|
1
|
Geraci E and Chablani L: Immunotherapy as
a second-line or later treatment modality for advanced non-small
cell lung cancer: A review of safety and efficacy. Crit Revi Oncol
Hematol. 152:1030092020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Leary C, Gasper H, Sahin KB, Tang M,
Kulasinghe A, Adams MN, Richard DJ and O'Byrne KJ: Epidermal growth
factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC).
Pharmaceuticals (Basel). 13:2732020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kazandjian D, Blumenthal GM, Yuan W, He K,
Keegan P and Pazdur R: FDA approval of gefitinib for the treatment
of patients with metastatic EGFR mutation-positive non-small cell
lung cancer. Clin Cancer Res. 22:1307–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khozin S, Blumenthal GM, Jiang X, He K,
Boyd K, Murgo A, Justice R, Keegan P and Pazdur R: U.S. Food and
drug administration approval summary: Erlotinib for the first-line
treatment of metastatic non-small cell lung cancer with epidermal
growth factor receptor exon 19 deletions or exon 21 (L858R)
substitution mutations. Oncologist. 19:774–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brazel D, Kroening G and Nagasaka M:
Non-small cell lung cancer with EGFR or HER2 exon 20 insertion
mutations: Diagnosis and treatment options. BioDrugs. 36:717–729.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burnett H, Emich H, Carroll C, Stapleton
N, Mahadevia P and Li T: Epidemiological and clinical burden of
EGFR Exon 20 insertion in advanced non-small cell lung cancer: A
systematic literature review. PLoS One. 16:e02476202021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang GJ, Li J, Xu HY, Sun Y, Liu L, Li HS,
Yang L, Zhang Y, Li GH and Wang Y: Osimertinib for Chinese advanced
non-small cell lung cancer patients harboring diverse EGFR exon 20
insertion mutations. Lung Cancer. 152:39–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duke ES, Stapleford L, Drezner N, Amatya
AK, Mishra-Kalyani PS, Shen YL, Maxfield K, Zirkelbach JF, Bi Y,
Liu J, et al: FDA approval summary: Mobocertinib for metastatic
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Clin Cancer Res. 29:508–512. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalvez F, Vincent S, Baker TE, Gould
AE, Li S, Wardwell SD, Nadworny S, Ning Y, Zhang S, Huang WS, et
al: Mobocertinib (TAK-788): A targeted inhibitor of EGFR exon 20
insertion mutants in non-small cell lung cancer. Cancer Discov.
11:1672–1687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Ramalingam SS, Kim TM, Kim SW,
Yang JC, Riely GJ, Mekhail T, Nguyen D, Garcia Campelo MR, Felip E,
et al: Treatment outcomes and safety of mobocertinib in
platinum-pretreated patients with EGFR exon 20 insertion-positive
metastatic non-small cell lung cancer: A phase 1/2 open-label
nonrandomized clinical trial. JAMA Oncol. 7:e2147612021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sava J: FDA and takeda to withdraw
mobocertinib for EGFR Exon20+ NSCLC. Targeted Oncology.
2023.Available from:. https://www.targetedonc.com/view/fda-and-takeda-to-withdraw-mobocertinib-for-egfr-exon20-nsclc
|
|
12
|
Wang M, Yang JCH, Mitchell PL, Fang J,
Camidge DR, Nian W, Chiu CH, Zhou J, Zhao Y, Su WC, et al:
Sunvozertinib, a selective EGFR inhibitor for previously treated
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Cancer Discov. 12:1676–1689. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
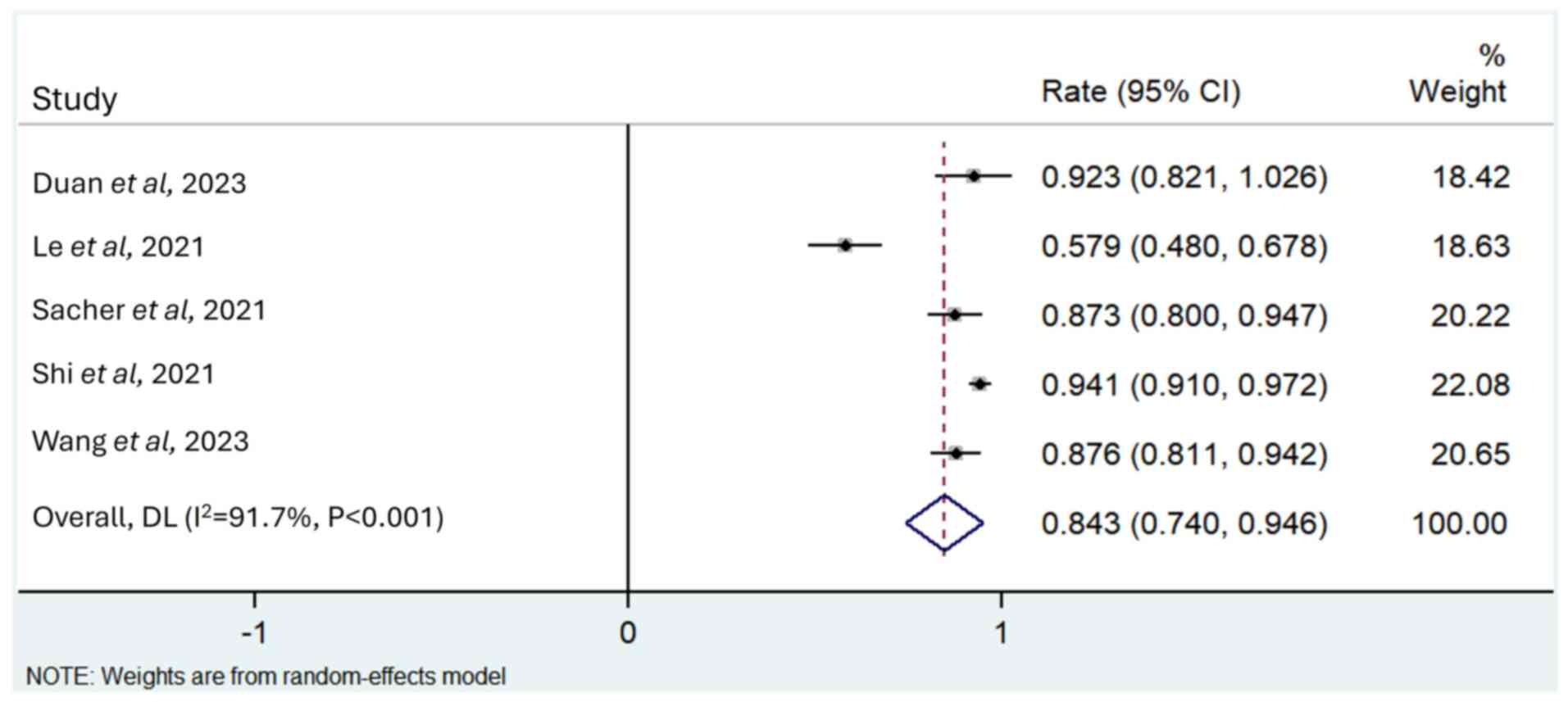
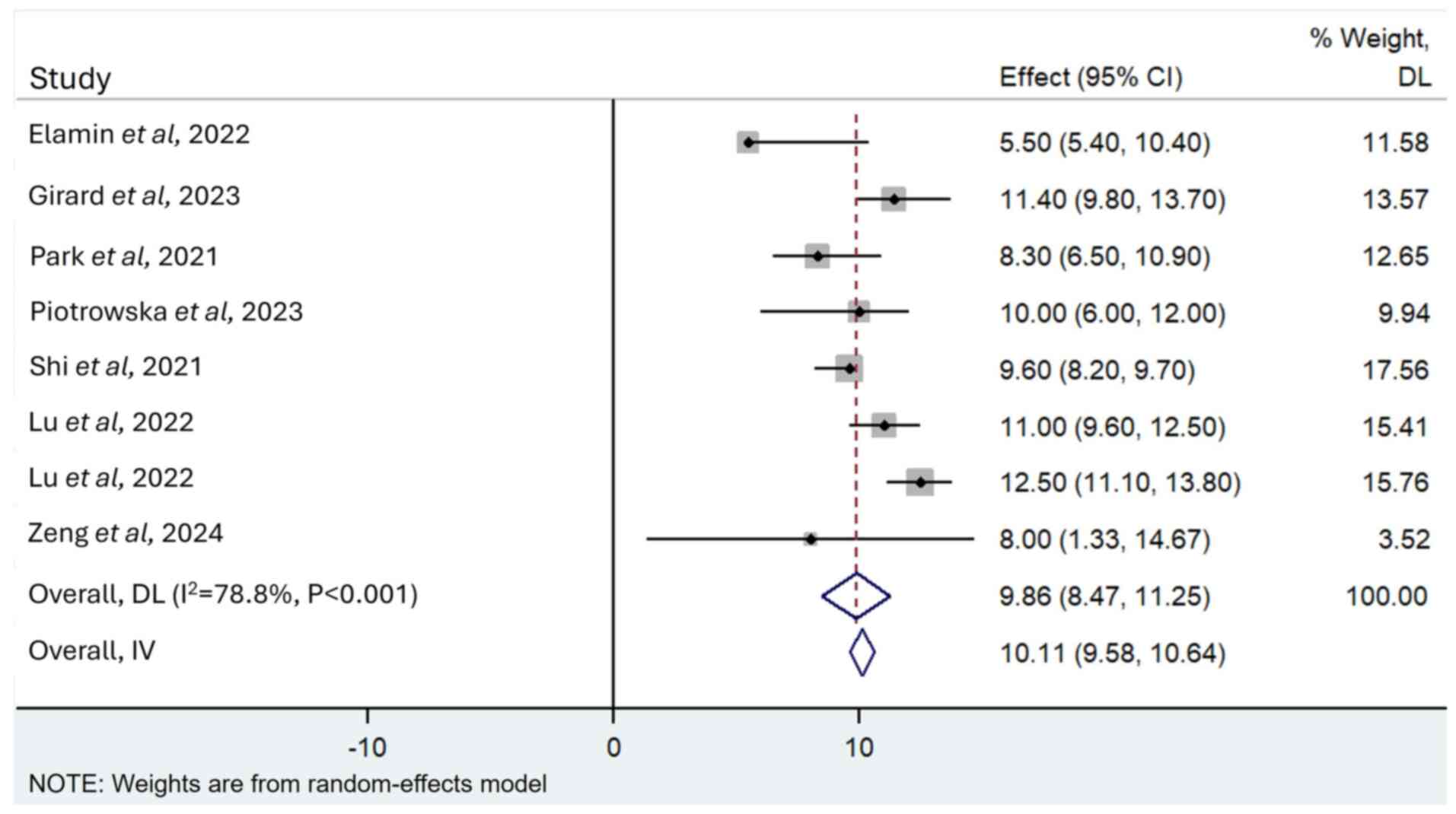
Shi Y, Hu X, Zhang S, Lv D, Wu L, Yu Q,
Zhang Y, Liu L, Wang X, Cheng Y, et al: Efficacy, safety, and
genetic analysis of furmonertinib (AST2818) in patients with EGFR
T790M mutated non-small-cell lung cancer: A phase 2b, multicentre,
single-arm, open-label study. Lancet Respir Med. 9:829–839. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
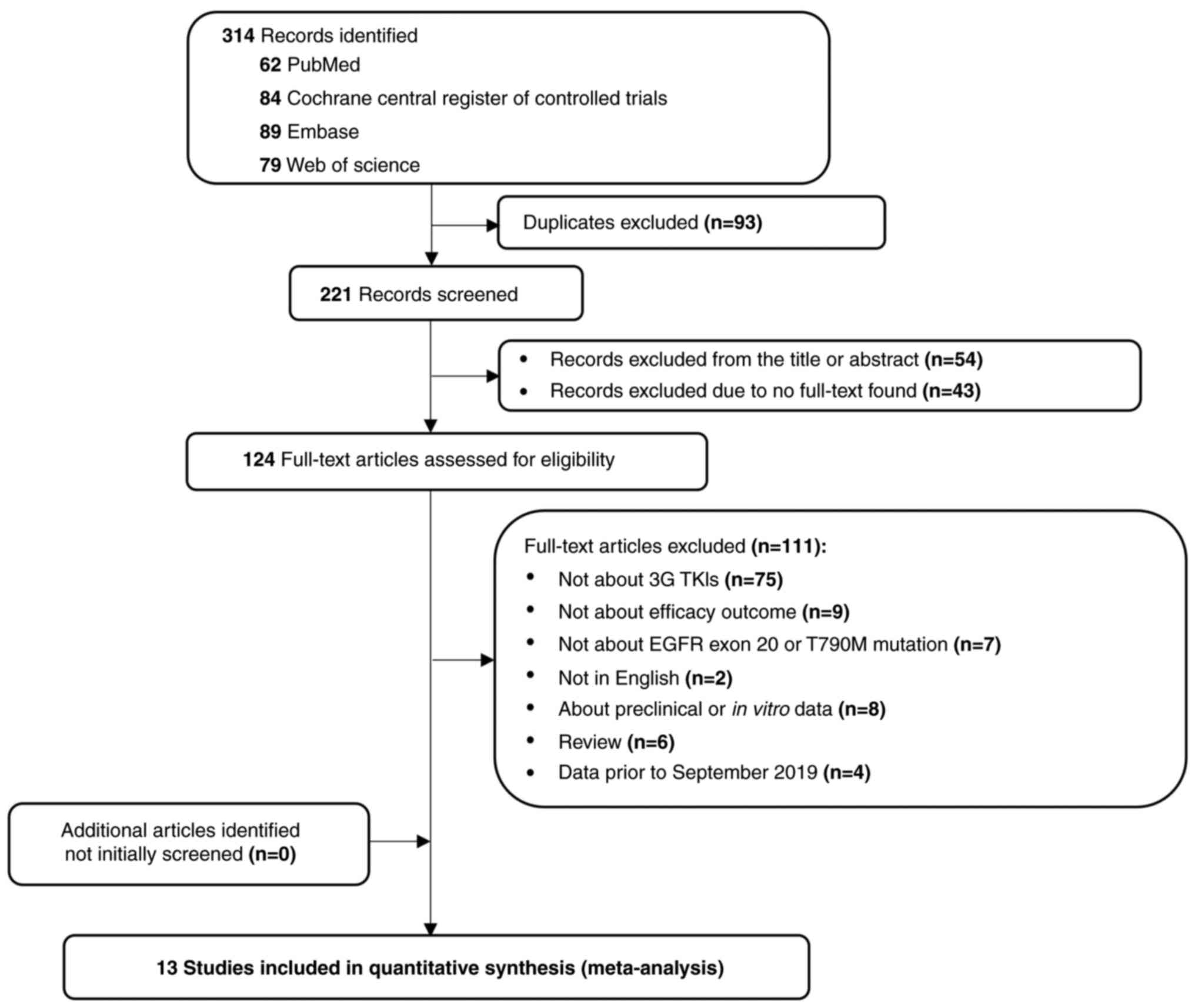
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
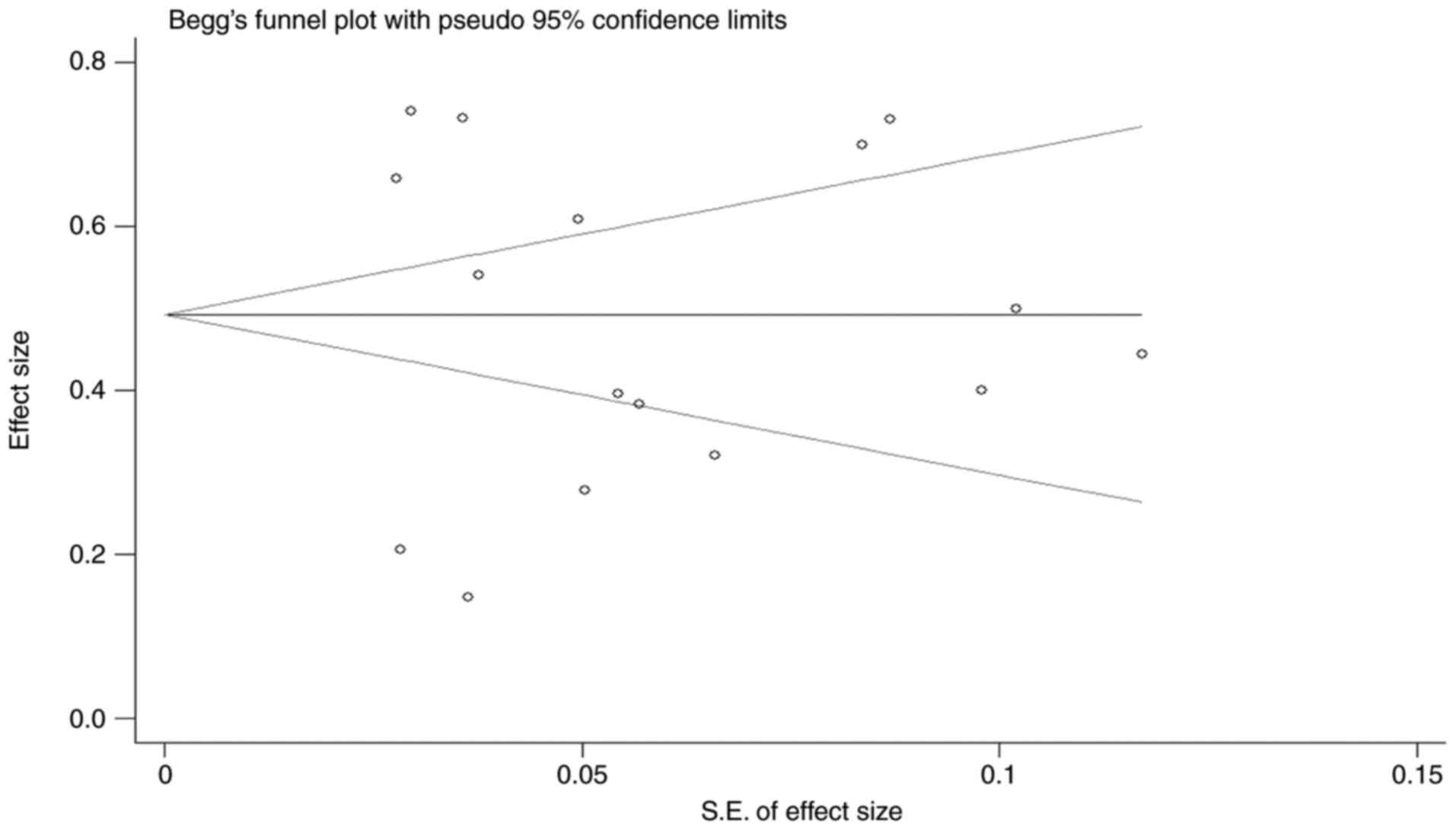
Lin L and Chu H: Quantifying publication
bias in meta-analysis. Biometrics. 74:785–794. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JPT, Chandler J, Cumpston M, Li T,
Page MJ and Welch VA: Cochrane Handbook for Systematic Reviews of
Interventions Version 6.4 (updated August 2023). Cochrane; 2023,
Available from:. www.training.cochrane.org/handbook
|
|
20
|
Park K, Haura EB, Leighl NB, Mitchell P,
Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al:
Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung
cancer progressing on platinum chemotherapy: Initial results from
the CHRYSALIS phase I study. J Clin Oncol. 39:3391–3402. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girard N, Park K, Tang K, Cho BC, Paz-Ares
L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK, et
al: LBA5 Amivantamab plus chemotherapy vs chemotherapy as
first-line treatment in EGFR Exon 20 insertion-mutated advanced
non-small cell lung cancer (NSCLC): Primary results from PAPILLON,
a randomized phase III global study. Ann Oncol. 34 (Suppl
2):S13042023. View Article : Google Scholar
|
|
22
|
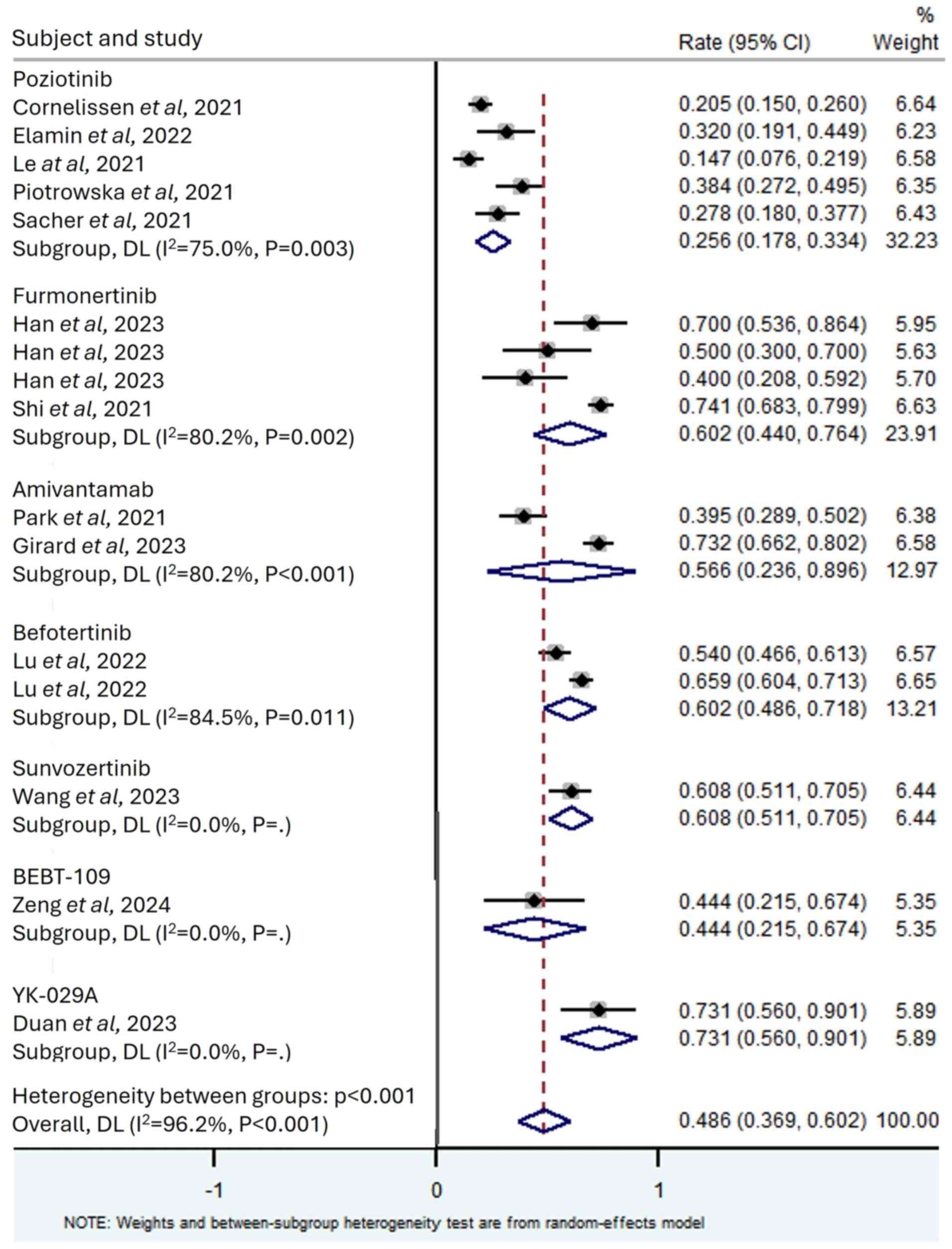
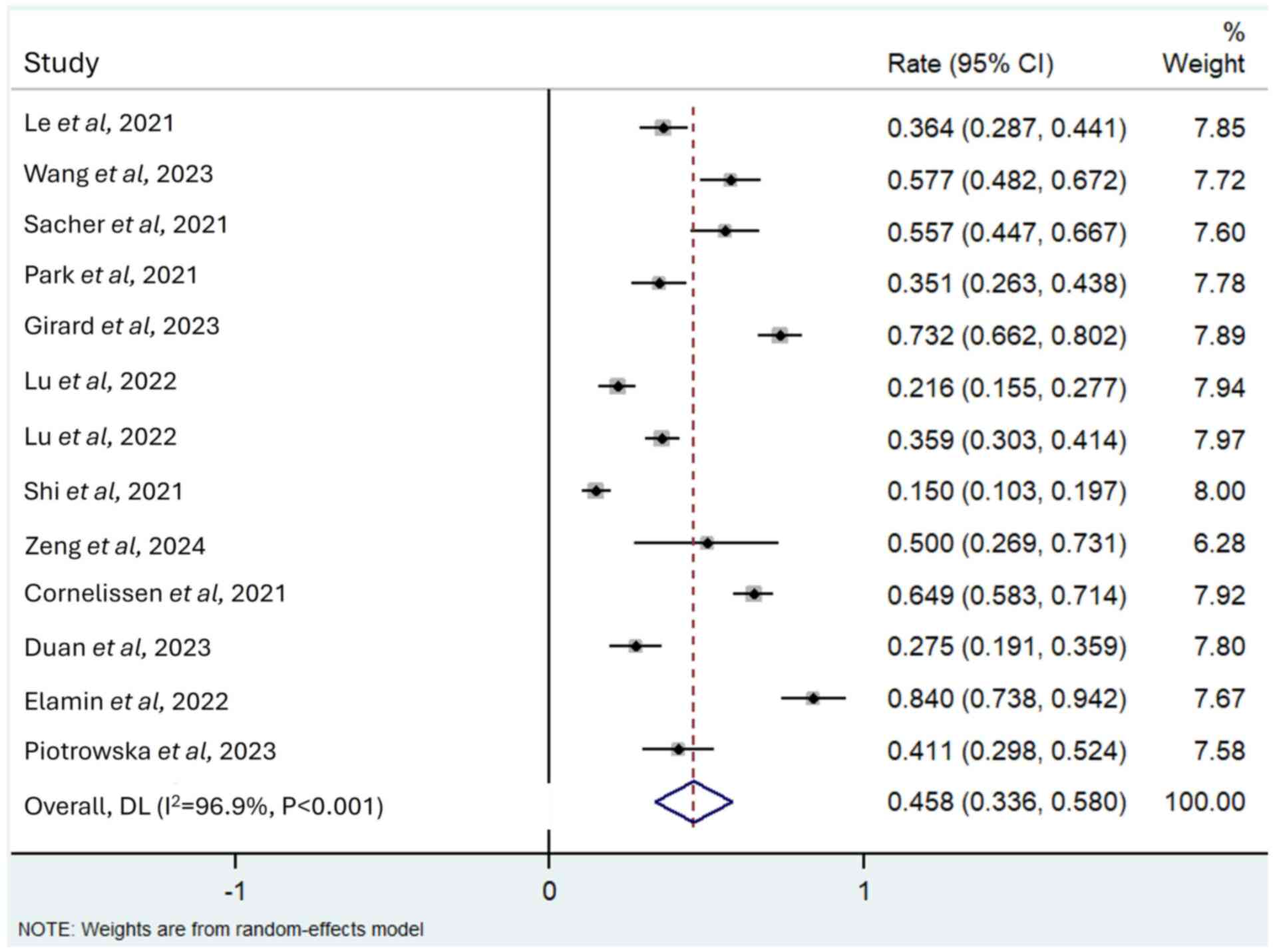
Lu S, Zhang Y, Zhang G, Zhou J, Cang S,
Cheng Y, Wu G, Cao P, Lv D, Jian H, et al: Efficacy and safety of
befotertinib (D-0316) in patients with EGFR T790M-mutated NSCLC
that had progressed after prior EGFR tyrosine kinase inhibitor
therapy: A phase 2, multicenter, single-arm, open-label study. J
Thorac Oncol. 17:1192–1204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng L, Song L, Liu L, Wu F, Xu Q, Yan H,
Lin S, Jiang W, Wang Z, Deng L, et al: First-in-human phase I study
of BEBT-109 in previously treated EGFR exon 20 insertion-mutated
advanced non-small cell lung cancer. Med. 5:445–458.e3. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Fan Y, Sun M, Wang Y, Zhao Y, Jin
B, Hu Y, Han Z, Song X, Liu A, et al: Sunvozertinib for patients in
China with platinum-pretreated locally advanced or metastatic
non-small-cell lung cancer and EGFR exon 20 insertion mutation
(WU-KONG6): Single-arm, open-label, multicentre, phase 2 trial.
Lancet Respir Med. 12:217–224. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le X, Shum E, Suga JM, Brahmer JR, Dooms
C, Mamdani H, Nechushtan H, Riess JW, Spira A, Barrett JA, et al:
Abstract CT169: Poziotinib administered twice daily improves safety
and tolerability in patients with EGFR or HER2 exon 20 mutant NSCLC
(ZENITH20-5). Cancer Res. 81 (Suppl 13):CT1692021. View Article : Google Scholar
|
|
26
|
Cornelissen R, Garassino MC, Le X, Clarke
J, Tchekmedyian N, Goldman J, Lebel F, Bhat G and Socinski M:
MA11.04 updated efficacy, safety and dosing management of
poziotinib in previously treated EGFR and HER2 exon 20 NSCLC
patients. J Thorac Oncol. 16 (Suppl 1):S173–S174. 2021. View Article : Google Scholar
|
|
27
|
Sacher A, Le X, Cornelissen R, Shum E,
Suga J, Socinski M, Molina JR, Haura E, Clarke J, Bhat G, et al:
36MO Safety, tolerability and preliminary efficacy of poziotinib
with twice daily strategy in EGFR/HER2 Exon 20 mutant non-small
cell lung cancer. Ann Oncol. 32 (Suppl 1):S152021. View Article : Google Scholar
|
|
28
|
Elamin YY, Robichaux JP, Carter BW, Altan
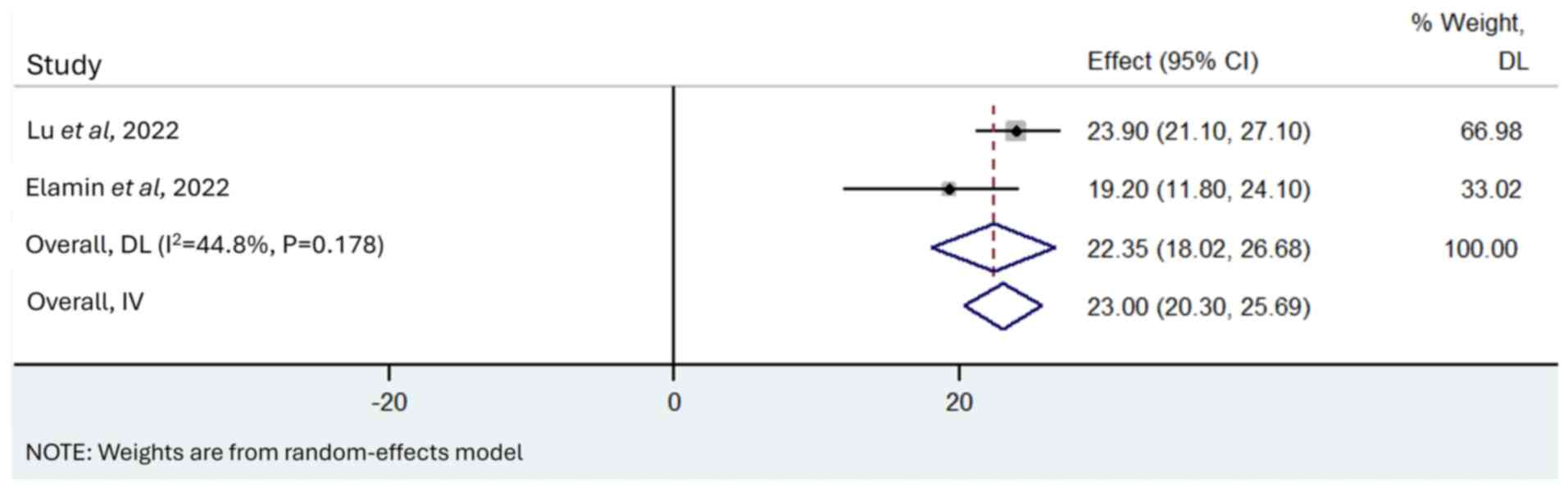
M, Tran H, Gibbons DL, Heeke S, Fossella FV, Lam VK, Le X, et al:
Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy,
resistance mechanisms, and impact of insertion location on drug
sensitivity. Cancer Cell. 40:754–767.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piotrowska Z, Tan DS, Smit EF, Spira AI,
Soo RA, Nguyen D, Lee VH, Yang JC, Velcheti V, Wrangle JM, et al:
Safety, tolerability, and antitumor activity of zipalertinib among
patients with non-small-cell lung cancer harboring epidermal growth
factor receptor exon 20 insertions. J Clin Oncol. 41:4218–4225.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan J, Wu L, Yang K, Zhao J, Zhao Y, Dai
X, Li M, Xie Y, Yao Y, Zhao M, et al: Safety, tolerability,
pharmacokinetics, and preliminary efficacy of YK-029A in
treatment-naive patients with advanced NSCLC harboring EGFR exon 20
insertion mutations: A phase 1 trial. J Thorac Oncol. 19:314–324.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han B, Zhou C, Zheng W, Wu L, Ma Z, Wang
H, Yu X, Ding G, Ma D, Nie L, et al: OA03.04 A phase 1b study of
furmonertinib, an oral, brain penetrant, selective EGFR inhibitor,
in patients with advanced NSCLC with EGFR exon 20 insertions. J
Thorac Oncol. 18 (Suppl 1):S492023. View Article : Google Scholar
|
|
32
|
Ou SI, Lin HM, Hong JL, Yin Y, Jin S, Lin
J, Mehta M, Nguyen D and Neal JW: Real-world response and outcomes
in patients with NSCLC with EGFR exon 20 insertion mutations. JTO
Clin Res Rep. 4:1005582023.PubMed/NCBI
|
|
33
|
Riess JW, Gandara DR, Frampton GM, Madison
R, Peled N, Bufill JA, Dy GK, Ou SI, Stephens PJ, McPherson JD, et
al: Diverse EGFR exon 20 insertions and co-occurring molecular
alterations identified by comprehensive genomic profiling of NSCLC.
J Thorac Oncol. 13:1560–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kwon CS, Lin HM, Crossland V, Churchill
EN, Curran E, Forsythe A, Tomaras D and Ou SI: Non-small cell lung
cancer with EGFR exon 20 insertion mutation: A systematic
literature review and meta-analysis of patient outcomes. Curr Med
Res Opin. 38:1341–1350. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Mao T, Wang J, Zheng H, Hu Z, Cao P,
Yang S, Zhu L, Guo S, Zhao X, et al: Toward the next generation
EGFR inhibitors: An overview of osimertinib resistance mediated by
EGFR mutations in non-small cell lung cancer. Cell Commun Signal.
21:712023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang W, Huang Y, Hong S, Zhang Z, Wang M,
Gan J, Wang W, Guo H, Wang K and Zhang L: EGFR exon 20 insertion
mutations and response to osimertinib in non-small-cell lung
cancer. BMC Cancer. 19:5952019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Low JL, Lim SM, Lee JB, Cho BC and Soo RA:
Advances in the management of non-small-cell lung cancer harbouring
EGFR exon 20 insertion mutations. Ther Adv Med Oncol.
15:175883592211461312023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang F, Li C, Wu Q and Lu H: EGFR exon 20
insertion mutations in non-small cell lung cancer. Transl Cancer
Res. 9:2982–2991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah MP, Aredo JV, Padda SK, Ramchandran
KJ, Wakelee HA, Das MS and Neal JW: EGFR exon 20 insertion NSCLC
and response to platinum-based chemotherapy. Clin Lung Cancer.
23:e148–e153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi J, Yang H, Jiang T, Li X, Zhao C,
Zhang L, Zhao S, Liu X, Jia Y, Wang Y, et al: Uncommon EGFR
mutations in a cohort of Chinese NSCLC patients and outcomes of
first-line EGFR-TKIs and platinum-based chemotherapy. Chin J Cancer
Res. 29:543–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang G, Li J, Xu H, Yang Y, Yang L, Xu F,
Xia B, Zhu VW, Nagasaka M, Yang Y, et al: EGFR exon 20 insertion
mutations in Chinese advanced non-small cell lung cancer patients:
Molecular heterogeneity and treatment outcome from nationwide
real-world study. Lung Cancer. 145:186–194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Li J, Zhou Y, Cao S, Ling X, Zhang
Y, Nie W and Zhong H: Tumor genomics and response to chemotherapy
in advanced non-small cell lung cancer with exon 20 insertion
epidermal growth factor receptor mutations. Ann Transl Med.
8:12972020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anand U, Dey A, Chandel AKS, Sanyal R,
Mishra A, Pandey DK, De Falco V, Upadhyay A, Kandimalla R,
Chaudhary A, et al: Cancer chemotherapy and beyond: Current status,
drug candidates, associated risks and progress in targeted
therapeutics. Genes Dis. 10:1367–1401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Le X, Cornelissen R, Garassino M, Clarke
JM, Tchekmedyian N, Goldman JW, Leu SY, Bhat G, Lebel F, Heymach JV
and Socinski MA: Poziotinib in non-small-cell lung cancer harboring
HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2
trial. J Clin Oncol. 40:710–718. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ou SI, Le X, Nagasaka M, Reungwetwattana
T, Ahn MJ, Lim DWT, Santos ES, Shum E, Lau SCM, Lee JB, et al: Top
20 EGFR+ NSCLC clinical and translational science papers that
shaped the 20 years since the discovery of activating EGFR
mutations in NSCLC. An editor-in-chief expert panel consensus
survey. Lung Cancer (Auckl). 15:87–114. 2024.PubMed/NCBI
|
|
46
|
Locke M, Aung WY and Seetharamu N: YK-029A
in the landscape of treatments for non-small cell lung cancer
(NSCLC) with EGFR exon 20 insertion mutations. AME Clin Trials Rev.
2:1–5. 2024. View Article : Google Scholar
|