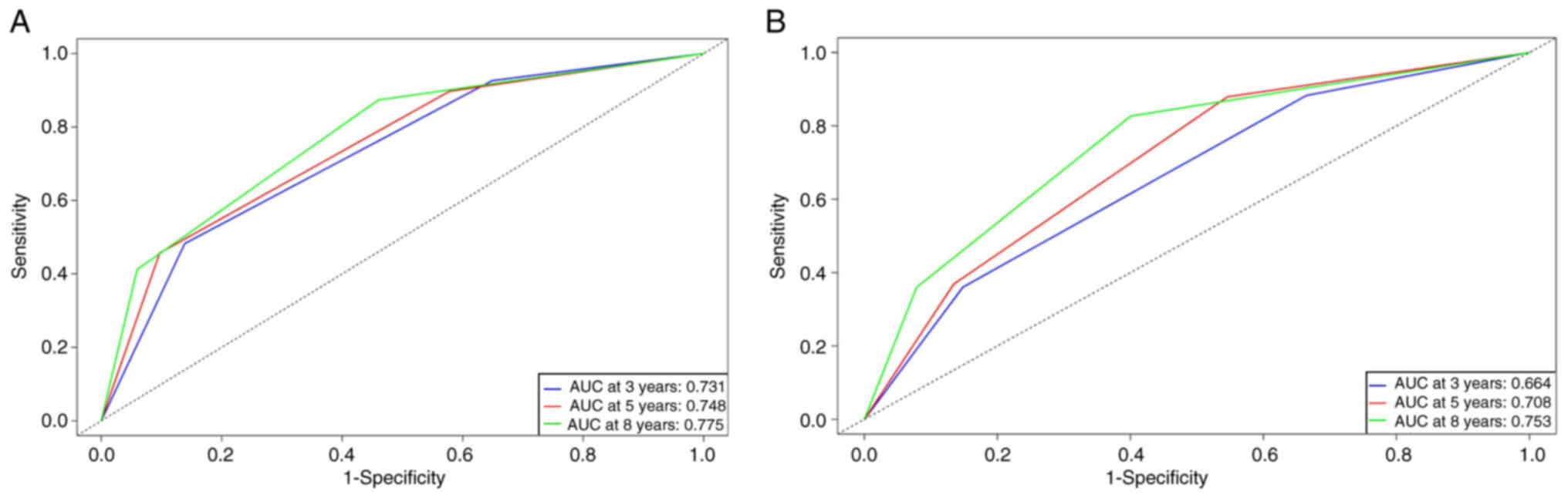
0.685–0.755) for the model-development group. ROC curve analysis
demonstrated area AUCs of 0.731, 0.748 and 0.775 at 3, 5 and 8
years, respectively (Fig. 3A).
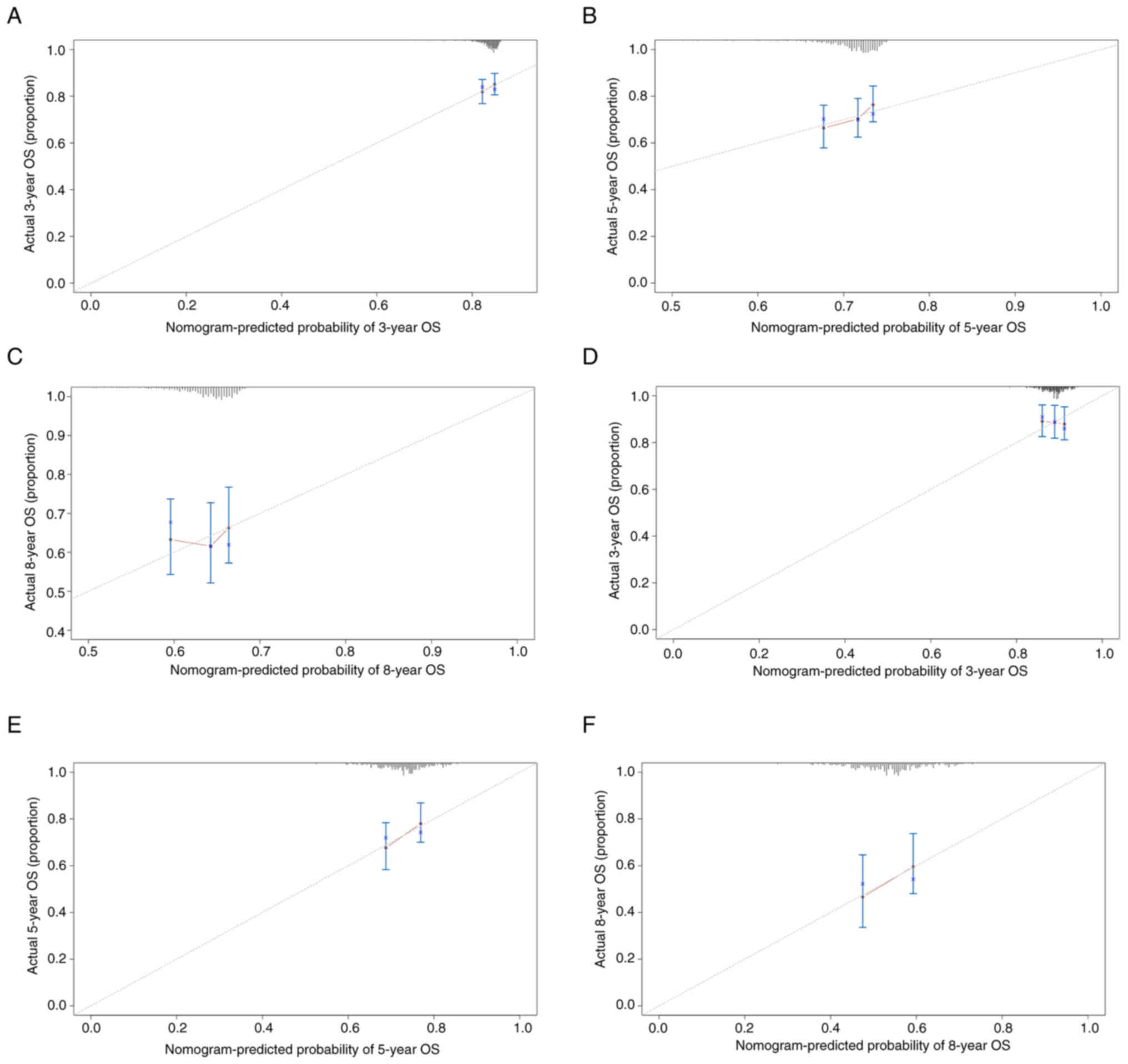
Calibration curves illustrated good consistency between predicted
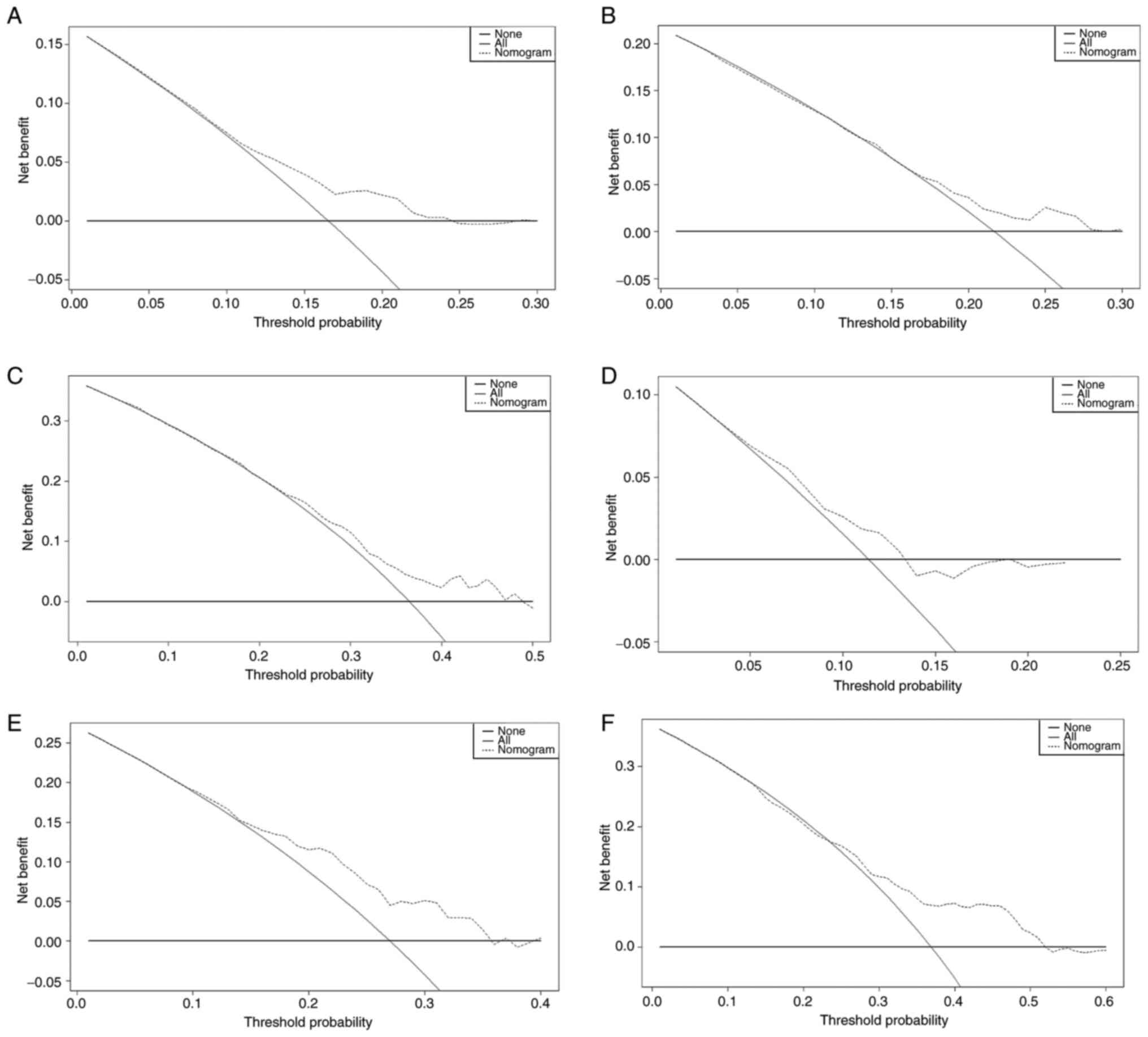
and actual values for the 3-, 5- and 8-year OS rates (Fig. 4A-C). DCA further indicated the
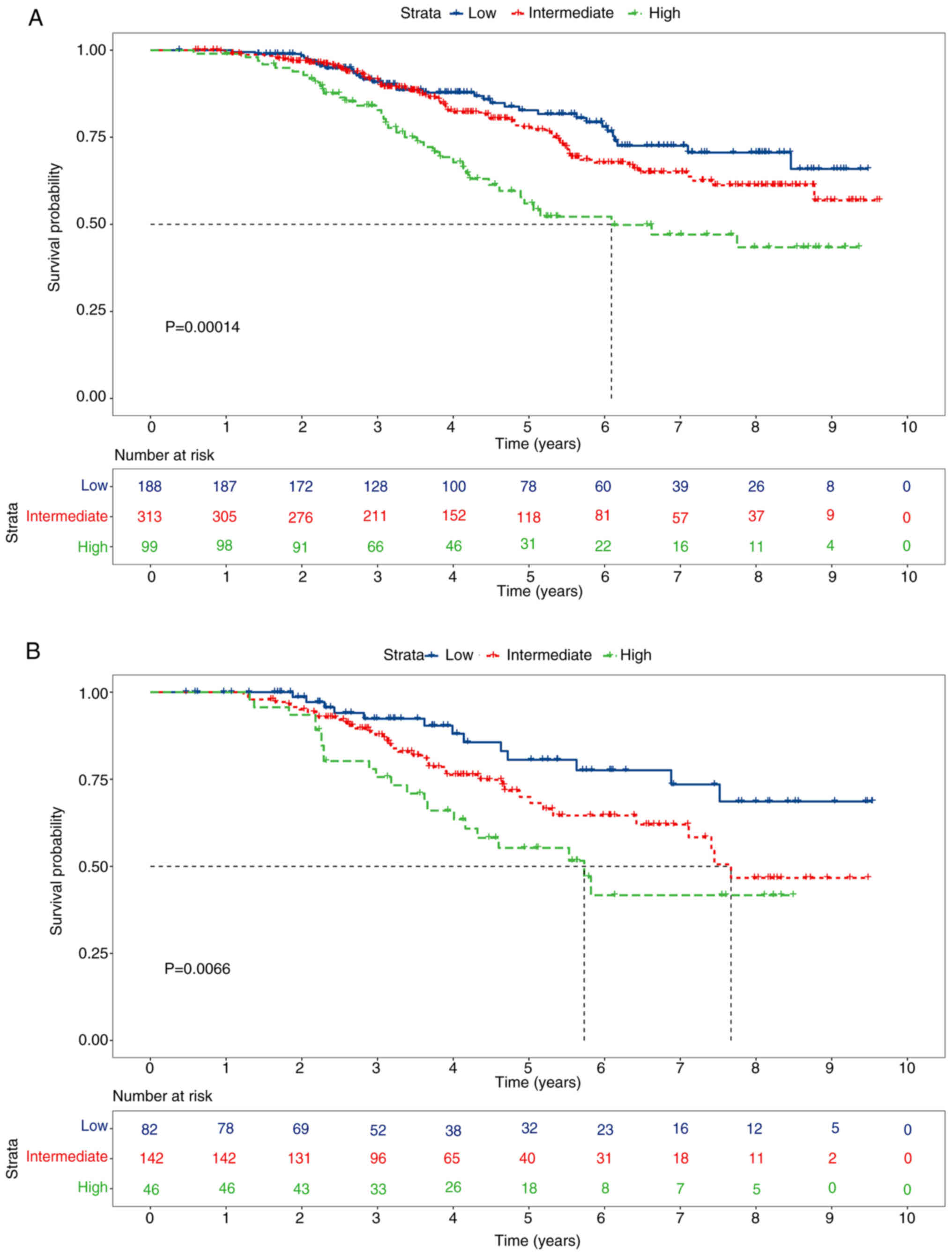
nomogram as a valuable predictive tool (Fig. 5A-C). Moreover, stratifying patients
into high-risk, intermediate-risk and low-risk groups showed
significant statistical differences in survival rates in the
training set (P<0.001; Fig.
6A).
Validation of the nomogram
To ascertain the reliability of the nomogram,
internal validation process was undertaken. The C-index for the
validation cohort was 0.664 (95% CI: 0.611–0.717), indicating that
the predicted results of the nomogram were consistent with the
actual observed outcomes, confirming the good discriminative
ability of the model. The corresponding AUCs for 3-, 5- and 8-year
ROC curves were 0.664, 0.708 and 0.753, respectively, attesting to
its strong classification capability (Fig. 3B). The calibration curves also
exhibited good alignment between predicted and observed values
(Fig. 4D-F). DCA curves validated
good clinical utility as well as a fine balance between benefits
and risks (Fig. 5D-F). Lastly, the
OS rates stratified by risk groups were consistent with those in
the model development group, also showing notable disparities
(P<0.01; Fig. 6B).
Discussion
Approximately 30% of HCC cases are AFP-NHCC,
representing a clinically distinct subgroup that is frequently
underdiagnosed owing to nonspecific clinical manifestations.
Consequently, the delayed diagnosis and treatment poses threat to
their survival (12,18). Among these patients, some exhibit
dynamic changes from AFP negativity to AFP positivity indicating a
unique population within patients with AFP-NHCC. AFP levels have
been associated with the pathological grading, progression and
prognosis of the patients, suggesting that this subgroup of
patients differ from AFP-negative patients (14,15).
After surgical resection for HCC, patients may sustain varying
degrees of damage to both the tumor and its surrounding tissues,
leading to inflammation. Additionally, the liver initiates a
regenerative process to repair the damaged areas following the
resection (19,20). These responses can both stimulate
hepatocyte proliferation and the secretion of AFP, ultimately
resulting in a temporary elevation of AFP levels post-surgery.
However, this is not indicative of a poor prognosis. Furthermore,
guidelines indicate that for AFP-positive liver cancer patients,
AFP levels typically return to normal within two months after
hepatic resection (21). To ensure
that fluctuations in AFP levels in AFP-negative HCC patients after
hepatic resection were not transient increases, the present study
established a two-month observation period as a basis for assessing
AFP level fluctuations. AFP levels, to a certain extent, reflect
the size of the tumor and their dynamic changes are related to the
disease status, serving as a sensitive indicator for assessing
treatment efficacy and prognosis (21–24).
Additionally, early-stage liver cancer or liver cancer confined to
a single lobe, with small lesions and no metastasis, generally
responds well to treatment. By contrast, advanced liver cancer,
characterized by larger lesions often accompanied by metastasis,
presents markedly greater treatment challenges. Despite various
treatment options such as immunotherapy and targeted therapy,
overall treatment outcomes remain limited and patient survival
rates are unsatisfactory (25–28).
Survival predictions for early-stage liver cancer can help doctors
devise more reasonable treatment plans and improve treatment
outcomes. However, for patients with advanced liver cancer, even
with survival predictions, the rapid progression of the disease and
poor treatment efficacy may lead to significant discrepancies
between predicted and actual survival outcomes. Furthermore,
clinical practice focusses more on early detection of disease
changes, enhancing treatment efficacy and improving patient
prognosis. Therefore, research on survival predictions for
early-stage liver cancer confined to a single lobe aligns more
closely with the needs of clinical practice. Therefore, the present
study aimed to focus on this special patient cohort and develop a
nomogram to predict their OS. Moreover, for these patients,
treatments such as liver transplantation and extended resection
should be considered and follow-up frequency should be increased
(29,30).
The nomogram developed in the present study
incorporated sex, tumor size, globulin, GGT and fibrinogen. It
assigned scores to each level of these key factors based on their
contribution to the outcome variable within the model. By
aggregating these scores, the total score was then translated into
the predicted OS probability using a function transformation
relationship. The model demonstrated robust predictive capability
through multidimensional validation. As evidenced by the
calibration curve and ROC analysis, the model displayed robust
diagnostic performance. DCA analysis also indicated satisfactory
predictive ability, while the variable availability made this model
user-friendly for practical clinical applications. KM analysis
further confirmed the ability of this model in clinical practice.
Stratifying patients based on total scores into low, intermediate
and high-risk groups revealed markedly distinct OS rates.
Previous studies (31–35)
have primarily focused on the prognosis of AFP-NHCC patients after
hepatectomy, neglecting the dynamic changes in AFP levels. To
address this gap, the present study developed a more accurate
predictive model for this subgroup to improve their prognosis. The
cohort was not only massive in scale but also had a long follow-up
period, lending greater credibility to the conclusions. In
addition, published reports (36–40)
have shown the trustworthiness of the aforementioned five
indicators in predicting OS for patients with HCC. Generally, male
patients have poorer prognoses. Beyond liver cancer, they are more
prone to non-reproductive system tumors and worse prognoses,
possibly due to factors related to Y chromosome genes and
testosterone (28,41,42).
Studies suggest that testosterone promotes CD8+ T cell exhaustion,
leading to faster tumor cell growth (43,44).
Additionally, another study indicates a correlation between higher
levels of Inc-FTX, a regulator transcribed from the X chromosome
inactivation center XIST, and longer prognosis in HCC patients.
Inc-FTX acts as a tumor suppressor, with higher expression levels
observed in the female liver (45).
Tumor size is a critical determinant of the 2-year postoperative
recurrence rate in isolated HCC. Specially, patients with a tumor
diameter >5 cm and AFP ≥20 ng/ml have a 4.5 times higher
mortality rate than those with a tumor diameter <5 cm (46,47).
Retrospective analysis of patients surviving postoperative HCC for
over 10 years found that isolated and small tumors are critical
factors for long-term postoperative survival (48). The globulin levels in HCC patients
are markedly elevated compared with healthy individuals. As HCC
continues to progress, it can activate the body's immune mechanism,
leading to the production of a large number of inflammatory
factors, further burdening the liver and affecting its normal
physiological functions. This leads to a further increase in
globulin levels, which in turn affects the patient's liver
function, thereby exacerbating HCC. GGT, a key enzyme associated
with liver metabolism, has recently been implicated in oxidative
stress, extracellular inflammation and tumor progression (49). Inflammation stimuli within or
surrounding HCC may induce abundant GGT production in hepatocytes
and the cancer cells themselves also synthesize GGT, further
increasing serum GGT levels. Moreira et al (50) demonstrated that serum GGT levels
increase with the progression of liver cancer and promote tumor
advancement in the male Wistar rat HCC animal model. Moreover, GGT
levels are closely linked to the prognosis of patients with
AFP-NHCC (51–53). AFP-low-level HCC patients, those
with high GGT levels, are more likely to experience lower survival
rates (54), in accordance with the
findings of the present study. Fibrinogen, a common
coagulation-related protein, apart from participating in blood
clotting, is closely associated with tumors (55). Studies (56–60)
reveal a significant increase in preoperative plasma fibrinogen in
various malignant tumors, closely correlating with tumor
progression, metastasis and prognosis. Elevated fibrinogen usually
implies a hypercoagulable and inflammatory state, which inevitably
affects the patient's postoperative recovery and prognosis,
prolongs the postoperative hospital stay and affects the OS of the
patient.
Limitations existed in the present study. First,
retrospective studies inevitably introduce selection bias,
mitigated to some extent by the ample sample size of the present
study. Second, although the model exhibited good internal
performance during validation, external validation in additional
cohorts is necessary to enhance the credibility and persuasiveness
of the model, as well as to validate its generalization ability.
Third, the present study did not include recurrence patterns or
surgery-related indicators, such as the extent of operation,
surgical margin, need of blood transfusion and tumor pathology.
More comprehensive indicators should be encompassed in any future
study. Nevertheless, the model still provided more timely treatment
guidance for patients with AFP-NHCC who exhibit dynamic AFP changes
due to the identification of high-risk patients in this
subgroup.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science and
Technology Major Project (grant nos. 2023ZD0502405 and
2023ZD0502402) and 2023 Young and middle-aged Talents Incubation
Project (Youth Innovation) of Beijing You'an Hospital, Capital
Medical University (grant no. BJYAYY-YN2023-13).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization and design were performed by RJ
and GL. Data collection was performed by QW, LS, GZ and ZC. Data
analysis and interpretation were conducted by QW, LS and GZ. The
manuscript was drafted by QW, ZC and LS. Critical review and
editing were carried out by RJ and GL. GL obtained funding.
Supervision was provided by RJ and GL. GL and RJ confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki and approved by the Ethics Committee of
Beijing You'an Hospital, Capital Medical University, China
(approval no. LL-2021-152-K) and followed the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards. The Institutional Review Board of Beijing You'an
Hospital, Capital Medical University, China waived informed consent
because of the retrospective nature of our study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Glossary
Abbreviations
Abbreviations:
|
OS
|
overall survival
|
|
AFP
|
α-fetoprotein
|
|
HCC
|
hepatocellular carcinoma
|
|
AFPN-HCC
|
AFP-negative HCC
|
|
RSF
|
random survival forest
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curves
|
|
DCA
|
decision curve analysis
|
|
KM
|
Kaplan-Meier
|
|
GGT
|
gamma-glutamyl transferase
|
|
TACE
|
transarterial chemoembolization
|
|
BCLC
|
Barcelona Clinic Liver Cancer
|
|
SD
|
standard deviation
|
|
C-index
|
concordance index
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marquardt JU, Andersen JB and Thorgeirsson
SS: Functional and genetic deconstruction of the cellular origin in
liver cancer. Nat Rev Cancer. 15:653–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gordon AC: Ectopic anus in the adult. Br J
Surg. 74:6541987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vitale A, Cabibbo G, Iavarone M, Viganò L,
Pinato DJ, Ponziani FR, Lai Q, Casadei-Gardini A, Celsa C, Galati
G, et al: Personalised management of patients with hepatocellular
carcinoma: A multiparametric therapeutic hierarchy concept. Lancet
Oncol. 24:e312–e322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Couri T and Pillai A: Goals and targets
for personalized therapy for HCC. Hepatol Int. 13:125–137. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
European Association for the Study of the
Liver, . EASL clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng W, Chen F, Li Y and Xu L: Development
of a clinical scoring model to predict the overall and relapse-free
survival of patients with hepatocellular carcinoma following a
hepatectomy. Mol Clin Oncol. 19:872023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roayaie S, Obeidat K, Sposito C, Mariani
L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M and
Mazzaferro V: Resection of hepatocellular cancer ≤2 cm: Results
from two Western centers. Hepatology. 57:1426–1435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song P, Tobe RG, Inagaki Y, Kokudo N,
Hasegawa K, Sugawara Y and Tang W: The management of hepatocellular
carcinoma around the world: A comparison of guidelines from 2001 to
2011. Liver Int. 32:1053–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
She S, Xiang Y, Yang M, Ding X, Liu X, Ma
L, Liu Q, Liu B, Lu Z, Li S, et al: C-reactive protein is a
biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int
J Oncol. 47:543–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farinati F, Marino D, De Giorgio M, Baldan
A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA,
Benvegnù L, et al: Diagnostic and prognostic role of
alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am
J Gastroenterol. 101:524–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cucchetti A, Piscaglia F, Grigioni AD,
Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF
and Pinna AD: Preoperative prediction of hepatocellular carcinoma
tumour grade and micro-vascular invasion by means of artificial
neural network: A pilot study. J Hepatol. 52:880–888. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan AWH, Zhong J, Berhane S, Toyoda H,
Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, et al:
Development of pre and post-operative models to predict early
recurrence of hepatocellular carcinoma after surgical resection. J
Hepatol. 69:1284–1293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moazzam Z, Alaimo L, Endo Y, Lima HA,
Shaikh CF, Ratti F, Marques HP, Cauchy F, Lam V, Poultsides GA, et
al: Variations in textbook oncologic outcomes after curative-intent
resection: Early versus intermediate hepatocellular carcinoma based
on barcelona clinic liver cancer criteria and child-pugh
classification. Ann Surg Oncol. 30:750–759. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
R Core Team R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: 2021, URL. http://www.R-project.org/
|
|
18
|
Wang M, Devarajan K, Singal AG, Marrero
JA, Dai J, Feng Z, Rinaudo JA, Srivastava S, Evans A, Hann HW, et
al: The doylestown algorithm: A test to improve the performance of
AFP in the detection of hepatocellular carcinoma. Cancer Prev Res
(Phila). 9:172–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz M: A biomathematical approach to
clinical tumor growth. Cancer. 14:1272–1294. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tateishi R, Shiina S, Yoshida H, Teratani
T, Obi S, Yamashiki N, Yoshida H, Akamatsu M, Kawabe T and Omata M:
Prediction of recurrence of hepatocellular carcinoma after curative
ablation using three tumor markers. Hepatology. 44:1518–1527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen T, Jin C, Facciorusso A, Donadon M,
Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, et al:
Multidisciplinary management of recurrent and metastatic
hepatocellular carcinoma after resection: An international expert
consensus. Hepatobiliary Surg Nutr. 7:353–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peterson ML, Ma C and Spear BT: Zhx2 and
Zbtb20: Novel regulators of postnatal alpha-fetoprotein repression
and their potential role in gene reactivation during liver cancer.
Semin Cancer Biol. 21:21–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rebouissou S and Nault JC: Advances in
molecular classification and precision oncology in hepatocellular
carcinoma. J Hepatol. 72:215–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W and Wei C: Advances in the early
diagnosis of hepatocellular carcinoma. Genes Dis. 7:308–319. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singal AG, Kanwal F and Llovet JM: Global
trends in hepatocellular carcinoma epidemiology: Implications for
screening, prevention and therapy. Nat Rev Clin Oncol. 20:864–884.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 Practice
guidance by the American association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu X, Li S, Xu Y, Zhang Y, Ma W, Liang C,
Lu H, Ji Y, Liu C, Chen D and Li J: Androgen maintains intestinal
homeostasis by inhibiting BMP signaling via intestinal stromal
cells. Stem Cell Reports. 18:4102023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrer-Fàbrega J, Forner A, Liccioni A,
Miquel R, Molina V, Navasa M, Fondevila C, García-Valdecasas JC,
Bruix J and Fuster J: Prospective validation of ab initio liver
transplantation in hepatocellular carcinoma upon detection of risk
factors for recurrence after resection. Hepatology. 63:839–849.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsilimigras DI, Sahara K, Moris D, Hyer
JM, Paredes AZ, Bagante F, Merath K, Farooq AS, Ratti F, Marques
HP, et al: Effect of surgical margin width on patterns of
recurrence among patients undergoing R0 hepatectomy for T1
hepatocellular carcinoma: An international multi-institutional
analysis. J Gastrointest Surg. 24:1552–1560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Han L, Xiao B, Li X and Ye Z: A
predictive nomogram of early recurrence for patients with
AFP-negative hepatocellular carcinoma underwent curative resection.
Diagnostics (Basel). 12:10732022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan X, Li Y, Qin W, Liao J, Fan J, Xie Y,
Wang Z, Li S and Liao W: Radiomics model based on contrast-enhanced
computed tomography imaging for early recurrence monitoring after
radical resection of AFP-negative hepatocellular carcinoma. BMC
Cancer. 24:7002024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gan W, Huang JL, Zhang MX, Fu YP, Yi Y,
Jing CY, Fan J, Zhou J and Qiu SJ: New nomogram predicts the
recurrence of hepatocellular carcinoma in patients with negative
preoperative serum AFP subjected to curative resection. J Surg
Oncol. 117:1540–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao S, Yu X, Shan Y, Fan R, Wu S and Lu C:
Albumin-bilirubin (ALBI) and monocyte to lymphocyte ratio
(MLR)-based nomogram model to predict tumor recurrence of
AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma.
8:1355–1365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang J, Liu FC, Li L, Zhou WP, Jiang BG
and Pan ZY: Nomograms to predict the long-time prognosis in
patients with alpha-fetoprotein negative hepatocellular carcinoma
following radical resection. Cancer Med. 9:2791–2802. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang D, Hanna DL, Usher J, LoCoco J,
Chaudhari P, Lenz HJ, Setiawan VW and El-Khoueiry A: Impact of sex
on the survival of patients with hepatocellular carcinoma: A
Surveillance, Epidemiology, and End Results analysis. Cancer.
120:3707–3716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goh BK, Teo JY, Chan CY, Lee SY, Jeyaraj
P, Cheow PC, Chow PK, Ooi LL and Chung AY: Importance of tumor size
as a prognostic factor after partial liver resection for solitary
hepatocellular carcinoma: Implications on the current AJCC staging
system. J Surg Oncol. 113:89–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB,
Ha TY, Song GW, Jung DH and Lee SG: The impact of tumor size on
long-term survival outcomes after resection of solitary
hepatocellular carcinoma: Single-institution experience with 2558
patients. J Gastrointest Surg. 19:1281–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Li Z, Hao S, Wang J, Chen W, Dai S,
Hou Z, Chen B, Zhang Y and Liu D: Inversed albumin-to-globulin
ratio and underlying liver disease severity as a prognostic factor
for survival in hepatocellular carcinoma patients undergoing
transarterial chemoembolization. Diagn Interv Radiol. 29:520–528.
2023.PubMed/NCBI
|
|
40
|
Yan H, Chen S, Qiong Y and Cai L:
Preoperative prealbumin-to-fibrinogen ratio to predict survival
outcomes in hepatocellular carcinoma patients after hepatic
resection. J Med Biochem. 41:290–298. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Lan Z, Liao W, Horner JW, Xu X, Liu
J, Yoshihama Y, Jiang S, Shim HS, Slotnik M, et al: Histone
demethylase KDM5D upregulation drives sex differences in colon
cancer. Nature. 619:632–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdel-Hafiz HA, Schafer JM, Chen X, Xiao
T, Gauntner TD, Li Z and Theodorescu D: Y chromosome loss in cancer
drives growth by evasion of adaptive immunity. Nature. 619:624–631.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kwon H, Schafer JM, Song NJ, Kaneko S, Li
A, Xiao T, Ma A, Allen C, Das K, Zhou L, et al: Androgen conspires
with the CD8+ T cell exhaustion program and contributes
to sex bias in cancer. Sci Immunol. 7:eabq26302022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Cheng L, Gao C, Chen J, Liao S,
Zheng Y, Xu L, He J, Wang D, Fang Z, et al: Androgen signaling
contributes to sex differences in cancer by inhibiting NF-κB
activation in T cells and suppressing antitumor immunity. Cancer
Res. 83:906–921. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu F, Yuan JH, Huang JF, Yang F, Wang TT,
Ma JZ, Zhang L, Zhou CC, Wang F, Yu J, et al: Long noncoding RNA
FTX inhibits hepatocellular carcinoma proliferation and metastasis
by binding MCM2 and miR-374a. Oncogene. 35:5422–5434. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shinkawa H, Tanaka S, Takemura S, Ishihara
T, Yamamoto K and Kubo S: Tumor size drives the prognosis after
hepatic resection of solitary hepatocellular carcinoma without
vascular invasion. J Gastrointest Surg. 24:1040–1048. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dai CY, Lin CY, Tsai PC, Lin PY, Yeh ML,
Huang CF, Chang WT, Huang JF, Yu ML and Chen YL: Impact of tumor
size on the prognosis of hepatocellular carcinoma in patients who
underwent liver resection. J Chin Med Assoc. 81:155–163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shimada S, Kamiyama T, Orimo T, Nagatsu A,
Asahi Y, Sakamoto Y, Kamachi H and Taketomi A: Long-term prognostic
factors of patients with hepatocellular carcinoma who survive over
10 years after hepatectomy. J Surg Oncol. 121:1209–1217. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brennan PN, Dillon JF and Tapper EB:
Gamma-glutamyl transferase (γ-GT)-an old dog with new tricks? Liver
Int. 42:9–15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moreira AJ, Rodrigues GR, Bona S, Fratta
LX, Weber GR, Picada JN, Dos Santos JL, Cerski CT, Marroni CA and
Marroni NP: Ductular reaction, cytokeratin 7 positivity, and
gamma-glutamyl transferase in multistage hepatocarcinogenesis in
rats. Protoplasma. 254:911–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McCaffrey P, Gilmore DH and Beringer TR:
Relief care and risk of death in psychogeriatric patients. BMJ.
298:15221989. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Z, Song P, Xia J, Inagaki Y, Tang W
and Kokudo N: Can gamma-glutamyl transferase levels contribute to a
better prognosis for patients with hepatocellular carcinoma? Drug
Discov Ther. 8:134–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang L, Mo Z, Hu Z, Zhang L, Qin S, Qin X
and Li S: Diagnostic value of fibrinogen to prealbumin ratio and
gamma-glutamyl transpeptidase to platelet ratio in the progression
of AFP-negative hepatocellular carcinoma. Cancer Cell Int.
20:772020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carr BI, Guerra V, Giannini EG, Farinati
F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M,
Borzio F, et al: Low alpha-fetoprotein HCC and the role of GGTP.
Int J Biol Markers. 29:e395–e402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Son HJ, Park JW, Chang HJ, Kim DY, Kim BC,
Kim SY, Park SC, Choi HS and Oh JH: Preoperative plasma
hyperfibrinogenemia is predictive of poor prognosis in patients
with nonmetastatic colon cancer. Ann Surg Oncol. 20:2908–2913.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang HG, Li J, Shi SB, Chen P, Ge LP,
Jiang Q and Tang XP: Value of fibrinogen and D-dimer in predicting
recurrence and metastasis after radical surgery for non-small cell
lung cancer. Med Oncol. 31:222014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hefler-Frischmuth K, Lafleur J, Hefler L,
Polterauer S, Seebacher V, Reinthaller A and Grimm C: Plasma
fibrinogen levels in patients with benign and malignant ovarian
tumors. Gynecol Oncol. 136:567–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M and
Tajiri H: Elevated plasma fibrinogen levels are associated with a
poor prognosis in patients with hepatocellular carcinoma. Oncology.
85:269–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tanaka N, Kikuchi E, Matsumoto K, Hayakawa
N, Ide H, Miyajima A, Nakamura S and Oya M: Prognostic value of
plasma fibrinogen levels in patients with localized upper tract
urothelial carcinoma. BJU Int. 111:857–864. 2013. View Article : Google Scholar : PubMed/NCBI
|