Introduction
Lung cancer remains a leading cause of mortality
worldwide, with non-small cell lung cancer (NSCLC) accounting for
~85% of all lung cancer cases (1).
Over the past decades, notable advancements have been made in
understanding the molecular biology of NSCLC, particularly the
identification of key driver mutations such as those in the
epidermal growth factor receptor (EGFR) gene (2). Historically, research breakthroughs in
the early 2000s led to the development of targeted therapies,
beginning with first-generation EGFR tyrosine kinase inhibitors
(TKIs) such as erlotinib and gefitinib, which showed marked
efficacy in treating patients with common EGFR mutations, such as
exon 19 in-frame deletion and exon 21 L858R alterations
substitutions (3,4). However, rarer EGFR mutations, such as
EGFR exon 20 insertions, which represent 4–10% of EGFR mutations in
NSCLC, have proven more resistant to these therapies, leading to
worse clinical outcomes (5,6). These mutations exhibit inherent
resistance to first- and second-generation EGFR-TKIs, and even
third-generation inhibitors such as osimertinib, which have been
effective in overcoming resistance to earlier drugs, have shown
limited success in addressing exon 20 insertions in clinical trials
(7).
Therefore, newer EGFR-TKIs have been developed to
target this unique mutation profile. Mobocertinib (also known as
TAK-788) is an oral EGFR-TKI and was the first and only agent
approved globally for the treatment of advanced exon 20 insertion
mutant NSCLC. Early clinical trials reported that treatment with
160 mg mobocertinib once daily was associated with an overall
response rate (ORR) of 28%, a disease control rate (DCR) of 78%, a
mean progression-free survival (PFS) of 7.3 months and an overall
survival (OS) of 20.2 months (8–10).
However, it did not reach the primary endpoint in the phase III
study, although no new safety signals were observed. Thus, the U.S.
Food and Drug Administration (FDA) and Takeda, the sponsor of
mobocertinib, voluntarily withdrew the drug for the indicated
patients in October 2023 (11).
Nevertheless, emerging medications such as
sunvozertinib (DZD9008) and furmonertinib (AST2818) are potential
candidates as they have been reported to inhibit EGFR exon 20
insertion mutations with an acceptable safety profile in initial
studies (12,13). However, as the comprehensive data on
the efficacy and safety of these emerging drugs in treating
patients with NSCLC with EGFR exon 20 insertions are still lacking,
the present study aimed to perform a systematic review and
meta-analysis to evaluate the efficacy and safety outcomes of the
key emerging EGFR-TKIs, namely sunvozertinib, furmonertinib,
poziotinib, amivantamab, zipalertinib, befotertinib, YK-029A,
BEBT-109 and BLU-451, for the treatment of patients with NSCLC with
EGFR exon 20 insertion mutations.
Materials and methods
Protocol
The present systematic review and meta-analysis was
performed following the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses guidelines (14). The present review protocol was
registered in the PROSPERO database (registration ID no.
CRD42023472851).
Search strategy and criteria
A total of four databases [the Cochrane Library
(https://www.cochranelibrary.com/), Web
of Science (https://www.webofscience.com/wos/), PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Embase
(https://www.embase.com/)] were used for relevant
literature searches due to their comprehensive coverage of
biomedical literature and their relevance to oncology research. The
main search items to refine the search included ‘EGFR-TKIs’,
‘Zipalertinib’, ‘Sunvozertinib’, ‘Furmonertinib’, ‘Poziotinib’,
‘Amivantamab’, ‘Befotertinib’, ‘YK-029A’, ‘BEBT-109’, ‘BLU-451’,
‘NSCLC’ and ‘EGFR exon 20 insertions’. The search covered the
period from September 2019 to the study conduction date, April
2024, representing a timeframe during which third-generation
EGFR-TKIs emerged as promising candidates in clinical trials. The
April 2024 endpoint represents the intended cut-off for data
collection at the time of planning the present meta-analysis.
Whilst no additional data were included beyond the actual
completion of the present study, this timeframe ensured a
comprehensive review of relevant clinical trials within the
predefined scope. Detailed search queries were tailored for each
database, considering their specific search algorithms and indexing
practices (Table I).
 | Table I.Detailed search queries used for the
literature search. |
Table I.
Detailed search queries used for the
literature search.
| Database | Search
strategy | Records, n |
|---|
| PubMed | #((‘EGFR-tyrosine
kinase inhibitor’[All Fields] OR ‘EGFR-TKI’[All Fields] OR
‘Zipalertinib’ [All Fields] OR ‘Sunvozertinib’[All Fields] OR
‘Furmonertinib’[All Fields] OR ‘Poziotinib’ [All Fields] OR
‘Amivantamab’[All Fields] OR ‘Befotertinib’[All Fields]) OR
‘YK-029A’ [All Fields]) OR ‘BEBT-109’[All Fields]) OR ‘BLU-451’[All
Fields]) AND (‘Non-small cell lung cancer’[All Fields] OR
‘NSCLC’[All Fields]) AND ‘exon 20’[All Fields] AND (‘clinical
trial’[Publication Type] OR ‘randomized controlled
trial’[Publication Type])) AND ((y_5[Filter]) AND
(clinicaltrial[Filter] OR randomized controlled
trial[Filter]))# | 15 |
| Cochrane | #(‘EGFR tyrosine
kinase inhibitor’ OR ‘EGFR-TKI’ OR ‘Zipalertinib’ OR
‘Sunvozertinib’ OR ‘Furmonertinib’ OR ‘Poziotinib’ OR ‘Amivantamab’
OR ‘Befotertinib’ OR ‘YK-029A’ OR ‘BEBT-109’ OR ‘BLU-451’):ti, ab,
kw AND ((‘Non-small cell lung cancer’ OR ‘NSCLC’) AND (‘exon
20’)):ti,ab,kw AND English:la (Word variations have been searched)
with Publication Year from 2019 to 2024, with Cochrane Library
publication date Between Sep 2019 and Apr 2024, in Trials (Word
variations have been searched)# | 61 |
| Embase | #(‘egfr-tyrosine
kinase inhibitor’ OR ‘egfr-tki’ OR ‘Zipalertinib’/exp OR
‘Sunvozertinib’ OR ‘Furmonertinib’/exp OR ‘Poziotinib’ OR
‘Amivantamab’/exp OR ‘Befotertinib’ OR ‘YK-029A’/exp OR
‘BEBT-109’/exp OR ‘BLU-451’) AND (‘non-small cell lung cancer’/exp
OR ‘non-small cell lung cancer’ OR ‘nsclc’) AND (‘exon 20’/exp OR
‘exon 20’) AND (2019:py OR 2020:py OR 2021:py OR 2022:py OR 2023:py
OR 2024:py) AND (‘clinical article’/de OR ‘clinical study’/de OR
‘clinical trial’/de OR ‘clinical trial topic’/de OR ‘cohort
analysis’/de OR ‘comparative effectiveness’/de OR ‘comparative
study’/de OR ‘controlled clinical trial’/de OR ‘controlled
study’/de OR ‘major clinical study’/de OR ‘observational study’/de
OR ‘phase 1 clinical trial’/de OR ‘phase 1 clinical trial topic’/de
OR ‘phase 2 clinical trial’/de OR ‘phase 2 clinical trial topic’/de
OR ‘phase 3 clinical trial’/de OR ‘phase 3 clinical trial topic’/de
OR ‘prospective study’/de OR ‘randomized controlled trial’/de OR
‘randomized controlled trial topic’/de) AND (‘article’/it OR
‘article in press’/it) AND (‘case control study’/de OR ‘clinical
trial’/de OR ‘comparative effectiveness’/de OR ‘comparative
study’/de OR ‘controlled clinical trial’/de OR ‘controlled
study’/de OR ‘longitudinal study’/de OR ‘major clinical study’/de
OR ‘multicenter study’/de OR ‘observational study’/de OR ‘phase 1
clinical trial’/de OR ‘phase 2 clinical trial’/de OR ‘phase 3
clinical trial’/de OR ‘prospective study’/de OR ‘randomized
controlled trial topic’/de OR ‘retrospective study’/de)# | 74 |
| Web of Science | #((((ALL=(‘EGFR
tyrosine kinase inhibitor’ OR ‘EGFR-TKI’ OR ‘Zipalertinib’ OR
‘Sunvozertinib’ OR ‘Furmonertinib’ OR ‘Poziotinib’ OR ‘Amivantamab’
OR ‘Befotertinib’ OR ‘YK-029A’ OR ‘BEBT-109’ OR ‘BLU-451’)) AND
ALL=((‘Non-small cell lung cancer’ OR ‘NSCLC’) AND ‘exon 20’)) AND
LA=(English)) AND DOP=(2019-09-01/2024-04-1)) AND
DT=(Article)# | 76 |
Studies were included if the following inclusion
criteria were met: i) Population: Patients had a confirmed
diagnosis of EGFR exon 20 insertion mutation- or T790M
mutation-positive NSCLC; ii) intervention: Patients were treated
with third-generation EGFR-TKIs including zipalertinib,
sunvozertinib, furmonertinib, poziotinib, amivantamab,
befotertinib, YK-029A, BEBT-109 and BLU-451. Mobocertinib was not
included as its further development program was withdrawn due to
the FDA withdrawal (11); iii)
study type: Clinical trials published in English, including
randomised controlled trials and single arm prospective trials, and
sub-group analyses of previously published studies; and iv)
outcomes: Clinical tumour outcomes including ORR, DCR, PFS, OS and
treatment relevant adverse events (TRAEs) of grade ≥3. The tumour
response was evaluated using the Response Evaluation Criteria in
Solid Tumours version 1.1 (15).
Safety concerns were assessed for their incidence and severity
using the Common Terminology Criteria for Adverse Events (16). The exclusion criteria were as
follows: i) Studies focusing exclusively on osimertinib, as it is
an established treatment and not the primary focus of the present
review; ii) preclinical or animal studies; iii) case reports,
reviews, editorials or other non-original research articles.
Systematic reviews and meta-analysis were only used for reference
cross-checking; and iv) publications not available in English.
Data extraction and quality
assessment
A total of two investigators independently extracted
all required data for the included studies and subsequently
performed the quality assessment of the studies. The extracted data
included the authors, year of publication, study phase and
registration number, sample size, interventions and reported
outcomes. Efficacy outcomes included ORR, DCR, PFS and OS. The
safety outcome was the incidence of TRAEs of grade ≥3. The
Newcastle Ottawa Scale (NOS) was used to evaluate the quality of
prospective cohort studies and indirect comparison studies
(17).
Meta-analysis
All data in the present meta-analysis were analysed
using STATA 17.0 software (StataCorp LP). Heterogeneity was
measured using the χ2 test and I2 statistic.
P<0.05 was considered to indicate a statistically significant
difference. As a random-effects model accounts for variability
between studies in a more flexible and comprehensive way than a
fixed-effects model, a random-effects model was performed in the
present study. Disease-free survival (DFS) was collected as a
continuous variable and ultimately consolidated into a single DFS
group, presented as the median with its 95% confidence interval
(CI). Several studies reported the confirmed ORR and they were
analysed as the ORR in the present meta-analysis. Funnel plots and
Egger's test were used to quantitatively analyse publication bias
(18,19).
Results
Literature selection and study
characteristics
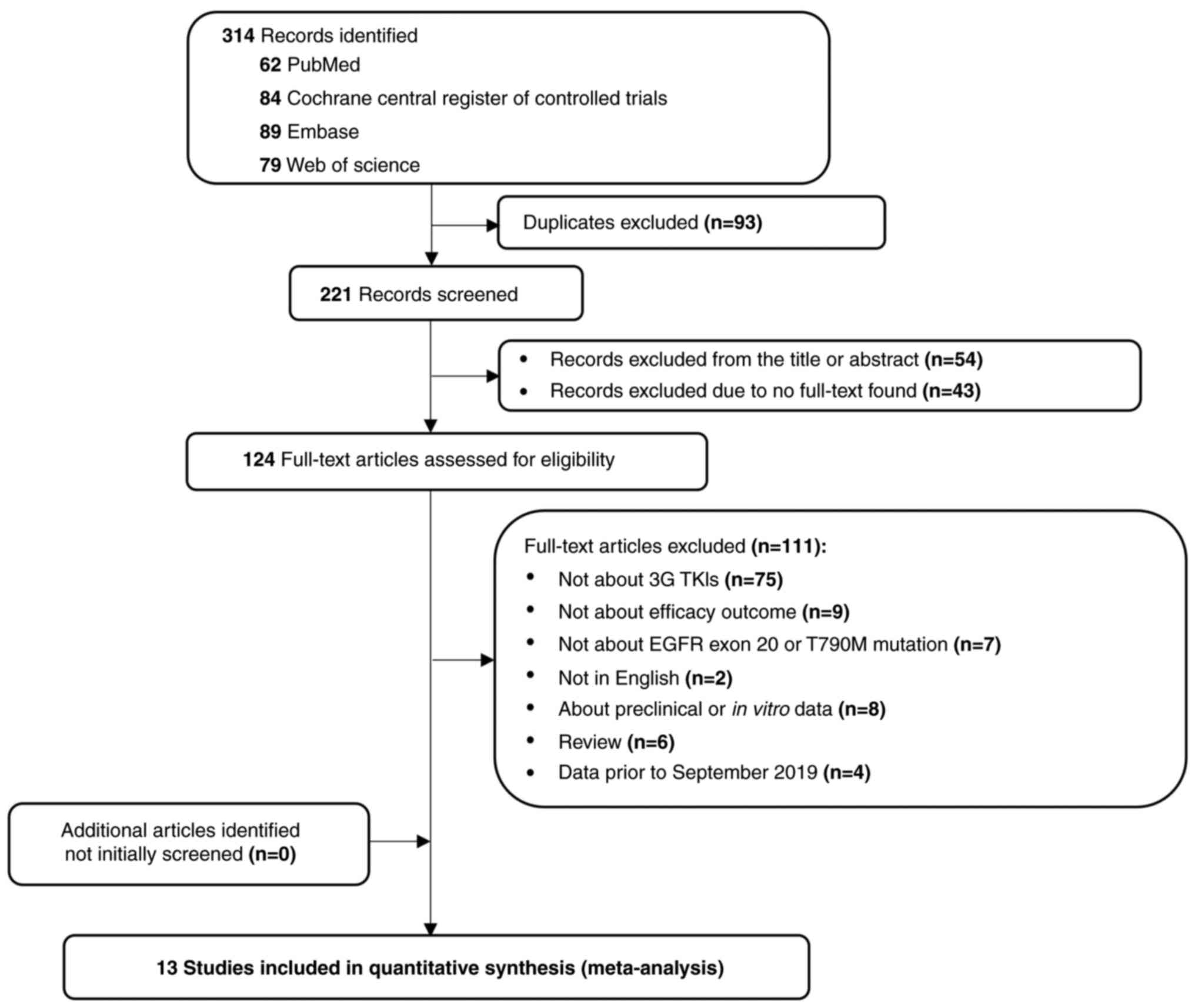
The search across the PubMed, Cochrane Library,
Embase and Web of Science databases yielded a total of 314
articles. After initial screening, 190 were excluded due to
duplicates or irrelevance, leaving 124 articles eligible for
full-text review. Following a rigorous evaluation, 13 studies were
ultimately included in the systematic review and meta-analysis
(13,20–31)
(Fig. 1). The included studies were
published between 2021 and 2024 and encompassed a total of 1,642
patients diagnosed with NSCLC with EGFR exon 20 insertion
mutations. The characteristics of the selected studies are
summarized in Table II. The
included studies mainly consisted of Phase II trials (13,22,24–28),
accounting for 7/13 (53.8%), followed by Phase I trials (20,23,30,31),
accounting for 4/13 (30.8%). The study design included 12 single
arm studies and one randomized controlled trial; however, only the
results of amivantamab were extracted from this randomized
controlled trial. Most of the included literature reported PFS
(13,20,23,28,29),
but due to insufficient follow-up time, only 3 articles reported OS
(20,22,28).
No efficacy or safety data on zipalertinib or BLU-451 were
reported.
 | Table II.Characteristics of clinical studies
of patients with non-small cell lung cancer with EGFR exon 20
insertions using emerging third-generation EGFR-tyrosine kinase
inhibitors. |
Table II.
Characteristics of clinical studies
of patients with non-small cell lung cancer with EGFR exon 20
insertions using emerging third-generation EGFR-tyrosine kinase
inhibitors.
| First author/s,
year | Phase | Registration
no. | Study design | Intervention | Sample size | ORR, % | Median PFS, months
(95% CI) | Median OS, months
(95% CI) | (Refs.) |
|---|
| Park et al,
2021 | I | NCT02609776 | Single arm | Amivantamab | 81 | 40 | 8.3 (6.5–10.9) | 22.8 (14.6-not
reached) | (20) |
| Girard et
al, 2023 | III | NCT04538664 | RCT | Amivantamab | 153 | 73 | 11.4
(9.8–13.7) | NR | (21) |
| Lu et al,
2022 | II | NCT04206072 | Single arm | Befotertinib 50
mg/day | 176 | 54 | 11.0
(9.6–12.5) | 23.9
(21.1–27.1) | (22) |
|
|
|
|
| Befotertinib 100
mg/day | 290 | 65.9 | 12.5
(11.1–13.8) | NR |
|
| Shi et al,
2021 | II | NCT03452592 | Single arm | Furmonertinib | 220 | 74.1 | 9.6 (8.2–9.7) | NR | (13) |
| Zeng et al,
2024 | I | CTR20192575 | Single arm | BEBT-109 | 18 | 44.4 | 8.3 (1.3–14.7) | NR | (23) |
| Wang et al,
2024 | II | NCT05712902 | Single arm | Sunvozertinib | 97 | 61 | 9.7 | NR | (24) |
| Le et al,
2021 | II | NCT03318939 | Single arm | Poziotinib | 95 | 14.7 | NR | NR | (25) |
| Cornelissen et
al, 2021 | II | NCT03318939 | Single arm | Poziotinib | 205 | 20.5 | NR | NR | (26) |
| Sacher et
al, 2021 | II | NCT03318939 | Single arm | Poziotinib | 79 | 27.8 | 7.2 | NR | (27) |
| Elamin et
al, 2022 | II | NCT03066206 | Single arm | Poziotinib | 50 | 32.0 | 5.5 (5.4–10.4) | 19.2
(11.8–24.1) | (28) |
| Piotrowska et
al, 2023 | I/II | NCT04036682 | Single arm | Zipalertinib | 73 | 38.4 | 10.0
(6.0–12.0) | NR | (29) |
| Duan et al,
2024 | I | NCT05767866 | Single arm | YK-029A | 26 | 73.1 | 9.3 | NR | (30) |
| Han et al,
2023 | I | NCT04858958 | Single arm | Furmonertinib
treatment-naïve 240 mg/day | 30 | 69.0 | 10.7 | NR | (31) |
|
|
|
|
| Furmonertinib
previously treated 240 mg/day | 24 | 50.0 | 7.0 | NR |
|
|
|
|
|
| Furmonertinib
previously treated 160 mg/day | 25 | 40.9 | 5.8 | NR |
|
Quality assessment
To maintain the integrity and reliability of the
findings, the risk-of-bias of each study was assessed. The NOS was
used to evaluate the quality of prospective cohort studies and
indirect comparison studies. Each study scored above six on the
nine-point system, thereby indicating moderate-to-high quality. The
quality assessment details are presented in Table III.
 | Table III.Quality assessment of the studies
included in the meta-analysis. |
Table III.
Quality assessment of the studies
included in the meta-analysis.
|
| Selection of
cohorts |
|
|
|
|
|
|
|---|
|
|
|
| Outcome |
|
|
|---|
| First author/s,
year | Representativeness
of the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Demonstration that
the outcome of interest was not present at the start of the
study | Comparability of
cohorts on the basis of the design or analysis |
|
|
|
|---|
| Assessment of
outcome | Was follow-up long
enough for outcomes to occur | Adequacy of follow
up of cohorts | NOS score | (Refs.) |
|---|
| Park et al,
2021 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (20) |
| Girard et
al, 2023 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (21) |
| Lu et al,
2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | (22) |
| Shi et al,
2021 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (13) |
| Zeng et al,
2024 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (23) |
| Wang et al,
2024 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 | (24) |
| Le et al,
2021 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 | (25) |
| Cornelissen et
al, 2021 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 | (26) |
| Sacher et
al, 2021 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 | (27) |
| Elamin et
al, 2022 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (28) |
| Piotrowska et
al, 2023 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | (29) |
| Duan et al,
2024 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 | (30) |
| Han et al,
2023 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 | (31) |
Tumour response
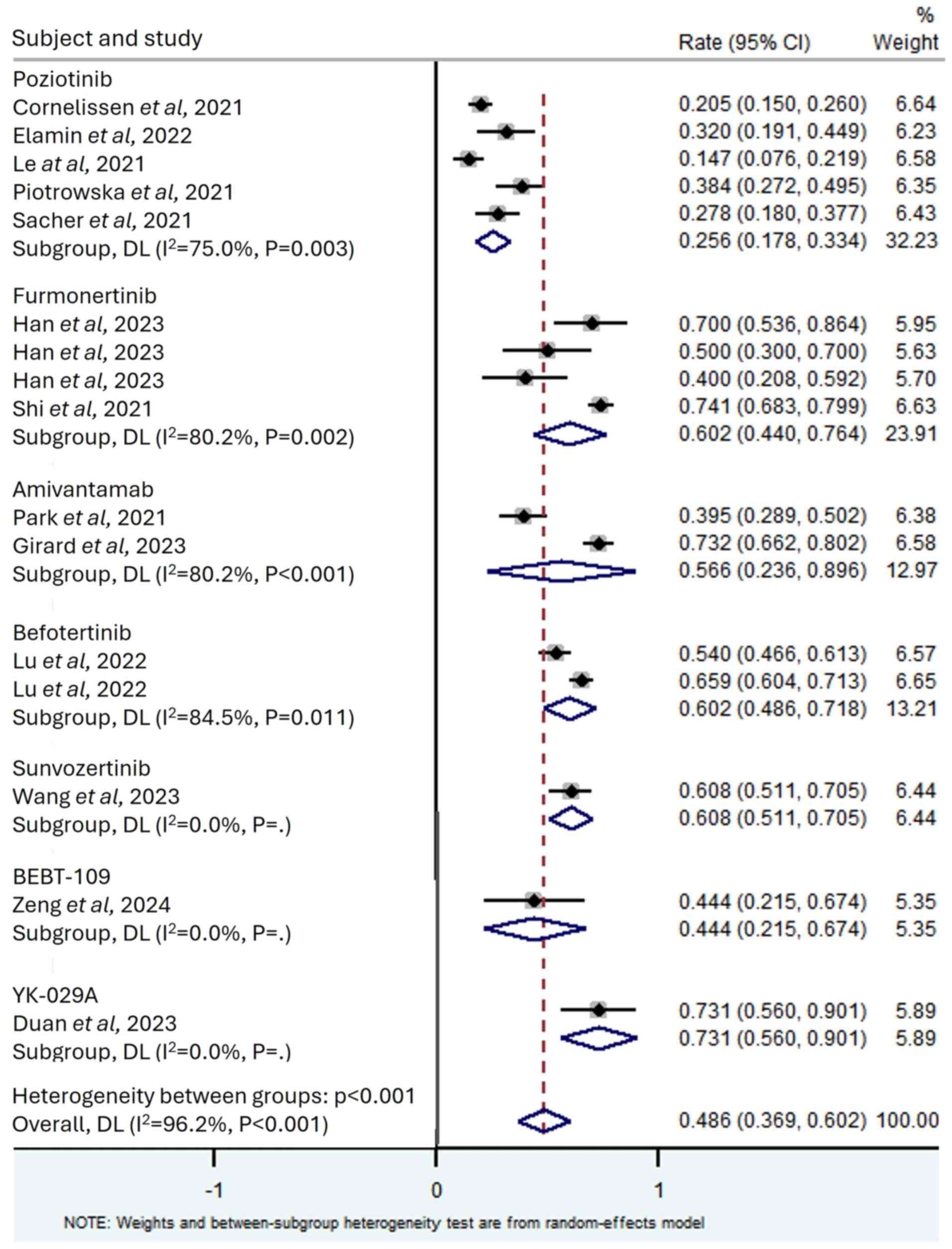
All studies included in the present analysis
reported the ORR for the assessed EGFR-TKIs for patients with NSCLC
with exon 20 insertions, which ranged from 14.7–74.1%. The
meta-analysis results revealed significant heterogeneity among all
studies (I2=96.2%; P<0.001); therefore, a random
effects model was used for analysis, with a combined ORR of 0.486
(95% CI, 0.369–0.602; Fig. 2).
Subgroup analysis was performed on the drugs used in each study,
and a total of seven drugs were included. According to the size of
ORR, they were YK-029A (ORR, 0.731; 95% CI, 0.560–0.901;
I2=0%), sunvozertinib (ORR, 0.608; 95% CI, 0.511–0.705;
I2=0%), furmonertinib (ORR, 0.602; 95% CI, 0.440–0.764;
I2=80.2%), befotertinib (ORR, 0.602; 95% CI,
0.486–0.718; I2=84.5%), amivantamab (ORR, 0.566; 95% CI,
0.236–0.896; I2=96.3%), BEBT-109 (ORR, 0.444; 95% CI,
0.215–0.674; I2=0%) and poziotinib (ORR, 0.256; 95% CI,
0.178–0.334; I2=75.0%).
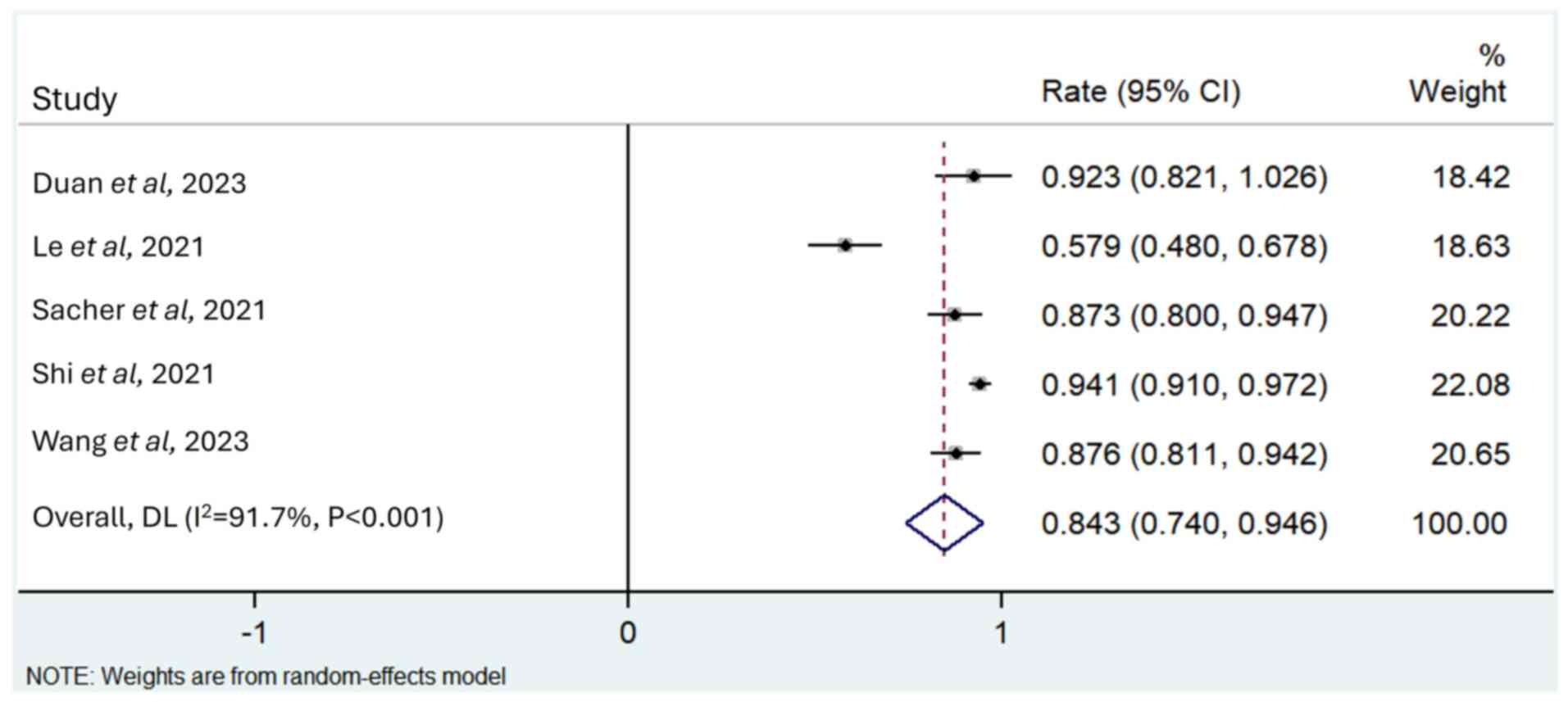
A total of five studies reported the DCR (13,24,25,27,30),
and a random effects model was used for analysis as there was
significant heterogeneity between studies (I2=91.7%;
P<0.001). The merged DCR value was 0.843 (95% CI, 0.740–0.946;
Fig. 3).
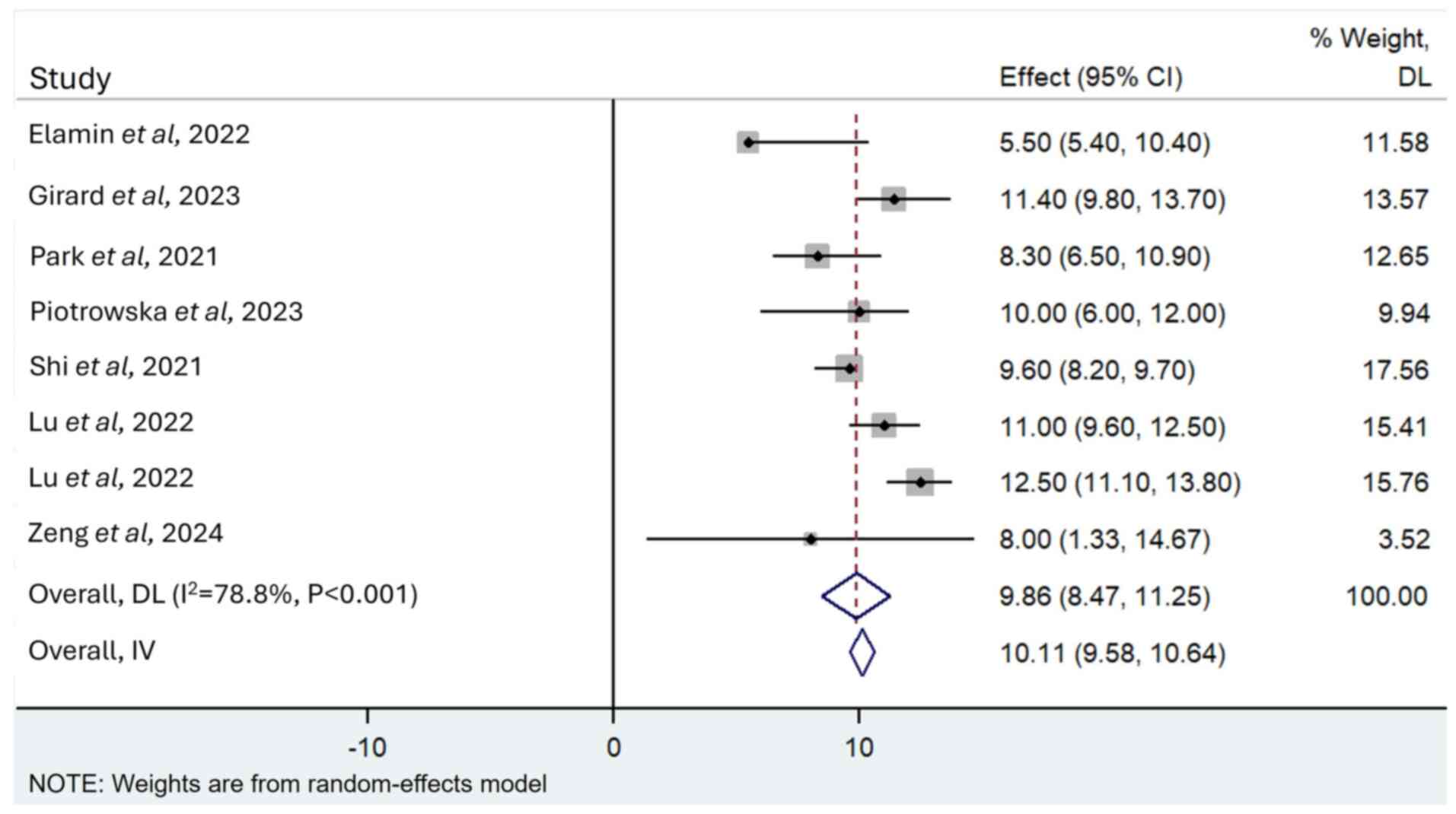
Survival
A total of 11 articles reported PFS (13,20,23,28,29),
with 7 studies reporting its 95% CI. A total of three articles
reported OS (20,22,28),
whilst one of them reported OS not reached (20). Using the random-effects model, the
pooled median PFS was 10.11 months (95% CI, 9.58–10.64 months;
I2=78.8%; P<0.001; Fig.
4). Using the random-effects model, the pooled median OS was
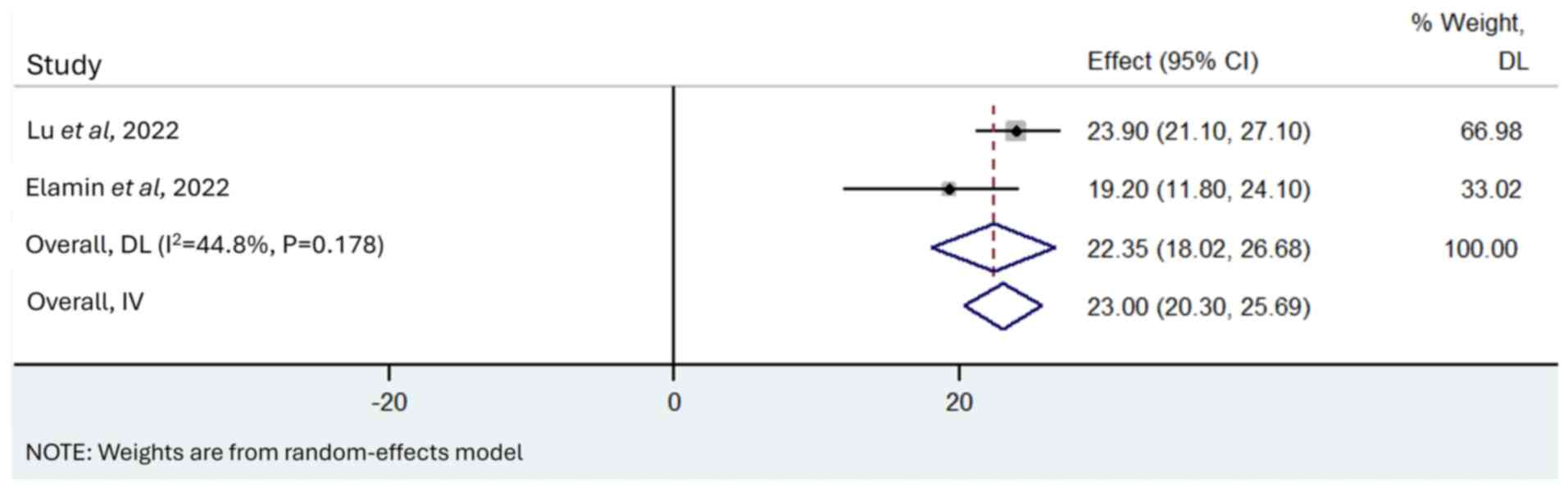
23.00 months (95% CI, 20.30–25.69 months; I2=44.8;
P=0.178; Fig. 5).
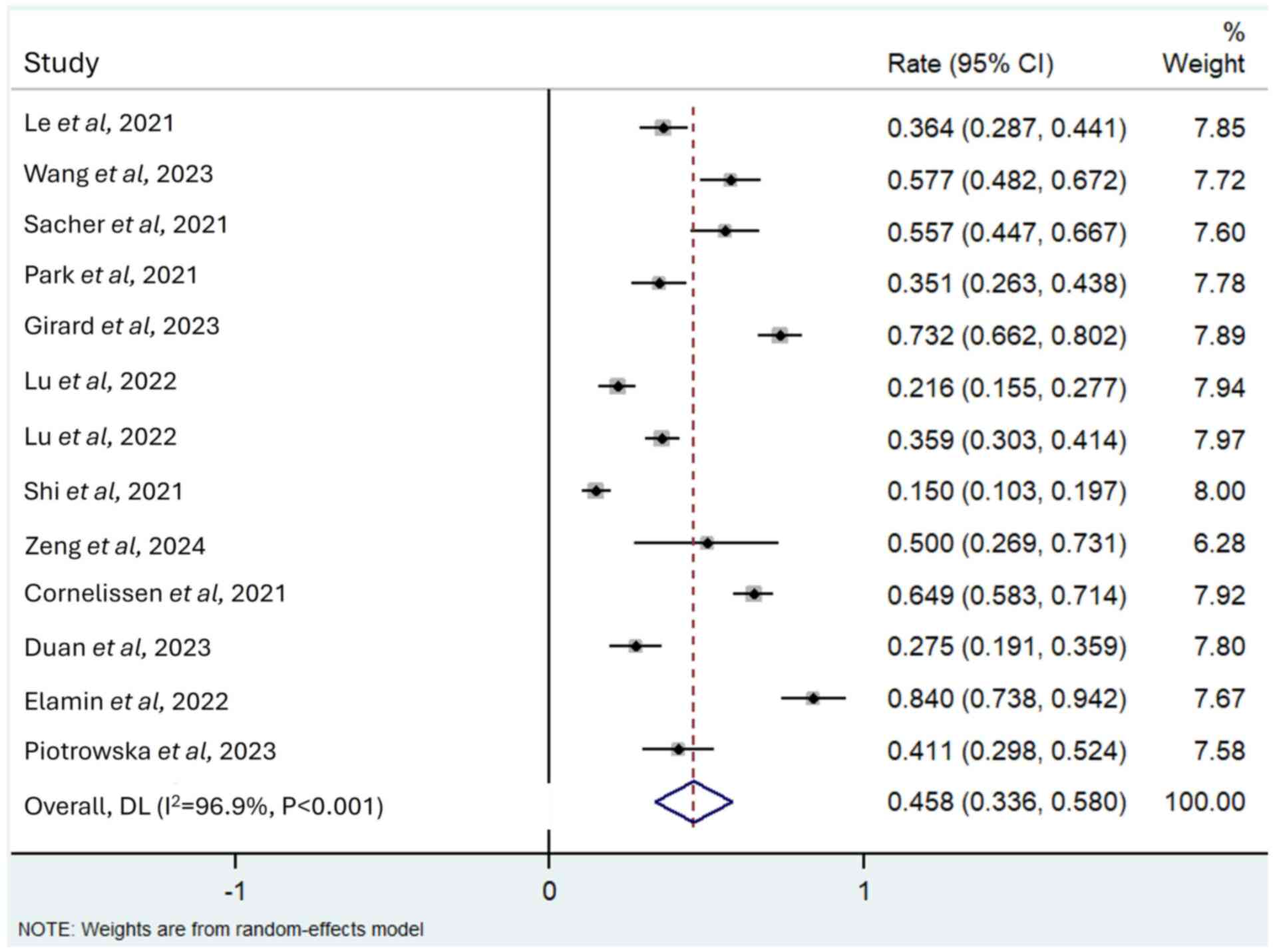
Safety
A total of 12 studies reported TRAEs of grade ≥3
(13,20–30),
with the incidence varying from 15.0–84.0%. The three most reported
TRAEs associated with the emerging EGFR-TKIs were diarrhoea, rash
and hypokalaemia. Overall, the pooled incidence of TRAEs of grade
≥3 was 0.458 (95% CI, 0.336–0.580; I2=96.9%; P<0.001;
Fig. 6), with diarrhoea (0.112; 95%
CI, 0.060–0.164) thrombocytopenia (0.065; 95% CI, −0.012–0.141) and
anaemia (0.040; 95% CI, 0.005–0.076) the most commonly reported.
Further details are presented in Table
IV.
 | Table IV.Pooled incidence of TRAEs of grade
≥3. |
Table IV.
Pooled incidence of TRAEs of grade
≥3.
| TRAEs grade ≥3 | Cohorts, n | I2,
% | P-value | Effect (95%
CI) |
|---|
| Diarrhoea | 10 | 93.6 | <0.001 | 0.112
(0.060–0.164) |
| Rash | 8 | 95.1 | <0.001 | 0.124
(0.074–0.173) |
| Hypokalaemia | 5 | 69.6 | 0.011 | 0.027
(0.005–0.049) |
| Decreased
appetite | 4 | 0.0 | 0.819 | 0.007
(0.001–0.013) |
| Anaemia | 4 | 76.7 | 0.005 | 0.040
(0.005–0.076) |
| Vomiting | 3 | 12.3 | 0.320 | 0.012
(0.000–0.023) |
|
Thrombocytopenia | 3 | 96.0 | <0.001 | 0.065
(−0.012–0.141) |
| leukopenia | 2 | 25.6 | 0.261 | 0.014
(0.005–0.022) |
| Headache | 2 | 0.0 | 0.848 | 0.008
(0.001–0.015) |
| Elevated ALT | 2 | 13.1 | 0.316 | 0.009
(0.001–0.017) |
| Elevated AST | 2 | 13.1 | 0.316 | 0.009
(0.001–0.017) |
| Pulmonary
embolism | 2 | 87.9 | <0.001 | 0.033
(−0.003–0.068) |
| Nausea | 2 | 21.2 | 0.260 | 0.025
(0.000–0.049) |
| Dizziness | 2 | 0.0 | 0.571 | 0.004
(−0.002–0.010) |
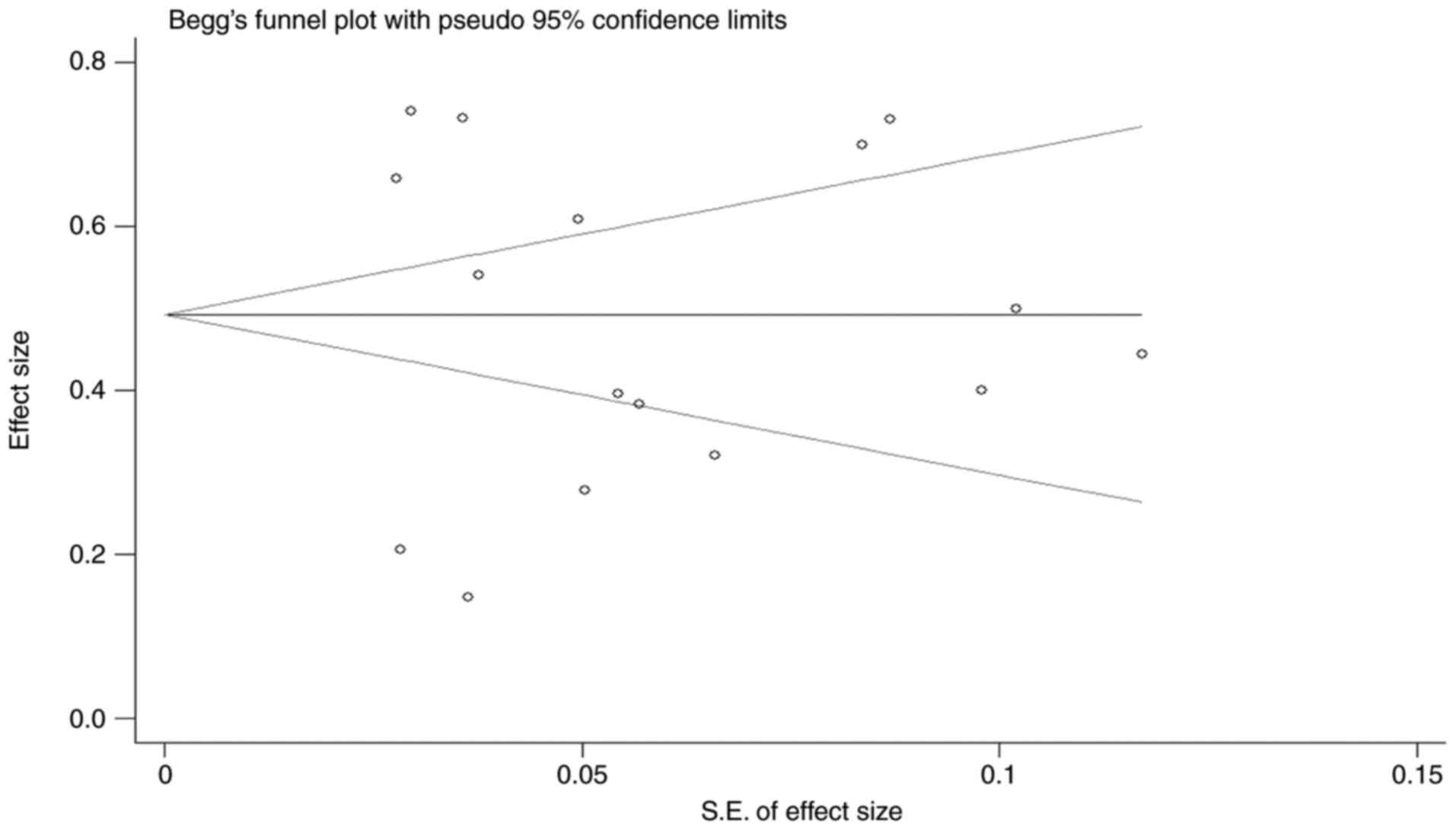
Publication bias
The number of studies included in the pooled ORR was
13. The Begg's funnel plot demonstrated an incomplete symmetrical
scatter distribution (Fig. 7).
Furthermore, Egger's test was used to quantitatively analyse
publication bias, and the P-value was 0.844, indicating that there
was no significant publication bias in the included literature.
Discussion
A total of 13 trials involving 9 investigational
agents were included in the pooled analysis. The sample size was
relatively small due to the early-stage development of these agents
and the rigorous clinical trial process, which limited the
availability of published data. The pooled analysis of these
included emerging third-generation EGFR-TKIs demonstrated a pooled
ORR of 48.6%, a median PFS of 10.11 months and a median OS of 23.00
months. Furthermore, a recently published real-world study reported
confirmed ORRs of 14.0–18.6%, a median PFS of 11.5–17.0 months and
a median OS of 3.3–5.2 months in patients with EGFR exon 20
insertion-mutant NSCLC (32). These
findings suggest that the emerging third-generation EGFR-TKIs may
offer good treatment outcomes for this patient population.
Therefore, despite the disappointing results observed in
mobocertinib trials, third-generation EGFR-TKIs remain a critical
treatment strategy for patients with EGFR exon 20 insertion
mutations.
EGFR exon 20 insertions are structurally similar to
EGFR T790M and tend to be insensitive to first- and
second-generation EGFR-TKIs (33).
In a previous meta-analysis, only low ORRs (0–9%) were reported for
patients with NSCLC exon 20 mutations who were administered first-
and second-generation EGFR-TKIs (34). The unsaturated acryloyl group of
third-generation EGFR-TKIs forms an irreversible covalent bond with
C79, removing the aromatic moiety and potentially solving the
spatial site-blocking problem caused by EGFR exon 20 insertions and
T790M. However, the oral irreversible third-generation EGFR-TKI,
osimertinib, which was developed to be selective for both EGFR
sensitizing mutations and EGFR T790M resistance mutations,
demonstrated conflicting results in clinical trials (35). The potential antitumour activity of
osimertinib was first reported in a small-sample size cohort, where
4/6 patients with NSCLC and an exon 20 insertion mutation receiving
80 mg osimertinib once daily, achieved a partial response and the
rest remained stable. The mean PFS was 6.2 months (36). However, in another retrospective
analysis with a larger sample size of a Chinese NSCLC cohort
harbouring diverse EGFR exon 20 insertions, osimertinib was
reported to have little effect, with an ORR of 6.5%, a DCR of 53.2%
and a mean PFS of only 2.3 months. Moreover, no dose-response was
reported for its use as a first-line treatment or above (7).
Prior to the recent approval of targeted therapies
specifically designed for EGFR exon 20 insertion mutations, the
cornerstone of treatment strategies for EGFR exon 20 insertion
mutations relied on conventional therapies such as traditional
EGFR-TKIs, immuno-oncology agents and cytotoxic chemotherapy
(37). At present, platinum-based
doublet chemotherapy remains the established standard of care for
most patients with NSCLC with EGFR exon 20 insertion mutations
(38). Real-world evidence has
reported that, as a first-line treatment, chemotherapy yields
comparable efficacy in EGFR exon 20 insertion mutant NSCLC as in
TKI-sensitive EGFR-mutant NSCLC, with an ORR ranging from 19-19.2%,
a DCR at 6 months of 41.3%, a median PFS of 6.4–7.6 months and an
OS of 19.9 months (39–42). However, the use of chemotherapy is
limited in the clinical setting due to its poorly tolerated side
effects. Thus, newer third-generation EGFR-TKIs have been developed
(43). In the present
meta-analysis, the third-generation EGFR-TKIs included were mostly
administered as second line or above treatments. The pooled
analysis indicated that the emerging EGFR-TKIs demonstrate a
superior treatment outcome for NSCLC with EGFR exon 20 insertions.
Although the PFS associated with these TKIs appears slightly
suboptimal, it represents a significant improvement over first-line
chemotherapy.
In the present study, among the included EGFR-TKIs,
poziotinib demonstrated the lowest ORR. Despite being an
irreversible pan-human epidermal growth factor receptor (HER)
inhibitor with antitumor activity in previously treated patients
with NSCLC harbouring HER2 exon 20 insertions in early clinical
trials, its ORR and DCR did not outweigh the associated risks,
leading to its denial of approval by the FDA (44). The present meta-analysis drew the
same conclusion. Moreover, YK-029A revealed the most favourable ORR
of 73.1%, followed by sunvozertinib (60.8%), furmonertinib (60.2%),
befotertinib (60.2%) and amivantamab (56.6%). Sunvozertinib,
furmonertinib, befotertinib, and amivantamab have now received
approval for the treatment of EGFR exon 20 insertion mutant NSCLC
(45). YK-029A is an oral
irreversible third-generation EGFR-TKI and an analogue of
Osimertinib, which is currently being considered as a breakthrough
therapeutic option in China for the first-line treatment of
advanced EGFR exon 20 insertion mutant NSCLC (46). The present meta-analysis supports
their potential as effective treatment options for this condition.
However, further real-world data is necessary to supplement the
evidence regarding their therapeutic effectiveness compared with
standard treatments.
Several limitations should be addressed in the
present meta-analysis. Firstly, there was high heterogeneity
observed among the included studies. Differences in study design,
including sample sizes and follow-up periods, as well as dosing
regimens and efficacy profiles of interventions may account for a
degree of the heterogeneity. For example, variations in baseline
characteristics and how outcomes such as ORR or PFS were measured
could influence the pooled results. However, between-study variance
was evaluated using random-effects models, ensuring the pooled
estimates remained robust despite the heterogeneity. This model
assumes that true effect sizes may vary across studies, which
provides more conservative estimates and wider confidence
intervals, leading to more robust conclusions. By adjusting for
study-specific differences, the random-effects model ensures that
the results are more generalizable and reflective of real-world
variability. Secondly, the lack of sufficient pathological data
prevented the analysis of the efficacy of third-generation
EGFR-TKIs for different histological types of EGFR exon 20 mutant
NSCLC. Thirdly, sensitivity analyses were not performed. Due to the
limited number of promising third-generation EGFR TKIs available
for analysis at the time of performing the meta-analysis, the
number of included studies was relatively small, thus performing
sensitivity analyses by removing individual studies would have
significantly reduced statistical power without providing
additional meaningful insights. Further analysis should be
performed when more data from clinical trials are released.
In conclusion, the emerging EGFR-TKIs for patients
with NSCLC with EGFR exon 20 insertion mutations have good
treatment outcome; however, the PFS outcome appears to be slightly
suboptimal. Few studies focused on first-line treatments and the
clinical need for this field remains to be elucidated. Further
analysis is needed when more new clinical data are released.
Acknowledgements
The authors thank Mr. Samuel Morice (Castle Hill
Hospital, Hull, UK) for English language support.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant no. 82102301) and the
Natural Science Foundation of Sichuan (grant no. 2024NSFSC0732) for
data collection and analysis.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KX, JW and DW conceived, designed and planned the
study. JW, JJ and ZD performed the literature search and screening.
KX, JJ ZD and QH extracted, analysed and interpreted the data. KX,
JW and JJ drafted the manuscript. KX and DW confirm the
authenticity of all the raw data. All authors critically reviewed
the manuscript. KX, JW and DW revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Geraci E and Chablani L: Immunotherapy as
a second-line or later treatment modality for advanced non-small
cell lung cancer: A review of safety and efficacy. Crit Revi Oncol
Hematol. 152:1030092020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Leary C, Gasper H, Sahin KB, Tang M,
Kulasinghe A, Adams MN, Richard DJ and O'Byrne KJ: Epidermal growth
factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC).
Pharmaceuticals (Basel). 13:2732020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kazandjian D, Blumenthal GM, Yuan W, He K,
Keegan P and Pazdur R: FDA approval of gefitinib for the treatment
of patients with metastatic EGFR mutation-positive non-small cell
lung cancer. Clin Cancer Res. 22:1307–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khozin S, Blumenthal GM, Jiang X, He K,
Boyd K, Murgo A, Justice R, Keegan P and Pazdur R: U.S. Food and
drug administration approval summary: Erlotinib for the first-line
treatment of metastatic non-small cell lung cancer with epidermal
growth factor receptor exon 19 deletions or exon 21 (L858R)
substitution mutations. Oncologist. 19:774–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brazel D, Kroening G and Nagasaka M:
Non-small cell lung cancer with EGFR or HER2 exon 20 insertion
mutations: Diagnosis and treatment options. BioDrugs. 36:717–729.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burnett H, Emich H, Carroll C, Stapleton
N, Mahadevia P and Li T: Epidemiological and clinical burden of
EGFR Exon 20 insertion in advanced non-small cell lung cancer: A
systematic literature review. PLoS One. 16:e02476202021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang GJ, Li J, Xu HY, Sun Y, Liu L, Li HS,
Yang L, Zhang Y, Li GH and Wang Y: Osimertinib for Chinese advanced
non-small cell lung cancer patients harboring diverse EGFR exon 20
insertion mutations. Lung Cancer. 152:39–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duke ES, Stapleford L, Drezner N, Amatya
AK, Mishra-Kalyani PS, Shen YL, Maxfield K, Zirkelbach JF, Bi Y,
Liu J, et al: FDA approval summary: Mobocertinib for metastatic
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Clin Cancer Res. 29:508–512. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalvez F, Vincent S, Baker TE, Gould
AE, Li S, Wardwell SD, Nadworny S, Ning Y, Zhang S, Huang WS, et
al: Mobocertinib (TAK-788): A targeted inhibitor of EGFR exon 20
insertion mutants in non-small cell lung cancer. Cancer Discov.
11:1672–1687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Ramalingam SS, Kim TM, Kim SW,
Yang JC, Riely GJ, Mekhail T, Nguyen D, Garcia Campelo MR, Felip E,
et al: Treatment outcomes and safety of mobocertinib in
platinum-pretreated patients with EGFR exon 20 insertion-positive
metastatic non-small cell lung cancer: A phase 1/2 open-label
nonrandomized clinical trial. JAMA Oncol. 7:e2147612021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sava J: FDA and takeda to withdraw
mobocertinib for EGFR Exon20+ NSCLC. Targeted Oncology.
2023.Available from:. https://www.targetedonc.com/view/fda-and-takeda-to-withdraw-mobocertinib-for-egfr-exon20-nsclc
|
|
12
|
Wang M, Yang JCH, Mitchell PL, Fang J,
Camidge DR, Nian W, Chiu CH, Zhou J, Zhao Y, Su WC, et al:
Sunvozertinib, a selective EGFR inhibitor for previously treated
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Cancer Discov. 12:1676–1689. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q,
Zhang Y, Liu L, Wang X, Cheng Y, et al: Efficacy, safety, and
genetic analysis of furmonertinib (AST2818) in patients with EGFR
T790M mutated non-small-cell lung cancer: A phase 2b, multicentre,
single-arm, open-label study. Lancet Respir Med. 9:829–839. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin L and Chu H: Quantifying publication
bias in meta-analysis. Biometrics. 74:785–794. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JPT, Chandler J, Cumpston M, Li T,
Page MJ and Welch VA: Cochrane Handbook for Systematic Reviews of
Interventions Version 6.4 (updated August 2023). Cochrane; 2023,
Available from:. www.training.cochrane.org/handbook
|
|
20
|
Park K, Haura EB, Leighl NB, Mitchell P,
Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al:
Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung
cancer progressing on platinum chemotherapy: Initial results from
the CHRYSALIS phase I study. J Clin Oncol. 39:3391–3402. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girard N, Park K, Tang K, Cho BC, Paz-Ares
L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK, et
al: LBA5 Amivantamab plus chemotherapy vs chemotherapy as
first-line treatment in EGFR Exon 20 insertion-mutated advanced
non-small cell lung cancer (NSCLC): Primary results from PAPILLON,
a randomized phase III global study. Ann Oncol. 34 (Suppl
2):S13042023. View Article : Google Scholar
|
|
22
|
Lu S, Zhang Y, Zhang G, Zhou J, Cang S,
Cheng Y, Wu G, Cao P, Lv D, Jian H, et al: Efficacy and safety of
befotertinib (D-0316) in patients with EGFR T790M-mutated NSCLC
that had progressed after prior EGFR tyrosine kinase inhibitor
therapy: A phase 2, multicenter, single-arm, open-label study. J
Thorac Oncol. 17:1192–1204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng L, Song L, Liu L, Wu F, Xu Q, Yan H,
Lin S, Jiang W, Wang Z, Deng L, et al: First-in-human phase I study
of BEBT-109 in previously treated EGFR exon 20 insertion-mutated
advanced non-small cell lung cancer. Med. 5:445–458.e3. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Fan Y, Sun M, Wang Y, Zhao Y, Jin
B, Hu Y, Han Z, Song X, Liu A, et al: Sunvozertinib for patients in
China with platinum-pretreated locally advanced or metastatic
non-small-cell lung cancer and EGFR exon 20 insertion mutation
(WU-KONG6): Single-arm, open-label, multicentre, phase 2 trial.
Lancet Respir Med. 12:217–224. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le X, Shum E, Suga JM, Brahmer JR, Dooms
C, Mamdani H, Nechushtan H, Riess JW, Spira A, Barrett JA, et al:
Abstract CT169: Poziotinib administered twice daily improves safety
and tolerability in patients with EGFR or HER2 exon 20 mutant NSCLC
(ZENITH20-5). Cancer Res. 81 (Suppl 13):CT1692021. View Article : Google Scholar
|
|
26
|
Cornelissen R, Garassino MC, Le X, Clarke
J, Tchekmedyian N, Goldman J, Lebel F, Bhat G and Socinski M:
MA11.04 updated efficacy, safety and dosing management of
poziotinib in previously treated EGFR and HER2 exon 20 NSCLC
patients. J Thorac Oncol. 16 (Suppl 1):S173–S174. 2021. View Article : Google Scholar
|
|
27
|
Sacher A, Le X, Cornelissen R, Shum E,
Suga J, Socinski M, Molina JR, Haura E, Clarke J, Bhat G, et al:
36MO Safety, tolerability and preliminary efficacy of poziotinib
with twice daily strategy in EGFR/HER2 Exon 20 mutant non-small
cell lung cancer. Ann Oncol. 32 (Suppl 1):S152021. View Article : Google Scholar
|
|
28
|
Elamin YY, Robichaux JP, Carter BW, Altan
M, Tran H, Gibbons DL, Heeke S, Fossella FV, Lam VK, Le X, et al:
Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy,
resistance mechanisms, and impact of insertion location on drug
sensitivity. Cancer Cell. 40:754–767.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piotrowska Z, Tan DS, Smit EF, Spira AI,
Soo RA, Nguyen D, Lee VH, Yang JC, Velcheti V, Wrangle JM, et al:
Safety, tolerability, and antitumor activity of zipalertinib among
patients with non-small-cell lung cancer harboring epidermal growth
factor receptor exon 20 insertions. J Clin Oncol. 41:4218–4225.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan J, Wu L, Yang K, Zhao J, Zhao Y, Dai
X, Li M, Xie Y, Yao Y, Zhao M, et al: Safety, tolerability,
pharmacokinetics, and preliminary efficacy of YK-029A in
treatment-naive patients with advanced NSCLC harboring EGFR exon 20
insertion mutations: A phase 1 trial. J Thorac Oncol. 19:314–324.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han B, Zhou C, Zheng W, Wu L, Ma Z, Wang
H, Yu X, Ding G, Ma D, Nie L, et al: OA03.04 A phase 1b study of
furmonertinib, an oral, brain penetrant, selective EGFR inhibitor,
in patients with advanced NSCLC with EGFR exon 20 insertions. J
Thorac Oncol. 18 (Suppl 1):S492023. View Article : Google Scholar
|
|
32
|
Ou SI, Lin HM, Hong JL, Yin Y, Jin S, Lin
J, Mehta M, Nguyen D and Neal JW: Real-world response and outcomes
in patients with NSCLC with EGFR exon 20 insertion mutations. JTO
Clin Res Rep. 4:1005582023.PubMed/NCBI
|
|
33
|
Riess JW, Gandara DR, Frampton GM, Madison
R, Peled N, Bufill JA, Dy GK, Ou SI, Stephens PJ, McPherson JD, et
al: Diverse EGFR exon 20 insertions and co-occurring molecular
alterations identified by comprehensive genomic profiling of NSCLC.
J Thorac Oncol. 13:1560–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kwon CS, Lin HM, Crossland V, Churchill
EN, Curran E, Forsythe A, Tomaras D and Ou SI: Non-small cell lung
cancer with EGFR exon 20 insertion mutation: A systematic
literature review and meta-analysis of patient outcomes. Curr Med
Res Opin. 38:1341–1350. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Mao T, Wang J, Zheng H, Hu Z, Cao P,
Yang S, Zhu L, Guo S, Zhao X, et al: Toward the next generation
EGFR inhibitors: An overview of osimertinib resistance mediated by
EGFR mutations in non-small cell lung cancer. Cell Commun Signal.
21:712023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang W, Huang Y, Hong S, Zhang Z, Wang M,
Gan J, Wang W, Guo H, Wang K and Zhang L: EGFR exon 20 insertion
mutations and response to osimertinib in non-small-cell lung
cancer. BMC Cancer. 19:5952019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Low JL, Lim SM, Lee JB, Cho BC and Soo RA:
Advances in the management of non-small-cell lung cancer harbouring
EGFR exon 20 insertion mutations. Ther Adv Med Oncol.
15:175883592211461312023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang F, Li C, Wu Q and Lu H: EGFR exon 20
insertion mutations in non-small cell lung cancer. Transl Cancer
Res. 9:2982–2991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah MP, Aredo JV, Padda SK, Ramchandran
KJ, Wakelee HA, Das MS and Neal JW: EGFR exon 20 insertion NSCLC
and response to platinum-based chemotherapy. Clin Lung Cancer.
23:e148–e153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi J, Yang H, Jiang T, Li X, Zhao C,
Zhang L, Zhao S, Liu X, Jia Y, Wang Y, et al: Uncommon EGFR
mutations in a cohort of Chinese NSCLC patients and outcomes of
first-line EGFR-TKIs and platinum-based chemotherapy. Chin J Cancer
Res. 29:543–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang G, Li J, Xu H, Yang Y, Yang L, Xu F,
Xia B, Zhu VW, Nagasaka M, Yang Y, et al: EGFR exon 20 insertion
mutations in Chinese advanced non-small cell lung cancer patients:
Molecular heterogeneity and treatment outcome from nationwide
real-world study. Lung Cancer. 145:186–194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Li J, Zhou Y, Cao S, Ling X, Zhang
Y, Nie W and Zhong H: Tumor genomics and response to chemotherapy
in advanced non-small cell lung cancer with exon 20 insertion
epidermal growth factor receptor mutations. Ann Transl Med.
8:12972020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anand U, Dey A, Chandel AKS, Sanyal R,
Mishra A, Pandey DK, De Falco V, Upadhyay A, Kandimalla R,
Chaudhary A, et al: Cancer chemotherapy and beyond: Current status,
drug candidates, associated risks and progress in targeted
therapeutics. Genes Dis. 10:1367–1401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Le X, Cornelissen R, Garassino M, Clarke
JM, Tchekmedyian N, Goldman JW, Leu SY, Bhat G, Lebel F, Heymach JV
and Socinski MA: Poziotinib in non-small-cell lung cancer harboring
HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2
trial. J Clin Oncol. 40:710–718. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ou SI, Le X, Nagasaka M, Reungwetwattana
T, Ahn MJ, Lim DWT, Santos ES, Shum E, Lau SCM, Lee JB, et al: Top
20 EGFR+ NSCLC clinical and translational science papers that
shaped the 20 years since the discovery of activating EGFR
mutations in NSCLC. An editor-in-chief expert panel consensus
survey. Lung Cancer (Auckl). 15:87–114. 2024.PubMed/NCBI
|
|
46
|
Locke M, Aung WY and Seetharamu N: YK-029A
in the landscape of treatments for non-small cell lung cancer
(NSCLC) with EGFR exon 20 insertion mutations. AME Clin Trials Rev.
2:1–5. 2024. View Article : Google Scholar
|