Hepatocellular carcinoma (HCC) is the sixth most
common malignant tumor in the world and the third greatest cause of
cancer-associated mortality (1).
The global incidence rate of HCC is expected to exceed 1 million
cases by 2025, mainly driven by chronic viral hepatitis and
metabolic related liver disease, of which metabolic related liver
disease cases account for 15–20% in western countries (2). HCC often remains asymptomatic in the
early stages, therefore, diagnosis typically occurs in the late
stages, and the 5-year survival rate of patients with advanced HCC
(aHCC) is <15%, underscoring the limitations of current aHCC
treatment options (3).
Since its confirmation in the SHARP trial,
sorafenib, a multi-kinase inhibitor, has been routinely used as the
first-line treatment for aHCC (4).
However, the effectiveness of sorafenib is limited. Compared with
the placebo, it increased the median overall survival (mOS) by 2.8
months. However, it typically has side effects (hand-foot skin
reaction in 30–60% of patients, diarrhea in 40–55%) that adversely
affect the quality of life (4). Due
to these constraints, new treatments for patients with aHCC have
been researched to increase survival rates and quality of life
(5).
Immune checkpoint inhibitors (ICIs) have potential
in treating aHCC because of their immune system modulatory activity
(5,6). The CheckMate 040 and KEYNOTE-224
trials (7,8) revealed that nivolumab and
pembrolizumab are both safe and effective in treating aHCC.
Despite advances in immunotherapy and targeted
therapy for aHCC, a considerable proportion of patients are unable
to obtain effective treatment because of variables such as the
complicated tumor microenvironment (TME) of HCC (9). As a result, novel therapeutic
strategies integrate multiple existing therapeutic approaches to
use the complementary advantages of each strategy while addressing
their limitations. Radiotherapy (RT) + ICI combinations have shown
promise in non-small cell lung cancer and melanoma, improving
survival by modulating the TME (10). Therefore, RT + ICI combinations in
HCC may enhance tumor response to immunotherapy. Clinicians have
hypothesized combining RT and ICIs as a potential synergistic
method to improve the treatment of aHCC (11–13).
The justification for combining RT and ICIs in
treating aHCC stems from their complementary mechanisms and
antitumor efficacy. This combination therapy may be a more
effective and comprehensive method of combating aHCC (14). RT has traditionally been considered
a local treatment, but has been discovered to promote systemic
immune responses via the ‘abscopal effect’, in which the tumor in
the irradiated area degenerates due to immune activation (15,16).
Radiation-induced cell death causes the production of
tumor-associated antigens and damage-associated molecular patterns
(DAMPs), which initiate an antitumor immune response (17,18).
Furthermore, radiation can promote tumor immunogenicity by
increasing expression of major histocompatibility complex class I
and T cell infiltration and altering the TME (11,19).
These radiation-induced changes convert immunologically ‘cold’
tumors (lacking immune cell infiltration and antigen presentation)
into ‘hot’ tumors (with immune recognition and attack capacity),
thereby increasing their susceptibility to ICIs (20,21).
Moreover, ICIs boost the systemic immune response, overcoming the
limitations of radiation, which may not totally eradicate
malignancy.
However, there are challenges associated with
optimizing the combination of RT and ICIs for aHCC (26,27).
Determining the appropriate sequencing, dosage and timing of RT in
comparison with those of ICIs is key (28,29).
Additionally, discovering predictive biomarkers for response to
combination therapy is essential for tailored treatment approaches
(30).
The present review aimed to summarize processes
through which RT and ICIs may interact to improve clinical outcomes
and methods to enhance this combination technique, as well as the
potential of low-dose radiation treatment (LDRT) as a complementary
or alternative strategy to high-dose regimens, particularly when
paired with ICIs (31–33). Furthermore, the present review aimed
to highlight the role of advanced imaging techniques and
specialized equipment in accurately targeting tumor tissue and
minimizing damage to surrounding healthy tissue, which is key for
the successful implementation of LDRT (34,35).
Additionally, the present review aimed to summarize
clinical trials assessing the safety and efficacy of combining RT
and ICIs in aHCC, including those that have assessed the impact of
different sequencing and timing strategies on treatment outcomes
(36–39). Identification and validation of
reliable biomarkers for predicting response to combined RT and ICIs
may improve patient selection and therapy optimization (40–47).
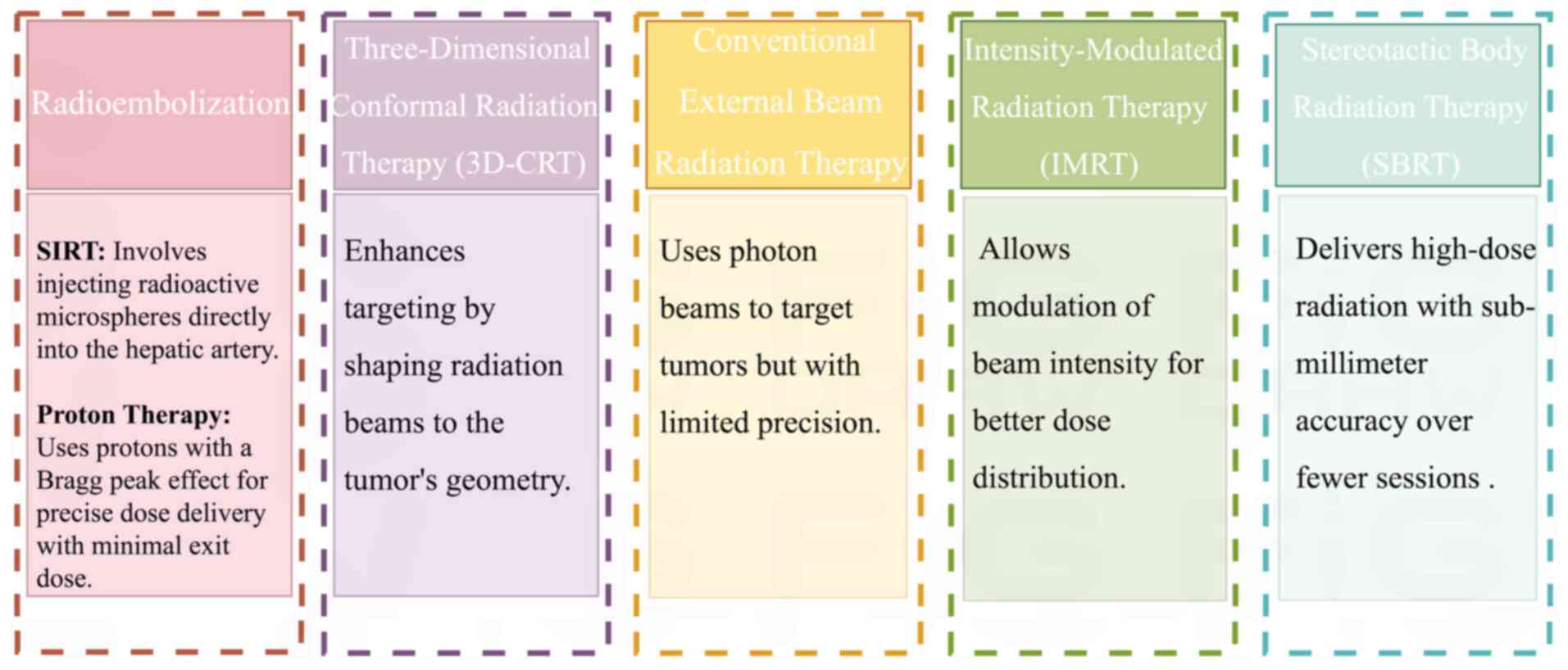
Historically, the use of external beam radiation
therapy (EBRT) for HCC has been limited because of the
radiosensitivity of the liver and the risk of radiation-induced
liver disease (RILD) (48).
Attempts to cure HCC with conventional radiation have shown limited
efficacy and severe toxicity because it is challenging to provide
an adequate radiation dose to tumors without destroying the
surrounding healthy liver tissue (49,50).
Developments in imaging and radiation transmission technology
improved the accuracy of tumor targeting (51–53).
Steroetactic body radiation therapy (SBRT) and three-dimensional
conformal radiation have promise in the treatment of HCC (54,55).
According to reports, local control rates have improved, and
hazardous reactions are now acceptable (56,57).
Blomgren et al (58), for
example, reported that high-dose fractionated radiation achieves
local tumor suppression in different types of liver cancer,
including HCC, with manageable side effects.
Despite these advancements, irradiation for HCC has
limitations, including radiation-induced hepatotoxicity,
variability in tumor radiation sensitivity and technical complexity
(59,60). Technical complexity refers to the
challenges involved in accurately targeting tumors in the liver
while sparing surrounding healthy tissue. This is difficult due to
the anatomical variations of the liver, its proximity to key
structures and the need to optimize radiation planning to minimize
damage to healthy liver tissue while ensuring sufficient tumor dose
delivery. These challenges require advanced imaging technology and
highly precise radiation delivery systems, which can vary depending
on tumor size, location and liver function (61).
Liver toxicity has switched from ‘classical’ to
‘non-classical’ RILD, with the former primarily characterized by
widespread damage due to whole-liver irradiation, while the latter
manifests as localized damage and elevated liver enzymes. This is
often associated with an increased production of pro-inflammatory
cytokines, such as TNF-α and interleukins, which contribute to
hepatocyte injury and the activation of fibrogenic pathways. By
contrast, non-classical RILD involves more localized liver damage
and is associated with elevated levels of transaminases and
jaundice (62). This form of
toxicity is hypothesized to result from endothelial cell damage,
increased vascular permeability and a dysregulated immune response
in the irradiated liver region (62). Baseline liver function is the
primary predictor of liver toxicity after SBRT, and patients with
Child-Pugh grade ≥B8 have a greater risk (63,64).
Cárdenes et al (63)
revealed that a Child-Pugh score of ≥8 is associated with severe
liver toxicity or mortality in ≤6 months. The American Society for
Radiation Oncology guidelines establish dosage limits on the basis
of various Child-Pugh scores: 15–18, 13–15 and 8–10 Gy for
non-cirrhotic, Child-Pugh A and B7 grades, respectively (65,66).
Technical advancements have markedly decreased these constraints.
Modern EBRT employs three-dimensional computed tomography (CT) and
intensity-modulated RT, as well as improved imaging technology, to
portray tumors while preserving normal liver tissue (67,68).
These approaches accommodate differing dosage distributions,
allowing the dose of the tumor-specific treatment to be gradually
increased while minimizing exposure to healthy liver parenchyma
(69) (Fig. 1).
Chemotherapy and targeted molecular therapies are
less popular in the treatment of aHCC due to key limitations.
Chemotherapy exhibits poor efficacy, with high rates of
chemo-resistance and considerable toxicity, particularly in
patients with underlying liver dysfunction, resulting in minimal
survival benefits (70). Targeted
therapies, such as sorafenib and other tyrosine kinase inhibitors,
offer modest improvements in OS while being associated with
substantial side effects and a narrow therapeutic window (71). Additionally, resistance to targeted
therapies often develops, driven by tumor heterogeneity and the
immunosuppressive TME. These factors, combined with their lack of
impact on modulating the TME, render chemotherapy and targeted
molecular therapies less favorable compared with emerging
strategies such as the combination of RT and ICIs. By contrast, RT
combined with ICIs is preferred for aHCC (27). RT techniques such as SBRT and proton
therapy provide precise dose delivery, ideal for targeting small-
and medium-sized tumors in complex locations, making it more
effective for tumors that are large or irregularly shaped (72). Second, RT enhances immune activation
by altering the TME, promoting immune cell infiltration and
improving tumor recognition, which synergizes with ICIs to boost
immune responses, particularly in advanced stages where immune
activity is often suppressed (73).
Additionally, combining RT with ICIs helps overcome resistance to
immunotherapy by enhancing T cell activation and preventing immune
exhaustion (27). Moreover, RT +
ICIs offers comprehensive treatment by targeting both primary
tumors and metastases and providing broader control over both
systemic and local disease, unlike therapies such as
radioembolization, which are more suited for localized disease or
portal vein invasion. Additionally, compared with tyrosine kinase
inhibitors (such as sorafenib), which primarily target
angiogenesis, RT + ICI combinations address both local tumor
control and systemic immune activation, offering a dual therapeutic
advantage (74). Finally, ongoing
clinical trials show promising results, indicating that RT + ICIs
provides enhanced antitumor activity and safety for patients with
aHCC (36,75). Furthermore, careful patient
selection, precise treatment planning and interdisciplinary
collaboration are needed to improve outcomes and minimize adverse
effects.
The combination of radiotherapy and systemic
treatments, particularly ICIs, represents a promising frontier in
the treatment of aHCC. Radiation can affect the TME, improve immune
system identification of tumor cells and interact with ICIs
(24). Clinical trials are being
performed to determine the efficacy and safety of RT in combination
with ICIs and other systemic treatments (36,75).
This holistic strategy aims to improve the local and systemic
control of aHCC while also addressing primary tumors and potential
metastatic illness (Fig. 2).
The introduction of ICIs has transformed the
therapeutic landscape for HCC, particularly for patients with
advanced-stage illness. Traditional systemic medicines provide some
survival advantage for these patients, emphasizing the need for
innovative therapeutic options. ICIs have emerged as promising
medications that induce an efficient antitumor response via the
human immune system (4,25).
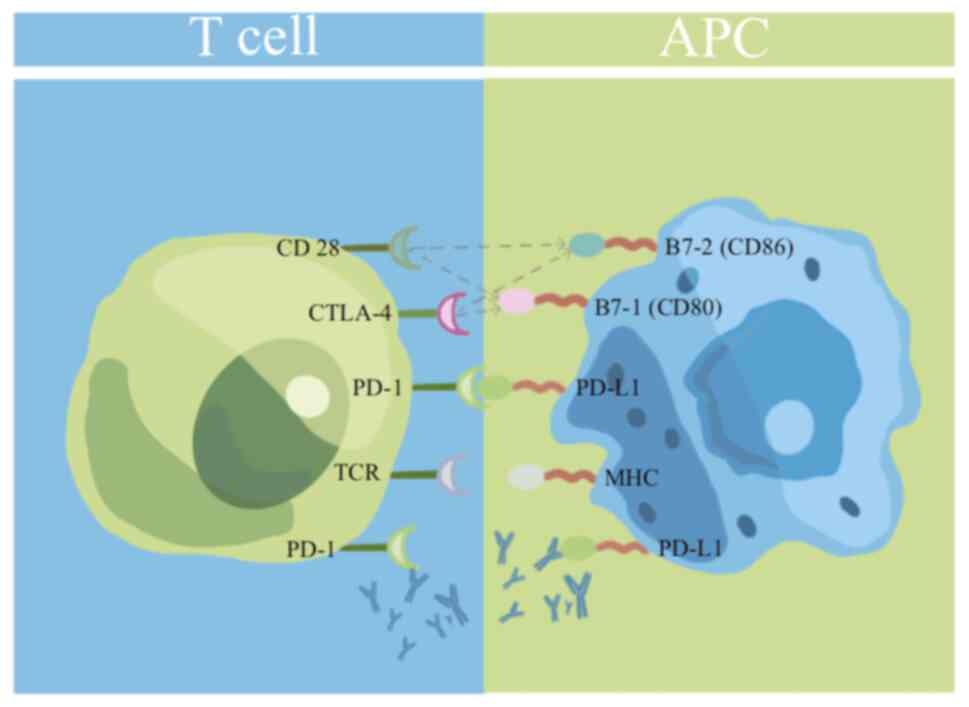
The ICI revolution in HCC is based on immune
checkpoint regulation, specifically PD-1/PD-L1 and CTLA-4 (76,77).
Tumors frequently exploit these molecular pathways, which are
necessary for maintaining immunological homeostasis, to avoid
immune surveillance. Checkpoint inhibitors revive depleted T cells
by eliminating inhibitory signals, boosting antitumor immunity
(78). (Fig. 3).
PD-1 drugs, including nivolumab and pembrolizumab,
have demonstrated clinical success in treating aHCC. The CheckMate
040 and KEYNOTE-224 studies (8,79)
reported objective response rates of 14–20 and 17%, respectively,
indicating the possibility of disrupting the PD-1 axis. However,
these response rates underscore the limited efficacy in treating
HCC relative to other types of cancer (80). This variability in patient reactions
highlights the complicated immunological landscape of aHCC and
presence of immune subgroups within the patient population. The
relatively low curative effect may be attributed to several
factors, including the inherent immunosuppressive environment, the
high heterogeneity of HCC tumors, the possibility of chronic liver
diseases that impair immune function and the presence of
immunosuppressive cells in the TME (81). Furthermore, HCC tumors may lack
sufficient novel antigens or tumor mutation burdens to elicit
robust immune responses (82). This
requires further research into the mechanisms of response and
resistance, as well as the creation of more tailored ICIs, to
overcome these limits and enhance the treatment outcomes for
patients with HCC.
Given the poor efficacy of ICIs as monotherapies,
researchers have investigated combination methods with other
treatment modalities, such as RT and anti-antigenic drugs, to
improve therapeutic outcomes (83–86). A
notable example is the IMbrave 150 study (87), which assessed the combination of
atezolizumab (an anti-PD-L1 antibody) and bevacizumab [an
anti-vascular endothelial growth factor (VEGF) drug]. After a
median follow-up of 61.9 months, the mOS was 21.0 months [95%
confidence interval (CI): 10.4–31.6 months]. The OS rates for 3, 4
and 5 years were 36.4, 25.7 and 25.7%, respectively (88). This combination is superior to
sorafenib in the first-line treatment of aHCC, and exhibits
synergistic potential. This technique improves the killing effect
of T cells on tumor cells and modulates the tumor vascular system,
potentially enhancing drug delivery and T cell infiltration,
allowing the simultaneous treatment of multiple aspects of tumor
biology (89).
The most recent advancements in ICIs emphasize the
prospect of PD-1/PD-L1 inhibition, and the concurrent investigation
of CTLA-4 blockade has potential, particularly in combination
therapy (23,90,91).
The HIMALAYA study (92), which
used durvalumab (an anti-PD-L1 antibody) and tremelimumab (an
anti-CTLA-4 antibody), highlights the concept of dual checkpoint
blocking in aHCC. This method targets independent but complementary
immune regulatory pathways, potentially overcoming resistance
mechanisms and increasing the breadth and depth of antitumor immune
responses.
The synergy of these pathways can be attributed to
their distinct effects on T cell priming and effector activity.
CTLA-4 suppression impacts the priming phase in lymphoid organs,
whereas PD-1/PD-L1 blocking improves effector T cell activity
(93,94). This complementary process provides a
theoretical foundation for development of future combined therapy
options.
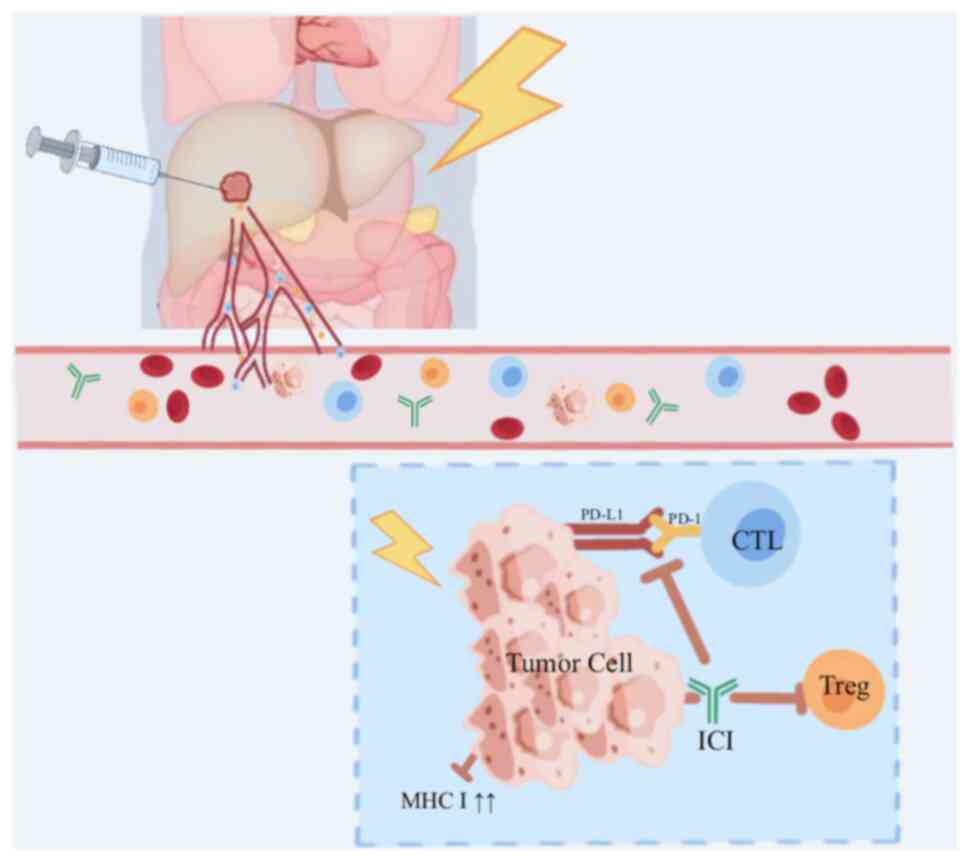
For patients with aHCC, who frequently have few
therapeutic options and a poor prognosis, combining RT + ICIs may
provide clinical advantages. In addition to enhancing local tumor
management, the synergistic effect can trigger systemic antitumor
responses, leading to the resolution of primary tumors and
metastatic lesions (95,96) (Fig.
4). This strategy is based on radiation-induced immunogenic
cell death (ICD), a unique form of cell death that can effectively
promote the immune system to target tumor cells.
Following RT, DAMPs are produced as a result of
radiation-induced cell death. DAMPs, such as calreticulin, high
mobility group box 1 protein (HMGB1) and ATP, are released from
tumor cells undergoing necrosis or apoptosis. These molecules serve
a key role in modulating immune responses in HCC by acting as
endogenous ligands for pattern-recognition receptors (PRRs),
including toll-like receptors, on immune cells (97–99).
Following receptor activation, DAMPs initiate innate immune
responses, which are key for the subsequent activation of adaptive
immunity (97). This
innate-to-adaptive immune transition is key in the context of ICI
therapy, as it helps bridge the gap between the two immune
systems.
DAMPs are key for the maturation of dendritic cells
(DCs) and the enhancement of antigen presentation (98,100).
They promote the activation of DCs and other antigen-presenting
cells, improving T cell priming and activation (101,102). Garg et al (97) have revealed that DAMPs, such as
surface-exposed calreticulin, secreted ATP and passively released
HMGB1, interact with phagocytosis receptors, purinergic receptors
and PRRs, respectively. These interactions are key for inducing
ICD, leading to the activation of potent anticancer immunity
(97,103–106). This helps overcome the immune
suppression in the advanced stages of HCC. Immune activation is an
important strategy to overcome immune suppression in aHCC (107). Chen et al (108) demonstrated that heat shock protein
70 (HSP70) levels (a DAMP) are markedly higher in the serum of
patients with HCC compared with healthy individuals, while antibody
levels remain unchanged, suggesting that HSP70 may counter immune
suppression via non-antibody mechanisms. Ren et al (109) revealed that during HCC
development, damaged or dying cells release DAMPs, which serve as
ligands for cyclic GMP-AMP synthase (cGAS). This activates the
cGAS-stimulator of interferon genes signaling pathway, potentially
enhancing the anti-tumor immune response of the body (109). Furthermore, when synergized with
ICIs such as PD-1/PD-L1 inhibitors, DAMPs enhance immune responses
by restoring T cell function and counteracting immune exhaustion
(97).
RT further illustrates the importance of DAMPs in
immune activation. Radiation-induced ICD results in the release of
DAMPs, such as calreticulin, HMGB1 and ATP, which enhance the
ability of the immune system to target tumor cells. These DAMPs
activate both innate and adaptive immune responses, effectively
transforming irradiated tumors into in situ vaccines
(99,110). Calreticulin, which translocates to
the cell surface following radiation, serves as an ‘eat-me’ signal
for DCs, prompting them to recognize and engulf dying tumor cells.
HMGB1 and ATP stimulate immune activation by binding receptors on
DCs, leading to the initiation of robust anti-tumor immunity
(98).
The complementary effects of radiation-induced DAMPs
(such as calreticulin, HMGB1, ATP) on the TME further elucidate the
synergistic interaction between RT and ICIs. Singh et al
(111) reported that intercellular
adhesion molecule 1 (ICAM-1) serves a key role in breast carcinoma
metastasis, promoting adhesion between breast cancer and
endothelial cells, enhancing circulating tumor cell cluster
formation and tumor cell survival. RT can modify the TME by
altering cytokine secretion patterns, increasing the expression of
adhesion molecules such as ICAM-1 and vascular CAM-1, and altering
chemokine gradients (111). These
modifications increase the recruitment and activation of effector
immune cells, such as cytotoxic T lymphocytes and natural killer
cells. Moreover, ICIs avoid T cell failure by inhibiting inhibitory
signals (PD-1/PD-L1 and CTLA-4), increasing long-term antitumor
activity and enhancing these effects. Exosomes regulate tumor
growth, invasion, metastasis, angiogenesis and immune therapy
resistance, serving a key role in intercellular communication in
the TME (112,113). They carry proteins, DNA, microRNA
and long non-coding RNA, altering recipient cell functions and
phenotypes. RT factors enhance or inhibit these effects,
influencing tumor progression and increasing their vulnerability to
ICIs (114).
Preclinical investigations have established the
existence of synergistic effects of RT + ICIs in models of aHCC
(22,23,115).
Pedros et al (115)
revealed that in mouse models of melanoma and prostate cancer, the
CTLA4 signaling pathway within Treg cells is key for suppressing
tumor immunity. Tregs lacking relevant key proteins weaken their
inhibitory function, leading to inhibited tumor growth, increased
levels of effector T cells and enhanced function (115). Studies have indicated increased
infiltration of CD8+ T cells and production of effector
cytokines, including IFN-γ and decreased Tregs within the TME
(22,23). Furthermore, radiation stimulates the
diversification of the T cell receptor (TCR) pool, identifying a
broader range of tumor antigens (116,117). Binder et al (117) revealed that RT stimulates the
diversification of TCR pool and expands the recognition range of
tumor antigens. When combined with ICIs (such as anti-CTLA-4), RT
can increase the diversity of TCR clones within tumors and enhance
the anti-tumor effect of the immune system (116,117). This diversification decreases the
chance of immune escape and increases the persistent response.
Clinically, the abscopal effect, a phenomenon in
which local RT causes regression of distant unirradiated tumors,
demonstrates the efficacy of this combination method in systemic
treatment (118). Although
uncommon, the abscopal effect demonstrates that combining RT + ICIs
induces broad antitumor immunity. ICIs have the ability to elicit
broad antitumor immune responses. For patients with aHCC with
numerous foci and metastases, this combined therapy may provide a
solution for both local and distant tumor burden (119).
The continuous updating of technology and
developments in treatment procedures provide a foundation for
further improving the overall effectiveness of RT and ICIs.
Advanced radiation procedures, such as SBRT, accurately administer
high radiation doses while causing minimum injury to adjacent
tissue (120). When combined with
novel ICIs, these strategies provide therapeutic advantages,
including tailored cancer vaccines, off-the-shelf T cell therapies,
and oncolytic viruses, which operate through complementary but
non-overlapping mechanisms (113).
Furthermore, treatment planning and prediction can be improved by
computational technologies such as radiomics and artificial
intelligence.
For patients with aHCC, RT in conjunction with ICIs
may improve survival rates and quality of life by balancing local
tumor focus resolution with systemic metastasis management. Future
research should refine treatment regimens, identify resistance
mechanisms and develop biomarkers that can predict treatment
efficacy.
The combined strategy of RT + ICIs in treating aHCC
is increasingly supported by preclinical and clinical evidence due
to the synergistic effect of radiation-induced immunomodulation and
IC suppression (84,121). Preclinical studies have clarified
how RT increases tumor immunogenicity (10,22,122).
Radiation, for example, causes ICD, as evidenced by the release of
DAMPs, including calreticulin, HMGB1 and ATP. These DAMPs stimulate
DC maturation and antigen presentation, effectively bridging innate
and acquired immunity (100,123). Furthermore, RT increases
tumor-associated antigen expression and upregulates key MHC-like
molecules, making cytotoxic T cells more visible in the tumor
(124).
In aHCC-specific models, the combination of RT +
ICIs has demonstrated substantial antitumor activity (22,125).
Combining radiation with anti-PD-1 or anti-CTLA-4 antibodies has
increased CD8+ T cell infiltration and decreased Tregs
within the TME (116,126,127). Twyman-Saint et al (116) reported the tumor regression effect
of anti-CTLA4 antibody and radiation therapy on patients with
metastatic melanoma, and reproduced this effect in a mouse model.
Drug resistance is associated with the upregulation of PD-L1 and T
cell depletion in melanoma cells (115). The optimal therapeutic response
requires the combination of RT, anti-CTLA4 and anti-PD-L1/PD-1
(116,126,127). Also, RT changes the
immune-suppressing TME by decreasing immune-suppressing cytokines
and myeloid-derived suppressor cells (10,128).
Early-phase trials have investigated the safety of
RT + ICIs and preliminary efficacy in patients with aHCC,
translating these preclinical findings into clinical settings
(36,129). Juloori et al (36) conducted a phase I trial in patients
with aHCC, comparing SBRT followed by nivolumab alone or nivolumab
with ipilimumab (36). This study
revealed adequate safety. Within 6 months of starting SBRT, 15.4%
of patients experienced dose-limiting effects. The combination of
SBRT with nivolumab and ipilimumab had ORR of 57%, a median
progression-free survival time of 11.6 months and a mOS time of
41.6 months. Tai et al (129) conducted a phase I trial in
patients with aHCC, evaluating the combination of SBRT and
nivolumab (129). This combination
was well tolerated, and the toxicity was moderate, consisting
primarily of fatigue and skin events. Preliminary efficacy results
suggested an encouraging ORR.
Other phase II trials have evaluated the efficacy of
RT + ICI combinations in larger populations (38,130,131). Li et al (38) evaluated a combined therapy of SBRT,
camrelizumab (an immune checkpoint inhibitor) and apatinib (a
targeted anti-angiogenic drug) in HCC patients with portal vein
tumor thrombus. The study reported a median overall survival of
12.7 months (95% CI: 10.2-not reached) and a median
progression-free survival of 4.6 months (95% CI: 3.3–7.0). While
the treatment was generally tolerable, over 22% of patients
experienced grade 3 or higher adverse effects, such as hypertension
or liver enzyme elevations.
Owing to the lack of extensive phase III data,
retrospective analyses provide key insight into the true efficacy
of RT-ICIs (37,132,133). Ning et al (134) conducted a retrospective analysis
on the outcomes of 36 patients with aHCC treated with RT and
anti-PD-1 drugs and reported markedly higher ORR and OS rates when
compared with ICIs alone.
Optimizing the combination of RT and ICIs for aHCC
remains an issue. Determining the appropriate sequencing, dosage
and timing of RT in comparison with those of ICIs is key (28,29).
Furthermore, discovering predictive biomarkers for response to
combination medication is key for tailored treatment (80).
Clinical trials will likely offer high-level data to
demonstrate the efficacy and safety of combined RT + ICIs in aHCC
(Table I). These studies are key
for future clinical practice and to redefine the landscape of aHCC
treatment.
The synergistic combination of RT and ICIs is a
feasible technique for improving treatment for patients with aHCC.
Numerous variables must be addressed to obtain the best therapeutic
benefit while minimizing toxicity, including dosage, timing,
staging and patient-specific characteristics.
The dosage and manner of RT must be optimized to
maximize synergy with ICIs in patients with aHCC. Historically,
higher doses/fraction, such as those utilized in hypofractionated
SBRT, have been employed to produce strong ICD and increase
antitumor immune responses (135,136). Robbins et al (137) used a 40 Gy regimen delivered in
five parts, demonstrating a move toward providing greater doses
over shorter treatment duration to enhance immune activation and
improve patient compliance (137).
The choice of RT mode is important in maximizing
treatment outcomes. Advanced technologies, such as proton treatment
and SBRT, provide precision targeting, maximizing the tumor dose
while minimizing damage to adjacent healthy tissue. Several factors
influence the choice between these techniques, including the size
and location of the tumor, the liver function of the patient and
the specific immunological goals (34,35).
Integrating LDRT as a supplement to these RT techniques may affect
immune activation. Given the ability of LDRT to regulate the TME to
promote immune-mediated tumor elimination (138), its combination may improve the
overall therapeutic efficacy of these targeted RT technologies.
Although LDRT holds promise, its precise delivery and monitoring
presents challenges that limit its wider clinical application
(139). The technical complexity
of LDRT requires highly accurate targeting of tumor tissue while
minimizing damage to surrounding healthy tissue, demanding advanced
imaging techniques and specialized equipment, which may not be
available in all clinical settings. Additionally, the variability
in tumor shape, locations and movement, such as respiratory motion,
complicates consistent treatment delivery, necessitating real-time
adjustments (140). Achieving the
required dosimetric precision is a challenge, as high doses of
radiation delivered to small, localized areas can cause toxicity to
nearby structures (141).
Furthermore, real-time monitoring of treatment effects on both
tumor and normal tissue remains difficult, as current imaging
technology may not provide sufficient resolution or immediate
feedback, leading to potential inaccuracies.
The sequence and timing of RT and ICIs are key
factors in enhancing the synergy of combination therapy in patients
with aHCC. ICI after radiotherapy is more effective than taking ICI
before radiotherapy in other tumors (142). This technique exploits
immunostimulatory effects, increasing immunotherapeutic drug
efficacy (126,143–145). Juloori et al (36) initiated ICIs 14 days after SBRT
completion to capitalize on the peak period of radiation-induced
antigen release and immune cell infiltration, which normally occurs
1–2 weeks after RT. By delivering ICIs during this window,
treatment takes advantage of the increased immunogenicity caused by
RT.
By contrast, RT following ICI therapy has resulted
in less notable effects. ICIs alone may not fully activate the
immune system to exploit subsequent RT-induced ICD and antigen
presentation (116). As a result,
ICIs after RT remains the most commonly used approach in clinical
practice. Theelen et al (146), which examined the efficacy of
pembrolizumab with or without RT in patients with metastatic
non-small cell lung cancer, lends support to this viewpoint; 564
individuals receiving pembrolizumab were evaluated, with 89 (15.8%)
receiving RT either before or during therapy. There was no notable
difference in the OS rate between patients who underwent RT
(mOS;10.6 months) and patients who did not receive RT (mOS;10.9
months), with an adjusted hazard ratio of 1.13 (95% CI: 0.82–1.54;
P=0.46). Similarly, the two groups had no notable difference in
progression-free survival or ORR (147). These findings indicate that RT
after or during ICI therapy may not provide considerable benefits
in terms of survival or tumor response.
Determining the best sequence and timing of RT and
ICIs is important in aHCC management (143). The timing of these procedures
influences immediate treatment outcomes and may have an impact on
the long-term prognoses of patients. To achieve the best clinical
results, the treatment plan must be tailored to each patient and
the immune dynamics of the TME, while also identifying and
validating reliable biomarkers for predicting response to RT + ICIs
(30).
Identifying biomarkers that predict response to RT +
ICIs is important for patient selection and therapy optimization.
Circulating tumor DNA is a potential biomarker that allows
non-invasive monitoring of tumor load and molecular alterations
throughout treatment (40–42). Analysis of cytokine profiles and
circulating immune cell subsets offer information regarding
systemic immunological activation and overall immune status,
potentially indicating ICI timing and dose. In a study of 11
individuals receiving radioembolization or systemic therapy,
assessments at 16 time points revealed that changes in tumor
methylation score (TMS) more accurately predict tumor progression
than α-fetoprotein (AFP), with Area Under the Receiver Operating
Characteristic curve (AUROC) values of 0.800 and 0.783,
respectively. Moreover, combining AFP and TMS into a composite
score improved the accuracy of distinguishing tumor evolution, with
an AUROC of 0.892 (43).
Tumor tissue biomarkers, such as immune cell
infiltration patterns, molecular expression of ICs and tumor
mutation and novel antigen loads, directly indicate TME features
and susceptibility to combined RT + ICI methods (44,45).
Imaging modalities such as CT, magnetic resonance imaging (MRI) and
positron emission tomography (PET) serve a key role in improving RT
planning by providing detailed, high-resolution images of the tumor
and surrounding structures. CT imaging is essential for precise
localization and accurate dose delivery as it allows 3D
visualization of the size and position of the tumor. MRI is useful
in delineating liver tumors due to its high soft tissue contrast,
helping to distinguish tumors from adjacent liver tissue, which is
key in liver-directed therapy. PET imaging, combined with CT,
offers insight into tumor metabolism and activity, allowing more
accurate identification of active tumor regions that may require
higher radiation doses. These imaging techniques, when integrated
into RT planning, enable more accurate tumor targeting, improved
dose distribution and decreased toxicity to surrounding healthy
tissue (46,47).
Biomarker discovery and application may improve the
understanding of the synergistic mechanism of RT and ICIs in aHCC,
optimize treatment timing and sequence and improve patient
selection accuracy and clinical efficacy. This holistic strategy
demonstrates potential for enhancing customized medicine and
providing more effective treatment options for patients with
aHCC.
With the widespread use of immune checkpoint
inhibitors (ICIs), the incidence of immune-related adverse events
(irAEs) has risen, affecting 60–80% of patients, with severe cases
in 10–15%. Active management strategies, such as corticosteroids,
are effective in addressing these events (148,149). Fatigue, dermatological responses,
colitis, hepatitis, pneumonitis and endocrinopathy are among the
most common AEs (150,151). Although the incidence of AEs may
increase, these can be effectively managed with close monitoring
and appropriate interventions, hence optimizing safety (152,153) (Fig.
5).
Clinical trials have assessed the safety of
combining RT and ICIs in the treatment of aHCC and support the
viability of this strategy (36–39).
There was no notable difference between the rates of irAEs in
patients who received a combination treatment plan and those who
received only ICIs. These findings suggest that RT does not worsen
immune-associated toxicity. Hepatotoxicity has garnered attention
among irAEs since both can impair liver function (154,155). However, combination therapy does
not always result in a disproportionate increase in hepatotoxicity
(132,134,156). Trials of the treatment of aHCC
with RT and ICI therapy have revealed that the incidence of severe
hepatotoxicity is often tolerable (132,134,156). The aforementioned studies have
also stressed the importance of regular hepatic function monitoring
and aggressive risk-reduction methods. Additionally, Li et
al (156) conducted a
multicenter trial to evaluate the safety of a combination of RT +
ICIs for patients with portal vein tumor thrombus: The findings
revealed that the combined treatment was practical and had
manageable safety. Compared with the historical control group that
received either method alone, the number of AEs did not increase
appreciably. The aforementioned study revealed that attentive
monitoring and prompt treatment are key for improving patient
outcomes.
The aforementioned clinical investigations indicate
that while RT + ICIs may pose additional risks, these side effects
are typically controllable and preclude the practicality of a
combined strategy. Numerous studies have shown that the safety of
this combination therapy can be improved through proactive
techniques such as dose modification, vigilant monitoring and
patient education (157,158). This enables practitioners to
increase treatment outcomes while still ensuring patient safety.
The combination of RT + ICIs is a promising therapeutic option for
patients with aHCC.
Combination strategies of RT + ICIs have improved
the efficacy of aHCC treatment and novel combinations and
strategies may maximize the benefits of currently available
treatment options.
Combination of trans arterial chemoembolisation
(TACE), RT and ICIs for the treatment of aHCC uses synergistic
effects to improve therapeutic efficacy. TACE decreases the tumor
load and releases tumor antigens, whereas SBRT increases the
anticancer immune response by inducing ICD and influencing the TME.
Subsequent ICI therapy increases T cell-mediated antitumor
immunity, potentially extending the therapeutic window. The
START-FIT trial investigated sequential TACE, SBRT and avelumab;
55% of patients exhibited tumors that decreased to a level that
would allow surgical intervention and 42% achieved complete
remission (75). This novel method
improves local tumor control while also combating off-site disease
development via systemic immune activation. This synergy occurs
through increased tumor antigen presentation, PD-L1 expression
levels following radiation and recruitment of tumor-infiltrating
lymphocytes. Larger randomized controlled studies are needed to
confirm the efficacy and safety of triple therapy as a strategy to
downstage initially unresectable aHCC, making it eligible for
curative treatments such as surgery or ablation.
Researchers have explored other triple therapies,
including RT, ICIs and other drugs, such as anti-VEGF therapy or
new immunomodulators designed to target aspects of tumor biology
and the immune microenvironment at the same time, potentially
leading to more potent and durable antitumor responses (37,84,159).
As understanding of the biology of aHCC and
patient-specific variables increases, there is a greater emphasis
on establishing tailored treatments (160,161). Tailoring therapy seeks to maximize
the efficacy of combining RT + ICIs while limiting toxicity. The
development and validation of biomarkers predictive of response to
combination therapy is a key focus (162,163). Several possible biomarkers are
being investigated, including the tumor mutational load, PD-L1
expression and genetic changes (164,165). The goal is to create a
comprehensive biomarker panel that will guide treatment selection
and sequencing, ensuring patients receive the most suitable and
effective treatment.
There is emphasis on assessing therapies beyond
clinical efficacy, including patient-centered outcomes such as
quality of life, accessibility and cost-effectiveness (166,167). Understanding the real-world
applicability of these medicines is key to turning clinical trial
results into meaningful benefits for the patient population.
Involving patients in research and clinical decision-making ensures
their opinions and priorities are considered when developing and
implementing treatment. Shared decision-making models encourage
collaborative discussion between clinicians and patients to
consider individual preferences, quality of life implications and
life circumstances, resulting in increased patient satisfaction,
treatment adherence and overall outcomes (168,169).
Constraints have hampered the widespread use and
optimization of combined RT + ICIs in aHCC treatment. Determining
adequate markers for the prognosis of combination therapy is
difficult because of the large number of parameters to examine,
including tumor features, liver function, general performance
status, biomarker profiles and prior treatment history. HCC tumor
heterogeneity may result in varying responses to RT and ICIs,
making precise treatment outcome prediction difficult.
Another challenge in implementing RT + ICIs in
clinical settings is the lack of established treatment procedures.
Key topics requiring additional investigation include radiation
dose and segmentation schemes, treatment timing and sequencing,
ICIs and target volume specification. It is key to have
standardized protocols for clinical trials and consistent methods
across institutions.
Methodological limitations impede research on the
combination of RT + ICIs, affecting the universality and robustness
of findings. Several studies (37,133)
have limited sample sizes, which decreases their statistical power
and ability to establish confident findings. The paucity of
large-scale randomized controlled trials comparing combination
therapy with standard medication raises questions regarding its
true efficacy and safety. The heterogeneity of patient populations
and differences in measurement results and toxicity reports hinder
the interpretation and comparison. Short-term follow-up may not
demonstrate long-term outcomes, late toxicity or delayed responses
to ICIs.
Addressing these problems is key for promoting the
combination of RT and ICIs in the treatment of aHCC. Future
research should prioritize large-scale, multicenter randomized
controlled trials and use defined patient groups and standardized
programs. Developing and validating predictive biomarkers will
improve patient selection, allow individualized treatment options,
maximize therapeutic effects and reduce unnecessary toxicity.
Studying the optimal radiation dose, segmentation method and
treatment sequence may aid in maximizing the synergistic effect of
RT and ICIs.
RT + ICIs represents a substantial advancement in
treating aHCC. RT increases tumor antigenicity and eliminates
immunosuppressive barriers, which improves the efficacy of ICIs
(170). Clinical evidence from
early-phase trials reveals that combination treatment improves
antitumor responses while maintaining tolerable safety (126,142–144). However, issues such as optimum
patient selection, dosage technique, therapy sequencing and
toxicity control persist. Large-scale randomized controlled studies
should be prioritized to confirm therapeutic advantages and enhance
treatment strategies. The incorporation of biomarker-driven
approaches is key for personalizing medicines to patient and tumor
features, potentially increasing response rates while minimizing
side effects. Furthermore, prioritizing patient-centered outcomes,
such as quality of life, and real-world applicability (e.g.,
effectiveness in diverse patient populations, treatment
accessibility, and long-term safety), is critical for evaluating
the practical impact of these therapies. Continuous research
efforts to improve this combination, identify resistance mechanisms
and develop predictive biomarkers are needed. By addressing the
present constraints and supporting tailored treatment options,
longer-lasting responses, enhanced OS rate and improved quality of
life may be obtained.
Not applicable.
The present study was supported by the 2024 research project
from the Technology Department of Sichuan Provincial Science and
Central Guidance Local Exploration Fund Project (grant. no.
24ZYZYTS0262).
Not applicable.
RC, OJ and LS conceived the study. RC and XY
performed the literature review. XZ and RC wrote the manuscript. RC
and CM edited the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare they have no competing
interests.
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renne SL, Sarcognato S, Sacchi D, Guido M,
Roncalli M, Terracciano L and Di Tommaso L: Hepatocellular
carcinoma: A clinical and pathological overview. Pathologica.
113:203–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sangro B, Sarobe P, Hervas-Stubbs S and
Melero I: Advances in immunotherapy for hepatocellular carcinoma.
Nat Rev Gastroenterol Hepatol. 18:525–543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo M: Combination cancer immunotherapy
in hepatocellular carcinoma. Liver Cancer. 7:20–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dendy MS, Ludwig JM, Stein SM and Kim HS:
Locoregional therapy, immunotherapy and the combination in
hepatocellular carcinoma: Future directions. Liver Cancer.
8:326–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu S, Chao Y, Hu Y, Zhang Y, Hong W, Chen
Y, Chen R, Zeng Z and Du S: Radiotherapy enhances efficacy of PD-1
inhibitors in advanced hepatocellular carcinoma: A
propensity-matched real-world study. Chin Med J (Engl).
137:1332–1342. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharabi AB, Nirschl CJ, Kochel CM, Nirschl
TR, Francica BJ, Velarde E, Deweese TL and Drake CG: Stereotactic
radiation therapy augments antigen-specific PD-1-mediated antitumor
immune responses via cross-presentation of tumor antigen. Cancer
Immunol Res. 3:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ngwa W, Irabor OC, Schoenfeld JD, Hesser
J, Demaria S and Formenti SC: Using immunotherapy to boost the
abscopal effect. Nat Rev Cancer. 18:313–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Zhang H, Xin Zhang and Liu Y:
Abscopal effect: From a rare phenomenon to a new frontier in cancer
therapy. Biomark Res. 12:982024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Correction. Consensus guidelines for the
definition, detection and interpretation of immunogenic cell death.
J Immunother Cancer. 8:e000337corr12020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB,
Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, et al: Soluble
intracellular adhesion molecule-1 secreted by human umbilical cord
blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell
Death Differ. 19:680–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demaria S, Coleman CN and Formenti SC:
Radiotherapy: Changing the game in immunotherapy. Trends Cancer.
2:286–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonaventura P, Shekarian T, Alcazer V,
Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C and
Depil S: Cold Tumors: A therapeutic challenge for immunotherapy.
Front Immunol. 10:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedman D, Baird JR, Young KH, Cottam B,
Crittenden MR, Friedman S, Gough MJ and Newell P: Programmed cell
death-1 blockade enhances response to stereotactic radiation in an
orthotopic murine model of hepatocellular carcinoma. Hepatol Res.
47:702–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rimassa L, Finn RS and Sangro B:
Combination immunotherapy for hepatocellular carcinoma. J Hepatol.
79:506–515. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tojjari A, Yu J and Saeed A: Immunotherapy
and radiation therapy combinatorial approaches in hepatocellular
carcinoma. Cancers (Basel). 16:10582024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dawood ZS, Brown ZJ, Alaimo L, Lima HA,
Shaikh C, Katayama ES, Munir MM, Moazzam Z, Endo Y, Woldesenbet S
and Pawlik TM: Comparison of tumor response and outcomes of
patients with hepatocellular carcinoma after multimodal treatment
including immune checkpoint inhibitors-a systematic review and
meta-analysis. HPB (Oxford). 26:618–629. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee YH, Tai D, Yip C, Choo SP and Chew V:
Combinational immunotherapy for hepatocellular carcinoma:
Radiotherapy, immune checkpoint blockade and beyond. Front Immunol.
11:5687592020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bicak M, Cimen Bozkus C and Bhardwaj N:
Checkpoint therapy in cancer treatment: Progress, challenges, and
future directions. J Clin Invest. 134:e1848462024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Cancer Res.
25:2116–2126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Zhou J, Wu M, Hu C, Yang J, Li D,
Wu P, Chen Y, Chen P, Lin S, et al: Low-dose total body irradiation
can enhance systemic immune related response induced by
hypo-fractionated radiation. Front Immunol. 10:3172019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klug F, Prakash H, Huber PE, Seibel T,
Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et
al: Low-dose irradiation programs macrophage differentiation to an
iNOS(+)/M1 phenotype that orchestrates effective T cell
immunotherapy. Cancer Cell. 24:589–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Li K, Wang K, Yu H, Wang X, Shi M,
Liang Z, Yang Z, Hu Y, Li Y, et al: Low-dose radiotherapy combined
with dual PD-L1 and VEGFA blockade elicits antitumor response in
hepatocellular carcinoma mediated by activated intratumoral CD8(+)
exhausted-like T cells. Nat Commun. 14:77092023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mizumoto M, Okumura T, Hashimoto T, Fukuda
K, Oshiro Y, Fukumitsu N, Abei M, Kawaguchi A, Hayashi Y, Ookawa A,
et al: Proton beam therapy for hepatocellular carcinoma: A
comparison of three treatment protocols. Int J Radiat Oncol Biol
Phys. 81:1039–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wahl DR, Stenmark MH, Tao Y, Pollom EL,
Caoili EM, Lawrence TS, Schipper MJ and Feng M: Outcomes after
stereotactic body radiotherapy or radiofrequency ablation for
hepatocellular carcinoma. J Clin Oncol. 34:452–459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Juloori A, Katipally RR, Lemons JM, Singh
AK, Iyer R, Robbins JR, George B, Hall WA, Pitroda SP, Arif F, et
al: Phase 1 Randomized trial of stereotactic body radiation therapy
followed by nivolumab plus ipilimumab or nivolumab alone in
advanced/unresectable hepatocellular carcinoma. Int J Radiat Oncol
Biol Phys. 115:202–213. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Ji X, Sun J, Zhang A, Jia J, Zhang
T, Li W and Duan X: Stereotactic body radiotherapy combined with
lenvatinib with or without PD-1 inhibitors as initial treatment for
unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol
Phys. 120:1363–1376. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li JX, Su TS, Gong WF, Zhong JH, Yan LY,
Zhang J, Li LQ, He ML, Zhang RJ, Du YQ, et al: Combining
stereotactic body radiotherapy with camrelizumab for unresectable
hepatocellular carcinoma: A single-arm trial. Hepatol Int.
16:1179–1187. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiang CL, Chan ACY, Chiu KWH and Kong FS:
Combined stereotactic body radiotherapy and checkpoint inhibition
in unresectable hepatocellular carcinoma: A potential synergistic
treatment strategy. Front Oncol. 9:11572019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu Q, Xie J, Mei W and Zeng C: Methylated
circulating tumor DNA in hepatocellular carcinoma: A comprehensive
analysis of biomarker potential and clinical implications. Cancer
Treat Rev. 128:1027632024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu RH, Wei W, Krawczyk M, Wang W, Luo H,
Flagg K, Yi S, Shi W, Quan Q, Li K, et al: Circulating tumour DNA
methylation markers for diagnosis and prognosis of hepatocellular
carcinoma. Nat Mater. 16:1155–1161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Howell J, Atkinson SR, Pinato DJ, Knapp S,
Ward C, Minisini R, Burlone ME, Leutner M, Pirisi M, Buttner R, et
al: Identification of mutations in circulating cell-free tumour DNA
as a biomarker in hepatocellular carcinoma. Eur J Cancer.
116:56–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Angeli-Pahim I, Chambers A, Duarte S, Soma
D, Beduschi T, Sahin I, Hughes S and Zarrinpar A: Methylated ctDNA
quantification: Noninvasive approach to monitoring hepatocellular
carcinoma burden. J Am Coll Surg. 238:770–778. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fridman WH, Zitvogel L, Sautes-Fridman C
and Kroemer G: The immune contexture in cancer prognosis and
treatment. Nat Rev Clin Oncol. 14:717–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun R, Limkin EJ, Vakalopoulou M, Dercle
L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S,
et al: A radiomics approach to assess tumour-infiltrating CD8 cells
and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging
biomarker, retrospective multicohort study. Lancet Oncol.
19:1180–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han X, Guo Y, Ye H, Chen Z, Hu Q, Wei X,
Liu Z and Liang C: Development of a machine learning-based
radiomics signature for estimating breast cancer TME phenotypes and
predicting anti-PD-1/PD-L1 immunotherapy response. Breast Cancer
Res. 26:182024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim J and Jung Y: Radiation-induced liver
disease: Current understanding and future perspectives. Exp Mol
Med. 49:e3592017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lawrence TS, Robertson JM, Anscher MS,
Jirtle RL, Ensminger WD and Fajardo LF: Hepatic toxicity resulting
from cancer treatment. Int J Radiat Oncol Biol Phys. 31:1237–1248.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Emami B, Lyman J, Brown A, Coia L, Goitein
M, Munzenrider JE, Shank B, Solin LJ and Wesson M: Tolerance of
normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol
Phys. 21:109–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rammohan N, Randall JW and Yadav P:
History of technological advancements towards MR-Linac: The future
of image-guided radiotherapy. J Clin Med. 11:47302022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Khaledi N, Khan R and Gräfe JL: Historical
progress of stereotactic radiation surgery. J Med Phys. 48:312–327.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khoo VS, Dearnaley DP, Finnigan DJ,
Padhani A, Tanner SF and Leach MO: Magnetic resonance imaging
(MRI): considerations and applications in radiotherapy treatment
planning. Radiother Oncol. 42:1–15. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lawrence TS, Ten Haken RK, Kessler ML,
Robertson JM, Lyman JT, Lavigne ML, Brown MB, DuRoss DJ, Andrews
JC, Ensminger WD, et al: The use of 3-D dose volume analysis to
predict radiation hepatitis. Int J Radiat Oncol Biol Phys.
23:781–788. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Timmerman RD, Kavanagh BD, Cho LC, Papiez
L and Xing L: Stereotactic body radiation therapy in multiple organ
sites. J Clin Oncol. 25:947–952. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang L, Ke Q, Huang Q, Shao L, Chen J and
Wu J: Stereotactic body radiotherapy versus radiofrequency ablation
for hepatocellular carcinoma: A systematic review and
meta-analysis. Int J Hyperthermia. 37:1313–1321. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu DH, Liu L and Chen LH: Therapeutic
effects and prognostic factors in three-dimensional conformal
radiotherapy combined with transcatheter arterial chemoembolization
for hepatocellular carcinoma. World J Gastroenterol. 10:2184–2189.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Blomgren H, Lax I, Naslund I and Svanstrom
R: Stereotactic high dose fraction radiation therapy of
extracranial tumors using an accelerator. Clinical experience of
the first thirty-one patients. Acta Oncol. 34:861–870. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hall JT, Moon AM, Young M, Tan X,
Darawsheh R, Danquah F, Tepper JE and Yanagihara TK: Biochemical
safety of SBRT to multiple intrahepatic lesions for hepatocellular
carcinoma. J Hepatocell Carcinoma. 11:443–454. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hardy-Abeloos C, Lazarev S, Ru M, Kim E,
Fischman A, Moshier E, Rosenzweig K and Buckstein M: Safety and
efficacy of liver stereotactic body radiation therapy for
hepatocellular carcinoma after segmental transarterial
Radioembolization. Int J Radiat Oncol Biol Phys. 105:968–976. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Park SH, Kim JC and Kang MK: Technical
advances in external radiotherapy for hepatocellular carcinoma.
World J Gastroenterol. 22:7311–7321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Koay EJ, Owen D and Das P:
Radiation-induced liver disease and modern radiotherapy. Semin
Radiat Oncol. 28:321–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cárdenes HR, Price TR, Perkins SM,
Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K,
Deluca J and Johnstone PA: Phase I feasibility trial of
stereotactic body radiation therapy for primary hepatocellular
carcinoma. Clin Transl Oncol. 12:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Culleton S, Jiang H, Haddad CR, Kim J,
Brierley J, Brade A, Ringash J and Dawson LA: Outcomes following
definitive stereotactic body radiotherapy for patients with
Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol.
111:412–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Apisarnthanarax S, Barry A, Cao M, Czito
B, DeMatteo R, Drinane M, Hallemeier CL, Koay EJ, Lasley F, Meyer
J, et al: External beam radiation therapy for primary liver
cancers: An ASTRO clinical practice guideline. Pract Radiat Oncol.
12:28–51. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mayo CS, Moran JM, Bosch W, Xiao Y, McNutt
T, Popple R, Michalski J, Feng M, Marks LB, Fuller CD, et al:
American association of physicists in medicine task Group 263:
Standardizing nomenclatures in radiation oncology. Int J Radiat
Oncol Biol Phys. 100:1057–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schaub SK, Hartvigson PE, Lock MI, Hoyer
M, Brunner TB, Cardenes HR, Dawson LA, Kim EY, Mayr NA, Lo SS and
Apisarnthanarax S: Stereotactic body radiation therapy for
hepatocellular carcinoma: Current trends and controversies. Technol
Cancer Res Treat. 17:15330338187902172018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rim CH and Seong J: Application of
radiotherapy for hepatocellular carcinoma in current clinical
practice guidelines. Radiat Oncol J. 34:160–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Crane CH and Koay EJ: Solutions that
enable ablative radiotherapy for large liver tumors: Fractionated
dose painting, simultaneous integrated protection, motion
management, and computed tomography image guidance. Cancer.
122:1974–1986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Deng GL, Zeng S and Shen H: Chemotherapy
and target therapy for hepatocellular carcinoma: New advances and
challenges. World J Hepatol. 7:787–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee SU and Kim TH: Current evidence and
the potential role of proton beam therapy for hepatocellular
carcinoma. Clin Mol Hepatol. 29:958–968. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wennerberg E, Lhuillier C, Vanpouille-Box
C, Pilones KA, García-Martínez E, Rudqvist NP, Formenti SC and
Demaria S: Barriers to radiation-induced in situ tumor vaccination.
Front Immunol. 8:2292017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lu Z, Polan DF, Wei L, Aryal MP,
Fitzpatrick K, Wang C, Cuneo KC, Evans JR, Roseland ME, Gemmete JJ,
et al: PET/CT-based absorbed dose maps in (90)Y selective internal
radiation therapy correlate with spatial changes in liver function
derived from dynamic MRI. J Nucl Med. 65:1224–1230. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li
JCB, Wan CWS, Dai WC, Lam TC, Chen W, Wong NSM, et al: Sequential
transarterial chemoembolisation and stereotactic body radiotherapy
followed by immunotherapy as conversion therapy for patients with
locally advanced, unresectable hepatocellular carcinoma
(START-FIT): A single-arm, phase 2 trial. Lancet Gastroenterol
Hepatol. 8:169–178. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A Randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kudo M, Matilla A, Santoro A, Melero I,
Gracian AC, Acosta-Rivera M, Choo SP, El-Khoueiry AB, Kuromatsu R,
El-Rayes B, et al: CheckMate 040 cohort 5: A phase I/II study of
nivolumab in patients with advanced hepatocellular carcinoma and
Child-Pugh B cirrhosis. J Hepatol. 75:600–609. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yau T, Kang YK, Kim TY, El-Khoueiry AB,
Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al:
Efficacy and safety of nivolumab plus ipilimumab in patients with
advanced hepatocellular carcinoma previously treated with
sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol.
6:e2045642020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, et al:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ringelhan M, Pfister D, O'Connor T,
Pikarsky E and Heikenwalder M: The immunology of hepatocellular
carcinoma. Nat Immunol. 19:222–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk
O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G,
Bassaganyas L, Akers N, et al: Identification of an immune-specific
class of hepatocellular carcinoma, based on molecular features.
Gastroenterology. 153:812–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo
M: Challenges of combination therapy with immune checkpoint
inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ning C, Zhang X, Wang Y, Yang X, Yang X,
Chao J, Xun Z, Xue J, Wang Y, Sun H, et al: Radiation therapy with
combination therapy of immune checkpoint inhibitors and
antiangiogenic therapy for hepatocellular carcinoma. Int J Radiat
Oncol Biol Phys. 118:1461–1471. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and
Zhang B: Anti-angiogenic agents in combination with immune
checkpoint inhibitors: A promising strategy for cancer treatment.
Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Vafaei S, Zekiy AO, Khanamir RA, Zaman BA,
Ghayourvahdat A, Azimizonuzi H and Zamani M: Combination therapy
with immune checkpoint inhibitors (ICIs); a new frontier. Cancer
Cell Int. 22:22022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cheng AL, Qin S, Ikeda M, Galle PR,
Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al:
Updated efficacy and safety data from IMbrave150: Atezolizumab plus
bevacizumab vs. sorafenib for unresectable hepatocellular
carcinoma. J Hepatol. 76:862–873. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kudo M, Finn RS, Galle PR, Zhu AX, Ducreux
M, Cheng AL, Ikeda M, Tsuchiya K, Aoki KI, Jia J and Lencioni R:
IMbrave150: Efficacy and safety of atezolizumab plus bevacizumab
versus sorafenib in patients with barcelona clinic liver cancer
stage B unresectable hepatocellular carcinoma: An exploratory
analysis of the phase III study. Liver Cancer. 12:238–250. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ali S, Arshad M, Summer M, Zulfiqar M,
Noor S, Nazakat L and Javed MA: Recent developments on checkpoint
inhibitors, CAR T cells, and beyond for T cell-based
immunotherapeutic strategies against cancer. J Oncol Pharm Pract.
Mar 28–2025.(Epub ahead of print). View Article : Google Scholar
|
|
91
|
Osaki M and Sakaguchi S: Soluble CTLA-4
regulates immune homeostasis and promotes resolution of
inflammation by suppressing type 1 but allowing type 2 immunity.
Immunity. 58:889–908.e13. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sangro B, Chan SL, Kelley RK, Lau G, Kudo
M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK,
et al: Four-year overall survival update from the phase III
HIMALAYA study of tremelimumab plus durvalumab in unresectable
hepatocellular carcinoma. Ann Oncol. 35:448–457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Saygin I, Cakir E, Kazaz SN, Guvercin AR,
Eyupoglu I and Ustaoglu MM: Investigation of the status of immune
checkpoint molecules (PD-L1 and PD-1) in meningiomas by
immunohistochemistry. Turk J Med Sci. 54:735–743. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang Z, Liu X, Chen D and Yu J:
Radiotherapy combined with immunotherapy: The dawn of cancer
treatment. Signal Transduct Target Ther. 7:2582022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chang Y, Chang M, Bao X and Dong C:
Advancements in adoptive CAR immune cell immunotherapy
synergistically combined with multimodal approaches for tumor
treatment. Bioact Mater. 42:379–403. 2024.PubMed/NCBI
|
|
97
|
Garg AD, Nowis D, Golab J, Vandenabeele P,
Krysko DV and Agostinis P: Immunogenic cell death, DAMPs and
anticancer therapeutics: An emerging amalgamation. Biochim Biophys
Acta. 1805:53–71. 2010.PubMed/NCBI
|
|
98
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Garg AD, Galluzzi L, Apetoh L, Baert T,
Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R,
Cirone M, et al: Molecular and translational classifications of
DAMPs in immunogenic cell death. Front Immunol. 6:5882015.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Galluzzi L, Buque A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ghiringhelli F, Apetoh L, Tesniere A,
Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G,
Ullrich E, et al: Activation of the NLRP3 inflammasome in dendritic
cells induces IL-1beta-dependent adaptive immunity against tumors.
Nat Med. 15:1170–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Krysko DV, Agostinis P, Krysko O, Garg AD,
Bachert C, Lambrecht BN and Vandenabeele P: Emerging role of
damage-associated molecular patterns derived from mitochondria in
inflammation. Trends Immunol. 32:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Palm NW and Medzhitov R: Pattern
recognition receptors and control of adaptive immunity. Immunol
Rev. 227:221–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rock KL and Kono H: The inflammatory
response to cell death. Annu Rev Pathol. 3:99–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zitvogel L, Kepp O and Kroemer G: Decoding
cell death signals in inflammation and immunity. Cell. 140:798–804.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Oura K, Morishita A, Tani J and Masaki T:
Tumor immune microenvironment and immunosuppressive therapy in
hepatocellular carcinoma: A review. Int J Mol Sci. 22:58012021.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen R, Du J, Zhu H and Ling Q: The role
of cGAS-STING signalling in liver diseases. JHEP Rep. 3:1003242021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ren B, Luo S, Xu F, Zou G, Xu G, He J,
Huang Y, Zhu H and Li Y: The expression of DAMP proteins HSP70 and
cancer-testis antigen SPAG9 in peripheral blood of patients with
HCC and lung cancer. Cell Stress Chaperones. 22:237–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Amiri M, Molavi O, Sabetkam S, Jafari S
and Montazersaheb S: Stimulators of immunogenic cell death for
cancer therapy: Focusing on natural compounds. Cancer Cell Int.
23:2002023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Singh V, Kaur R, Kumari P, Pasricha C and
Singh R: ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and
cardiovascular disorders. Clin Chim Acta. 548:1174872023.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for cancer immunotherapy: Mechanism, combinations, and
clinical outcome. Front Pharmacol. 8:5612017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pandey P, Khan F, Qari HA, Upadhyay TK,
Alkhateeb AF and Oves M: Revolutionization in cancer therapeutics
via targeting major immune checkpoints PD-1, PD-L1 and CTLA-4.
Pharmaceuticals (Basel). 15:3352022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yu S, Jiang S, Zhou Y, Zhu Z and Yang X:
Impact of radiation on exosomes in regulating tumor immune
microenvironment. Adv Radiat Oncol. 9:1015492024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pedros C, Canonigo-Balancio AJ, Kong KF
and Altman A: Requirement of Treg-intrinsic CTLA4/PKCη signaling
pathway for suppressing tumor immunity. JCI Insight. 2:e956922017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Binder DC, Fu YX and Weichselbaum RR:
Radiotherapy and immune checkpoint blockade: Potential interactions
and future directions. Trends Mol Med. 21:463–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bae SH, Chun SJ, Chung JH, Kim E, Kang JK,
Jang WI, Moon JE, Roquette I, Mirabel X, Kimura T, et al:
Stereotactic body radiation therapy for hepatocellular carcinoma:
meta-analysis and international stereotactic radiosurgery society
practice guidelines. Int J Radiat Oncol Biol Phys. 118:337–351.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schaue D and McBride WH: Opportunities and
challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol.
12:527–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xiang YJ, Wang K, Zheng YT, Feng S, Yu HM,
Li XW, Cheng X, Cheng YQ, Feng JK, Zhou LP, et al: Effects of
stereotactic body radiation therapy plus PD-1 inhibitors for
patients with transarterial chemoembolization refractory. Front
Oncol. 12:8396052022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhai J, Gu X, Liu Y, Hu Y, Jiang Y and
Zhang Z: Chemotherapeutic and targeted drugs-induced immunogenic
cell death in cancer models and antitumor therapy: An update
review. Front Pharmacol. 14:11529342023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kroemer G, Galassi C, Zitvogel L and
Galluzzi L: Immunogenic cell stress and death. Nat Immunol.
23:487–500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Manda K, Glasow A, Paape D and Hildebrandt
G: Effects of ionizing radiation on the immune system with special
emphasis on the interaction of dendritic and T cells. Front Oncol.
2:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kim KJ, Lee HW and Seong J: Combination
therapy with anti-T-cell immunoglobulin and mucin-domain containing
molecule 3 and radiation improves antitumor efficacy in murine
hepatocellular carcinoma. J Gastroenterol Hepatol. 36:1357–1365.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen H, Zhao L, Fu K, Lin Q, Wen X,
Jacobson O, Sun L, Wu H, Zhang X, Guo Z, et al: Integrin
α(v)β(3)-targeted radionuclide therapy combined with immune
checkpoint blockade immunotherapy synergistically enhances
anti-tumor efficacy. Theranostics. 9:7948–7960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Shao Y, Han S, Hou Z, Yang C and Zhao Y:
Tumor-associated macrophages within the immunological milieu: An
emerging focal point for therapeutic intervention. Heliyon.
10:e368392024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tai WM, Yong WP, Lim C, Low LS, Tham CK,
Koh TS, Ng QS, Wang WW, Wang LZ, Hartano S, et al: A phase Ib study
of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib
in advanced hepatocellular carcinoma (HCC). Ann Oncol.
27:2210–2215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kim BH, Park HC, Kim TH, Koh YH, Hong JY,
Cho Y, Sinn DH, Park B and Park JW: Concurrent nivolumab and
external beam radiation therapy for hepatocellular carcinoma with
macrovascular invasion: A phase II study. JHEP Rep. 6:1009912023.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Buckstein M, Kim E, Ozbek U, Tabrizian P,
Gunasekaran G, Facciuto M, Rosenzweig K, Llovet JM and Schwartz M:
Combination transarterial chemoembolization and stereotactic body
radiation therapy for unresectable single large hepatocellular
carcinoma: Results from a prospective phase 2 trial. Int J Radiat
Oncol Biol Phys. 114:221–230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chiang CL, Chiu KW, Lee FA, Kong FS and
Chan AC: Combined stereotactic body radiotherapy and immunotherapy
versus transarterial chemoembolization in locally advanced
hepatocellular carcinoma: A propensity score matching analysis.
Front Oncol. 11:7988322021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Chiang CL, Lee FAS, Chan KSK, Lee VWY,
Chiu KWH, Ho RLM, Fong JKS, Wong NSM, Yip WWL, Yeung CSY, et al:
Survival outcome analysis of stereotactic body radiotherapy and
immunotherapy (SBRT-IO) versus SBRT-alone in unresectable
hepatocellular carcinoma. Liver Cancer. 13:265–276. 2023.PubMed/NCBI
|
|
134
|
Ning C, Jia J, Zhang X, Sun J, Wang Y, Xue
J, Zhang L, Hou X, Yang X, Sang X, et al: Efficacy and safety of
subsequent radiotherapy in patients with advanced-stage
hepatocellular carcinoma treated with immune checkpoint inhibitors.
Hepatobiliary Surg Nutr. 12:882–897. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Filatenkov A, Baker J, Mueller AM, Kenkel
J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S,
et al: Ablative tumor radiation can change the tumor immune cell
microenvironment to induce durable complete remissions. Clin Cancer
Res. 21:3727–3739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Vanpouille-Box C, Alard A, Aryankalayil
MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN,
Formenti SC and Demaria S: DNA exonuclease Trex1 regulates
radiotherapy-induced tumour immunogenicity. Nat Commun.
8:156182017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Robbins JR, Schmid RK, Hammad AY, Gamblin
TC and Erickson BA: Stereotactic body radiation therapy for
hepatocellular carcinoma: Practice patterns, dose selection and
factors impacting survival. Cancer Med. 8:928–938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Herrera FG, Ronet C, Ochoa de Olza M,
Barras D, Crespo I, Andreatta M, Corria-Osorio J, Spill A,
Benedetti F, Genolet R, et al: Low-Dose radiotherapy reverses tumor
immune desertification and resistance to immunotherapy. Cancer
Discov. 12:108–133. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Petragallo R, Bardach N, Ramirez E and
Lamb JM: Barriers and facilitators to clinical implementation of
radiotherapy treatment planning automation: A survey study of
medical dosimetrists. J Appl Clin Med Phys. 23:e135682022.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Han-Oh S, Hill C, Kang-Hsin Wang K, Ding
K, Wright JL, Alcorn S, Meyer J, Herman J and Narang A: Geometric
reproducibility of fiducial markers and efficacy of a
patient-specific margin design using deep inspiration breath hold
for stereotactic body radiation therapy for pancreatic cancer. Adv
Radiat Oncol. 6:1006552021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Marks LB, Bentzen SM, Deasy JO, Kong FM,
Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV,
Timmerman RD, et al: Radiation dose-volume effects in the lung. Int
J Radiat Oncol Biol Phys. 76 (3 Suppl):S70–S76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Higgins K, Hu C, Ross H, Jabbour S, Kozono
D, Owonikoko T, Dib E, Brownstein J, Kuzma C and Kotecha R:
Concurrent chemoradiation±atezolizumab (atezo) in limited-stage
small cell lung cancer (LS-SCLC): results of NRG Oncology/Alliance
LU005. Int J Radiat Oncol Biol Phys. 120:S22024. View Article : Google Scholar
|
|
143
|
Young KH, Baird JR, Savage T, Cottam B,
Friedman D, Bambina S, Messenheimer DJ, Fox B, Newell P, Bahjat KS,
et al: Optimizing timing of immunotherapy improves control of
tumors by hypofractionated radiation therapy. PLoS One.
11:e01571642016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Gunderson AJ and Young KH: Exploring
optimal sequencing of radiation and immunotherapy combinations. Adv
Radiat Oncol. 3:494–505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Aliru ML, Schoenhals JE, Venkatesulu BP,
Anderson CC, Barsoumian HB, Younes AI, K Mahadevan LS, Soeung M,
Aziz KE, Welsh JW and Krishnan S: Radiation therapy and
immunotherapy: What is the optimal timing or sequencing?
Immunotherapy. 10:299–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 Randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Theelen WSME, Chen D, Verma V, Hobbs BP,
Peulen HMU, Aerts JGJV, Bahce I, Niemeijer ALN, Chang JY, de Groot
PM, et al: Pembrolizumab with or without radiotherapy for
metastatic non-small-cell lung cancer: a pooled analysis of two
randomised trials. Erratum in: Lancet Respir Med. 9:e292021.
|
|
148
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, et
al: Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell
Lung Cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Sangro B, Chan SL, Meyer T, Reig M,
El-Khoueiry A and Galle PR: Diagnosis and management of toxicities
of immune checkpoint inhibitors in hepatocellular carcinoma. J
Hepatol. 72:320–341. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Jácome AA, Castro ACG, Vasconcelos JPS,
Silva MHCR, Lessa MAO, Moraes ED, Andrade AC, Lima FMT, Farias JPF,
Gil RA, et al: Efficacy and safety associated with immune
checkpoint inhibitors in unresectable hepatocellular carcinoma: A
meta-analysis. JAMA Netw Open. 4:e21361282021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li G, Zhao Y, Li K, Yang S, Xiang C, Song
J, Yang Y, Li G and Dong J: Effectiveness and safety of the PD-1
inhibitor lenvatinib plus radiotherapy in patients with HCC with
main PVTT: Real-world data from a tertiary centre. J Hepatocell
Carcinoma. 10:2037–2048. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Huang Y, Zhang Y, Zhang M, Zhao K, Feng L,
Guan J, Dong R, Liu J, Tian D, Liu M, et al: Combined immunotherapy
for hepatocellular carcinoma: How to maximize immune checkpoint
blockade synergic anti-tumor effect. Crit Rev Oncol Hematol.
189:1040702023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yang Y, Xiong L, Li M, Jiang P, Wang J and
Li C: Advances in radiotherapy and immunity in hepatocellular
carcinoma. J Transl Med. 21:5262023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z,
Huang X, Wu Y, Li T and Guo W: Pembrolizumab plus lenvatinib with
or without hepatic arterial infusion chemotherapy in selected
populations of patients with treatment-naive unresectable
hepatocellular carcinoma exhibiting PD-L1 staining: A multicenter
retrospective study. BMC Cancer. 21:11262021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Garin E, Tselikas L, Guiu B, Chalaye J,
Edeline J, de Baere T, Assenat E, Tacher V, Robert C,
Terroir-Cassou-Mounat M, et al: Personalised versus standard
dosimetry approach of selective internal radiation therapy in
patients with locally advanced hepatocellular carcinoma
(DOSISPHERE-01): A randomised, multicentre, open-label phase 2
trial. Lancet Gastroenterol Hepatol. 6:17–29. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lewandowski RJ and Salem R:
Radioembolisation with personalised dosimetry: Improving outcomes
for patients with advanced hepatocellular carcinoma. Lancet
Gastroenterol Hepatol. 6:2–3. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhu R, Wang X, Sun F, Zhu L and Guo W:
Exploring the role of DNA methylation located in
cuproptosis-related genes: Implications for prognosis and immune
landscape in hepatocellular carcinoma. Front Biosci (Landmark Ed).
29:1232024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhu J, Tang B, Lv X, Meng M, Weng Q, Zhang
N, Li J, Fan K, Zheng L, Fang S, et al: Identifying
apoptosis-related transcriptomic aberrations and revealing clinical
relevance as diagnostic and prognostic biomarker in hepatocellular
carcinoma. Front Oncol. 10:5191802021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Nebhan CA and Johnson DB: Predictive
biomarkers of response to immune checkpoint inhibitors in melanoma.
Expert Rev Anticancer Ther. 20:137–145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xu H, Liang XL, Liu XG and Chen NP: The
landscape of PD-L1 expression and somatic mutations in
hepatocellular carcinoma. J Gastrointest Oncol. 12:1132–1140. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Parikh ND, Singal AG, Hutton DW and Tapper
EB: Cost-effectiveness of hepatocellular carcinoma surveillance: An
assessment of benefits and harms. Am J Gastroenterol.
115:1642–1649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Lani L, Stefanini B and Trevisani F:
Surveillance for hepatocellular carcinoma in patients with
successfully treated viral disease of the liver: A systematic
review. Liver Cancer. 13:376–388. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zafar SY, Alexander SC, Weinfurt KP,
Schulman KA and Abernethy AP: Decision making and quality of life
in the treatment of cancer: A review. Support Care Cancer.
17:117–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Hamann J, Kissling W and Mendel R: Does it
matter whether physicians' recommendations are given early or late
in the decision-making process? An experimental study among
patients with schizophrenia. BMJ Open. 6:e0112822016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Nahm WJ, Bhatt A and Wu J: Role of
radiotherapy and its contribution to immunotherapy in
hepatocellular carcinoma. Chin Clin Oncol. 12:412023. View Article : Google Scholar : PubMed/NCBI
|