Introduction
Pulmonary pleomorphic carcinoma (PPC) is a rare
aggressive subtype of non-small cell lung cancer (NSCLC),
accounting for 0.1–0.4% of cases (1,2).
Histologically, PPC exhibits biphasic malignant components (such as
spindle/giant cells and conventional carcinoma), and is classified
by the 2021 World Health Organization criteria as a sarcomatoid
carcinoma requiring ≥10% spindle or giant cell morphology (3). A large-scale study utilizing the
Surveillance, Epidemiology and End Results database revealed that
PPC predominantly affects Caucasian populations (80%), with a
median age of 66 years at diagnosis and a male predominance
(male-to-female ratio, 1.38:1) (4).
PPC exhibits a significantly poorer prognosis compared with other
NSCLC subtypes, and optimal therapeutic strategies remain
undefined. For early stage disease, surgical resection is the
preferred treatment and is critical for preventing recurrence and
metastasis. Marked by aggressive behavior, invasiveness and
heterogeneity, PPC is associated with a poor prognosis, with a
median overall survival time of 9 months and a 5-year survival rate
of only 23% (5). These findings
underscore the need to explore systemic therapeutic approaches for
PPC. Radiologically, PPC typically presents as peripheral solid
masses, although cystic manifestations occur in exceptionally rare
cases (6). Notably, only three
cases of PPC-associated cystic lesions have been reported in the
English literature (as of 2024) (7–9).
Although spontaneous pneumothorax (SP) is a rare complication of
lung malignancies (10), primary
cystic PPC with recurrent SP has not yet been documented.
The present report details a histopathologically
confirmed case of PPC manifesting as cystic airspaces and recurrent
SP, challenging conventional diagnostic paradigms. The findings
emphasize the potential of PPC to mimic benign cystic lesions and
highlight the necessity of considering malignancy in patients with
cystic lung disease and recurrent SP.
Case report
A 66-year-old man (height, 166 cm; weight, 57 kg)
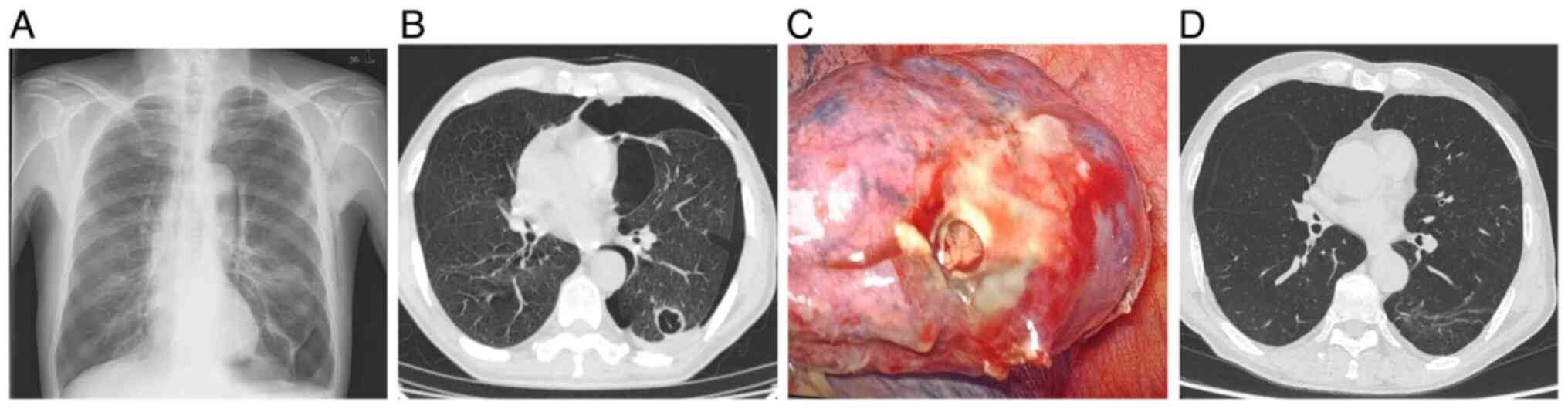
with a 40 pack-year smoking history presented with a SP in May 2022
(Fig. 1A). Initial computed
tomography (CT) results revealed bullous emphysema, prompting a
left-sided closed-tube thoracostomy. Despite initial resolution,
the patient experienced a recurrent pneumothorax within 2 weeks,
prompting further evaluation. High-resolution CT identified a
solitary cystic lesion in the left lower lobe with the following
malignant features: Uneven wall thickening (maximum 5.6 mm),
irregular nodular contours and heterogeneous internal septations
(Fig. 1B). Concurrently, serum
tumor markers were significantly elevated as follows:
Carcinoembryonic antigen (CEA; 4.32 ng/ml; normal range, 0–3.4
ng/ml), carbohydrate antigen 125 (CA125; 148.90 U/ml; normal range,
0–35 U/ml) and neuron-specific enolase (NSE; 34.66 ng/ml; normal
range, 0–16.3 ng/ml). Pulmonary function tests revealed mixed
ventilatory dysfunction [forced expiratory volume in 1 sec (FEV1)
1.01 liters (predicted normal, 2.73 liters; 37.1% predicted;
calculated postoperative FEV1, 29.3%)], corroborated by a shuttle
walk test (SWT) demonstrating limited functional capacity (320
meters). Video-assisted thoracoscopic surgery (VATS) revealed a
ruptured subpleural cyst, which led to a wedge resection (Fig. 1C).
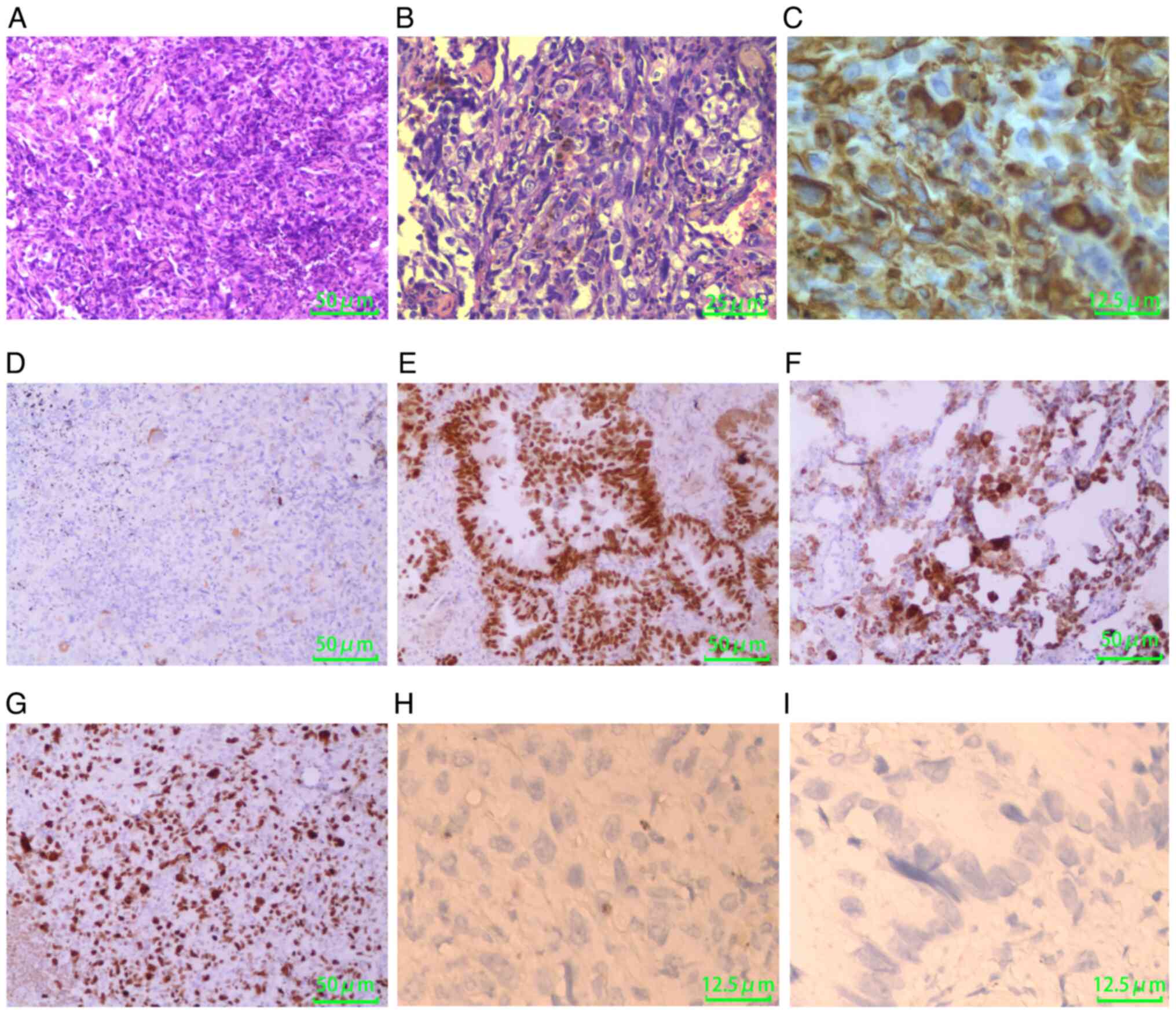
Histopathological examination of the wedge resection
confirmed the diagnosis of PPC with biphasic differentiation,
characterized by a predominant malignant spindle cell component
(80%) coexisting with conventional adenocarcinoma (20%), as
demonstrated by representative histological sections (Fig. 2A and B). Immunohistochemical
analysis (Data S1) revealed
diffuse vimentin expression within the sarcomatoid spindle cell
regions (Fig. 2C), while focal
cytokeratin immunoreactivity indicated residual epithelial
differentiation (Fig. 2D). The
adenocarcinoma component was validated through concurrent nuclear
transcription termination factor 1 (TTF-1) positivity (Fig. 2E) and cytoplasmic Napsin A
co-expression (Fig. 2F).
Proliferative metrics revealed aggressive biological behavior, with
a Ki-67 labeling index of 70% (Fig.
2G) and EGFR upregulation detected in 90% of tumor cells,
suggesting aberrant activation of oncogenic signaling pathways.
Molecular analysis revealed no actionable driver
mutations in EGFR, anaplastic lymphoma kinase (ALK), ROS1, KRAS,
MET, BRAF, ERBB2 or RET, and no expression of programmed
death-ligand 1 (PD-L1) (combined positive score=0) (Fig. 2H and I). In accordance with the
preferences of the patient, adjuvant therapy was not administered.
Surveillance consisted of semi-annual head and chest CT scans
combined with quarterly serum tumor marker monitoring. Notably,
clinical and imaging follow-up at 29 months demonstrated sustained
disease control, with no evidence of locoregional recurrence or
distant metastasis. Specifically, follow-up chest CT at 29 months
post-operation demonstrated the absence of locoregional recurrence
and pulmonary metastases (Fig.
1D).
Comprehensive immunohistochemical protocols
detailing tissue processing parameters, antibody validation data,
and quality control measures are documented in Data S1.
Discussion
PPC is the most prevalent subtype of pulmonary
sarcomatoid carcinoma (PSC) and comprises both epithelial carcinoma
and sarcomatous components (11).
The epithelial component includes traditional NSCLC features,
including squamous cell carcinoma and adenocarcinoma, while the
sarcomatous component (making up at least 10% of the tumor tissue)
consists of spindle cells or giant cells. Tumorigenesis and
malignant progression in cancer are synergistically driven by
genetic alterations and epigenetic dysregulation (12). PSC poses therapeutic challenges due
to its notable genetic heterogeneity and intricate epigenetic
landscape (3). Surgery is
considered the optimal treatment for resectable PSC (13), and due to the low sensitivity of PSC
to radiotherapy and chemotherapy, achieving negative surgical
margins is crucial for extending survival time in these patients,
irrespective of the tissue subtype (4,14).
Adjuvant chemotherapy is recommended for patients with surgically
resected stage II and III PSC (15). Traditional molecular targets such as
ALK and EGFR are infrequently observed in PSC and only a small
subset of patients are eligible for targeted therapy (16). Additionally, despite high levels of
PD-L1 expression in PSC, the response rates to programmed cell
death protein 1/PD-L1 inhibitors are only modest (40–55%) (17).
Cystic lung cancer is radiologically characterized
by thick-walled cystic airspaces with irregular walls and mural
nodules, which are predominantly observed in adenocarcinoma
subtypes (18). Thin-walled
solitary cysts in early stage lesions are frequently misdiagnosed
as bullae or benign cysts (19).
Notably, SP as an initial presentation of lung cancer remains
exceedingly rare. Yu et al (20) previously reported a recurrent
pneumothorax case where CT revealed a bullae-mimicking cystic
lesion in the right middle lobe that was later confirmed as
invasive adenocarcinoma. While PPC typically presents as solid
masses on CT, to the best of our knowledge, the present study
reports the first documented case of PPC manifesting as a primary
solitary cystic airspace with recurrent SP, highlighting its
imaging heterogeneity.
A review of the literature reveals limited reports
on purely cystic PPC. Iwamura et al (9) described a solitary subpleural cyst
exhibiting mural nodule enlargement over 5 years, diagnosed as
pT2aN0M0 PPC post-resection. The patient was not administered
adjuvant therapy and no recurrence was noted within 2 years. By
contrast, Yang et al (8)
documented a left lower lobe cystic lesion progressing to a 7-cm
solid mass over 3 years, staged as pT4N1M0 postoperatively,
suggesting a potential continuum from cystic to solid PPC. Although
the present case similarly featured a subpleural cystic lesion with
eventual pleural rupture, its biphasic histology (adenocarcinoma +
spindle cell carcinoma) aligns with prior reports, confirming the
cystic radiophenotypes across PPC subtypes.
It is of particular clinical note that the cases
reported by Iwamura et al (9) and Yang et al (8), and the present case, collectively
suggest that PPC may initially manifest as isolated cysts, evolving
into nodular or solid lesions over time. This implies that cystic
morphology might represent an uncommon early stage feature of PPC.
Mechanistically, cyst formation may involve check-valve-mediated
air entrapment (21), a
pathophysiological process shared with cystic adenocarcinoma,
necessitating an expanded classification of cystic lung cancer to
include PPC. However, Yamakawa et al (7) documented a PPC case exhibiting
triphasic histology (giant cells, spindle cells and focal
adenocarcinoma) that progressed paradoxically. Initially
manifesting as a solid mass, the mass subsequently developed
bilateral multifocal cystic metastases with recurrent pneumothorax,
defying conventional growth patterns. These findings underscore two
critical insights: i) High histological heterogeneity is associated
with aggressive behavior and metastatic potential; and ii)
metastatic PPC may exhibit multicystic cavities with pneumothorax,
necessitating differentiation from primary cystic lesions through
molecular profiling. Collectively, these observations delineate the
spectrum of the cystic manifestations of PPC, of which clinical
implications and biological underpinnings warrant further
large-scale investigations.
The present case involved dual challenges in both
diagnostic differentiation and surgical decision-making. The
initial task required distinguishing cystic lung cancer from benign
mimics such as bullae, cysts and tuberculous cavities, while the
second task centered on optimizing surgical safety given the severe
pulmonary impairment. In the present study, a 66-year-old male
patient with a 40 pack-year smoking history exhibited CT features
of malignancy in the left lower lobe of the lungs: A cystic lesion
exhibiting uneven wall thickening (maximum 5.6 mm) with irregular
contours and heterogeneous septations, consistent with high-risk
radiological markers. This suspicion of malignancy was further
supported by elevated CEA, CA125 and NSE serum levels (22). Progressive pneumothorax and evidence
of malignancy from the imaging prompted a multidisciplinary
consensus for VATS. Preoperative pulmonary function testing
revealed mixed ventilatory dysfunction (predicted FEV1 of 37.1%),
with a calculated postoperative FEV1 of 29.3%, which is below the
30% safety threshold established by European Respiratory
Society/European Society of Thoracic Surgeons and ACCP guidelines
(23,24). A 320-meter SWT further confirmed
elevated perioperative risk (25),
ultimately necessitating a lung-sparing wedge resection. The
intraoperative findings of visceral pleural penetration with
effusion suggested potential intrathoracic dissemination,
justifying the limited resection strategy. This approach
exemplifies precision risk-benefit analysis in patients with a
marginal pulmonary reserve.
The present case underscores the diagnostic
complexity of PPC. While intraoperative frozen sections confirmed
malignancy, a definitive diagnosis required extensive sampling and
immunohistochemical profiling of TTF-1, napsin A, cytokeratin and
vimentin, reflecting the intrinsic histological heterogeneity of
PPC. Despite pathological confirmation of pleural invasion (pT2a)
and occult metastasis risk, the patient declined adjuvant
chemotherapy, opting instead for personalized surveillance per the
Chinese NSCLC postoperative follow-up consensus (26). Although subclinical metastatic risk
persists, the 29-month recurrence-free survival of the patient may
be attributed to early R0 resection. Certain critical reflections
have emerged: First, the absence of contrast-enhanced CT may have
limited the evaluation of the mural enhancement patterns; second,
intraoperative cyst rupture despite saline lavage raised concerns
regarding pleural seeding, compounded by the absence of cytological
analysis of the effusion for staging completeness; and third,
recurrent pneumothorax served as an intervention catalyst, aligning
with the findings of Iwamura et al (9), where early surgical intervention in
stage IB PPC improved the outcome. Collectively, the present case
redefines the clinical trajectory of PPC, emphasizing that cystic
manifestations may represent an underrecognized early phenotype
requiring heightened vigilance in high-risk populations.
In conclusion, cystic airspaces represent a rare
radiological phenotype of PPC, frequently misdiagnosed as pulmonary
bullae. Early resection remains the cornerstone therapy of this
disease. Recurrent SP may indicate occult malignancy, necessitating
histopathological evaluation of any cystic lesions. These findings
redefine the imaging spectrum of PPC and mandate clinical vigilance
for cystic lung cancer, particularly in the context of
pneumothorax.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Wu Xianning,
Associate Chief Physician in the Department of Thoracic Surgery at
The First Affiliated Hospital of China Medical University (Hefei,
China), for providing guidance on manuscript preparation.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QL designed the study, collected the clinical data
for the case and compiled the first draft. JZ and XW performed
diagnostic evaluations through interpretation of imaging studies
and histopathological correlation, and acquired clinical data. YG
conducted histopathological analysis and immunohistochemical
evaluation of the surgical specimen. SZ and ZL were responsible for
preoperative patient management, participated in intraoperative
surgical decision-making, supervised data collection for
perioperative variables, and critically evaluated the manuscript's
clinical relevance. JL designed and performed the surgical
intervention, and critically revised the manuscript for
intellectual content. QL and JZ confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The clinical study in which the patient participated
was approved by the Ethics Committee of Bengbu Third People's
Hospital Affiliated to Bengbu Medical University (Bengbu, China;
approval no. 2024k12).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of clinical details and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kwon HJ, Lee S, Han YB, Lee J, Kwon S, Kim
H and Chung JH: Genomic landscape of pulmonary sarcomatoid
carcinoma. Cancer Res Treat. 56:442–454. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang X, Cheng Y, Yuan Z, Li Q, Huang Y
and Feng G: Clinical, pathological and treatment factors associated
with the survival of patients with pulmonary sarcomatoid carcinoma.
Oncol Lett. 19:4031–4039. 2020.PubMed/NCBI
|
|
3
|
Oi H, Taki T, Kuroe T, Sakamoto N,
Sakashita S, Kojima M, Sugiyama E, Umemura S, Sakai T, Izumi H, et
al: NETosis in pulmonary pleomorphic carcinoma. Cancer Sci.
116:524–532. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Liu J and Min L:
Clinicopathological characteristics, survival outcomes and
prognostic factors in pleomorphic carcinoma: A SEER
population-based study. BMC Pulm Med. 22:1162022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei Y, Wang L, Jin Z, Jia Q, Brcic L,
Akaba T and Chu Q: Biological characteristics and clinical
treatment of pulmonary sarcomatoid carcinoma: A narrative review.
Transl Lung Cancer Res. 13:635–653. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim C, Cho HH, Choi JY, Franks TJ, Han J,
Choi Y, Lee SH, Park H and Lee KS: Pleomorphic carcinoma of the
lung: Prognostic models of semantic, radiomics and combined
features from CT and PET/CT in 85 patients. Eur J Radiol Open.
8:1003512021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamakawa H, Yoshida M, Yabe M, Baba Y,
Baba E, Katagi H, Ishikawa T, Takagi M, Nakada T, Akiba T and
Kuwano K: Pulmonary pleomorphic carcinoma detected as a result of
pneumothorax and the subsequent occurrence of multiple cystic
metastases. Case Rep Med. 2014:2192732014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Mei J and Lin F: Pleomorphic
carcinoma of the lung: From thin-walled cavity to solid tumor. Ann
Transl Med. 7:2732019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwamura M, Nishimori M, Iwasa H, Otani M,
Nakaji K, Nitta N, Miyatake K, Yoshimatsu R, Yamanishi T, Matsumoto
T, et al: A case of pulmonary pleomorphic carcinoma associated with
cystic airspace. Radiol Case Rep. 18:2692–2696. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai RS, Perng RP and Chang SC: Primary
lung cancer complicated with pneumothorax. Jpn J Clin Oncol.
22:194–197. 1992.PubMed/NCBI
|
|
11
|
Li X, Wu D, Liu H and Chen J: Pulmonary
sarcomatoid carcinoma: Progress, treatment and expectations. Ther
Adv Med Oncol. 12:17588359209502072020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sindhu RK, Verma R, Salgotra T, Rahman MH,
Shah M, Akter R, Murad W, Mubin S, Bibi P, Qusti S, et al:
Impacting the remedial potential of nano delivery-based flavonoids
for breast cancer treatment. Molecules. 26:51632021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Q, Li J, Sun N, Xue Q, Gao Y, Zhao J,
Mao Y, Mu J, Wang D, Gao S and He J: Preoperative systemic
immune-inflammation index predicts survival and recurrence in
patients with resected primary pulmonary sarcomatoid carcinoma.
Transl Lung Cancer Res. 10:18–31. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferhatoglu F, Amirov F, Ozkan B, Kara M,
Toker A, Ak N, Aydın E, Paksoy N, Yilmazbayhan D and Aydiner A:
Clinicopathological and prognostic features of 67 cases with
pulmonary sarcomatoid carcinoma: An 18-year single-centre
experience. Oncol Res Treat. 44:590–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batchelor TJP: Commentary: Resected
pulmonary sarcomatoid carcinoma-a defined treatment paradigm, or
just the end of the beginning of the search? J Thorac Cardiovasc
Surg. 163:1683–1684. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schrock AB, Li SD, Frampton GM, Suh J,
Braun E, Mehra R, Buck SC, Bufill JA, Peled N, Karim NA, et al:
Pulmonary sarcomatoid carcinomas commonly harbor either potentially
targetable genomic alterations or high tumor mutational burden as
observed by comprehensive genomic profiling. J Thorac Oncol.
12:932–942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Daylan AE, Deng L, Yang J, Sharma
J, Su C, Li S, Zang X, Halmos B, Borczuk A and Cheng H:
Heterogeneous expression of PD-L1, B7×, B7-H3, and HHLA2 in
pulmonary sarcomatoid carcinoma and the related regulatory
signaling pathways. Cancers (Basel). 15:33722023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendoza DP, Heeger A, Mino-Kenudson M,
Lanuti M, Shepard JO, Sequist LV and Digumarthy SR:
Clinicopathologic and longitudinal imaging features of lung cancer
associated with cystic airspaces: A systematic review and
meta-analysis. AJR Am J Roentgenol. 216:318–329. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang K, Leng X, Yi H, Zhang G, Hu Z and
Mao Y: Lung cancer associated with cystic airspaces: Current
insights into diagnosis, pathophysiology, and treatment strategies.
Cancers (Basel). 16:39302024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu L, Lou Y and Zhu D: A case of
adenocarcinoma presenting with cystic lesion and recurrent
pneumothoraces. J Cardiothorac Surg. 19:5762024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheard S, Moser J, Sayer C, Stefanidis K,
Devaraj A and Vlahos I: Lung cancers associated with cystic
airspaces: Underrecognized features of early disease.
Radiographics. 38:704–717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saad HM, Tourky GF, Al-Kuraishy HM,
Al-Gareeb AI, Khattab AM, Elmasry SA, Alsayegh AA, Hakami ZH,
Alsulimani A, Sabatier JM, et al: The potential role of MUC16
(CA125) biomarker in lung cancer: A magic biomarker but with
adversity. Diagnostics (Basel). 12:29852022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brunelli A, Charloux A, Bolliger CT, Rocco
G, Sculier JP, Varela G, Licker M, Ferguson MK, Faivre-Finn C,
Huber RM, et al: ERS/ESTS clinical guidelines on fitness for
radical therapy in lung cancer patients (surgery and
chemo-radiotherapy). Eur Respir J. 34:17–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brunelli A, Kim AW, Berger KI and
Addrizzo-Harris DJ: Physiologic evaluation of the patient with lung
cancer being considered for resectional surgery: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest. 145
(5 Suppl):e166S–e190S. 2014.
|
|
25
|
Fennelly J, Potter L, Pompili C and
Brunelli A: Performance in the shuttle walk test is associated with
cardiopulmonary complications after lung resections. J Thorac Dis.
9:789–795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lunxu L, Shugeng G, Jianxing H, Jian H, Di
G, Hecheng L, Mingqiang K, Fengwei T and Kaican C; Chinese
Association of Thoracic Surgeons, : Thoracic Surgery Society of
China Medicine Education Association: Chinese thoracic surgery
experts consensus on postoperative follow-up plans for non-small
cell lung cancer patients. Chinese J Clinical Thoracic and
Cardiovascular Surgery. 28:4–10. 2021.
|