Introduction
Gastric cancer is a major global health challenge
due to its high mortality and morbidity rates, especially in East
Asia, Eastern Europe and South America. Despite an overall
decreasing trend in incidence and mortality in different countries
over the past decades, gastric cancer is the fifth most common
malignant tumor and the fourth leading cause of cancer-related
deaths worldwide (1–3). To improve early diagnosis and
prognosis, research into various biomarkers and immune-related
therapies is ongoing (4–6).
Autoantibodies against tumor-associated antigens
(TAAs) are antibodies produced in response to self-proteins that
are abnormally expressed or altered by cancer cells. These are
being researched for their potential as biomarkers in early cancer
detection, diagnosis, prognosis prediction and monitoring of
treatment efficacy (7,8). It has been reported that
autoantibodies against tumor-associated antigens can be detected
with increased sensitivity compared with the tumor-associated
antigens themselves (9,10). However, only a few autoantibodies
have been applied as biomarkers in clinical practice (11,12).
A large-scale screening for esophageal cancer
antigens through the serological identification of antigens was
performed in our previous studies using the recombinant cDNA
expression cloning (serological analysis of recombinant tumor cDNA
expression libraries; SEREX) method, which demonstrated that
autoantibody levels against these antigens (TROP2, SURF1, LOC146223
and HOOK2) were increased in the sera of patients with esophageal
cancer compared with those in healthy donors (13,14).
Cancer antigens not only for esophageal cancer are currently being
investigated, but also for other types of cancer, such as lung
cancer and colorectal cancer (15,16).
The p53 antibody, as a tumor marker using an autoantibody that
targets tumor antigens, has been applied for esophageal, colorectal
and breast cancer, but not gastric cancer in Japan (17,18).
Conversely, some of these autoantibodies, such as Anti-FIRΔexon2,
sorting nexin 15, and spermatogenesis and oogenesis specific basic
helix-loop-helix 1 antibodies, were increased in the sera of
patients with gastric cancer (14,19–21).
Therefore, the association of the esophageal cancer autoantibody
markers with gastric cancer were investigated.
Materials and methods
Collection of serum samples
Patients and sera
Blood samples were prospectively recruited from
patients with gastric cancer at the Toho University Omori Medical
Center (Tokyo, Japan) to search for new tumor markers. The present
study included 166 patients with gastric cancer who underwent
radical surgery, endoscopic submucosal dissection or probe
laparotomy at Toho University Omori Medical Center from May 2010 to
May 2013. Patients with other types of cancer and those aged ≤18
years were excluded. The control group comprised 96 healthy donors
from a health screening clinic, Port Square Kashiwado Clinic
(Chiba, Japan). Healthy donor blood samples were obtained from
consecutive patients who had undergone brain checkups between April
2013 and March 2014. According to the inclusion criteria for
healthy donors, those with a medication history and
lifestyle-related diseases were excluded. The patients with gastric
cancer comprised 118 male and 48 female patients (mean age, 64.9
years; range, 33–90 years). The number of patients in each
pathological tumor stage were: I, n=90; II, n=26; III, n=28; and
IV, n=22. The serum samples were analyzed at Chiba University
(Chiba, Japan). Patient hospital records were first accessed in
September 2019.
Ethical approval
The study was conducted under the guidelines of the
Declaration of Helsinki. The Ethics Committee of Faculty of
Medicine, Toho University (approval nos.
A18103_A17052_A16035_A16001_26095_25024_24038_22047 and
25131_23005; Tokyo, Japan), Toho University Omori Medical Center
(approval no. 26-255), Chiba University Graduate School of Medicine
(approval nos. 2018-320, 2020-1129, 2022-623 and 2023-836), and
Port Square Kashiwado Clinic, Kashiwado Memorial Foundation
(approval no. 2012-001) approved the collection of serum samples.
All patients signed written informed consent. The Ethics Committee
of Faculty of Medicine, Toho University (approval no.
A22038_A21089_A19030) and Toho University Omori Medical Center
(approval no. M22211) approved the retrospective analysis of
patients' medical records. Participants were allowed to decline to
be further enrolled in the present study (opt-out). Before
treatment, 5 ml blood samples were collected, centrifuged at 3,000
× g for 10 min, and serum was collected and stored at −80°C until
use.
Measurement of s-ENO1-Abs, s-SSNA-Abs
and conventional serum markers
Glutathione S-transferase (GST)-ENO1, GST-SSNA1 and
control GST were expressed in Escherichia coli and purified
using affinity-chromatography with glutathione-Sepharose (Cytiva)
as previously described (20). The
s-ENO1-Ab and s-SSNA1-Ab levels were measured using amplified
luminescence proximity homogeneous assay-linked immunosorbent assay
[AlphaLISA™; Revvity, Inc.; AlphaLISA Anti-Human HA Acceptor Beads,
cat. no. AL170M; AlphaScreen GSH Donor Beads, cat. no. 6765301;
AlphaLISA buffer, cat. no. AL000F; 384-well microtiter plates
(white opaque OptiPlate™), cat. no. 6008280]. AlphaLISA was
conducted using 384-well microtiter plates (Revvity, Inc.)
containing 2.5 µl 1/100-diluted sera and 2.5 µl GST, GST-ENO1 and
GST-SSNA1 proteins (10 µg/ml) in AlphaLISA buffer (25 mM HEPES, pH
7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/ml dextran-500 and 0.05%
Proclin-300) following the manufacturer's instructions (Revvity,
Inc.). The reaction mixture was incubated at room temperature for
6–8 h, and 2.5 µl anti-human IgG-conjugated acceptor beads (40
µg/ml) and 2.5 µl glutathione-conjugated donor beads (40 µg/ml)
were then added and incubated further for 7–21 days at room
temperature in the dark. The chemical emission at 607–623 nm (Alpha
photon count) which indicates the antigen-antibody binding level
was read using an EnSpire™ Alpha microplate reader (Revvity, Inc.)
as previously described (6,11,20,21–24).
Specific reactions were estimated by subtracting the Alpha values
of GST control from the GST-fusion protein values. Additionally,
the carcinoembryonic antigen (CEA) levels were assessed as
previously described (25). The
cut-off value for CEA was set at 5.0 ng/ml following the
manufacturer's instruction.
Comparison of the overall survival
rates according to the expression levels of the mRNA
Comparison of overall survival rates according to
mRNA expression levels were conducted, using reference data from
The Human Protein Atlas (https://www.proteinatlas.org/), from gastric cancer
tissue obtained during diagnosis.
Statistical analyses
Differences between the two variables were analyzed
using Fisher's exact test. Corresponding differences between the
two variables were identified using the Mann-Whitney U-test.
Receiver operating characteristic curve (ROC) analysis was utilized
to identify the predictive qualities of putative disease markers
and cut-off values were determined to maximize the total
sensitivity and specificity (Youden index). Optimal cut-off values
for serum antibody levels that affect overall survival were
identified using the X-tile software (version 3.6.1; Yale
University) as previously described (26). The Kaplan-Meier method was utilized
to analyze survival and survival curves were drawn. Additionally,
the survival distributions of the two groups were compared using
the log-rank test. Clinicopathological variables related to overall
survival were assessed by univariate analysis followed by
multivariate analysis using the Cox proportional hazards model.
Statistical analyses were conducted using EZR software (version
1.55; Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html)
(27). P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of s-ENO1-Abs and
s-SSNA1-Abs levels between patients with gastric cancer and healthy
donors
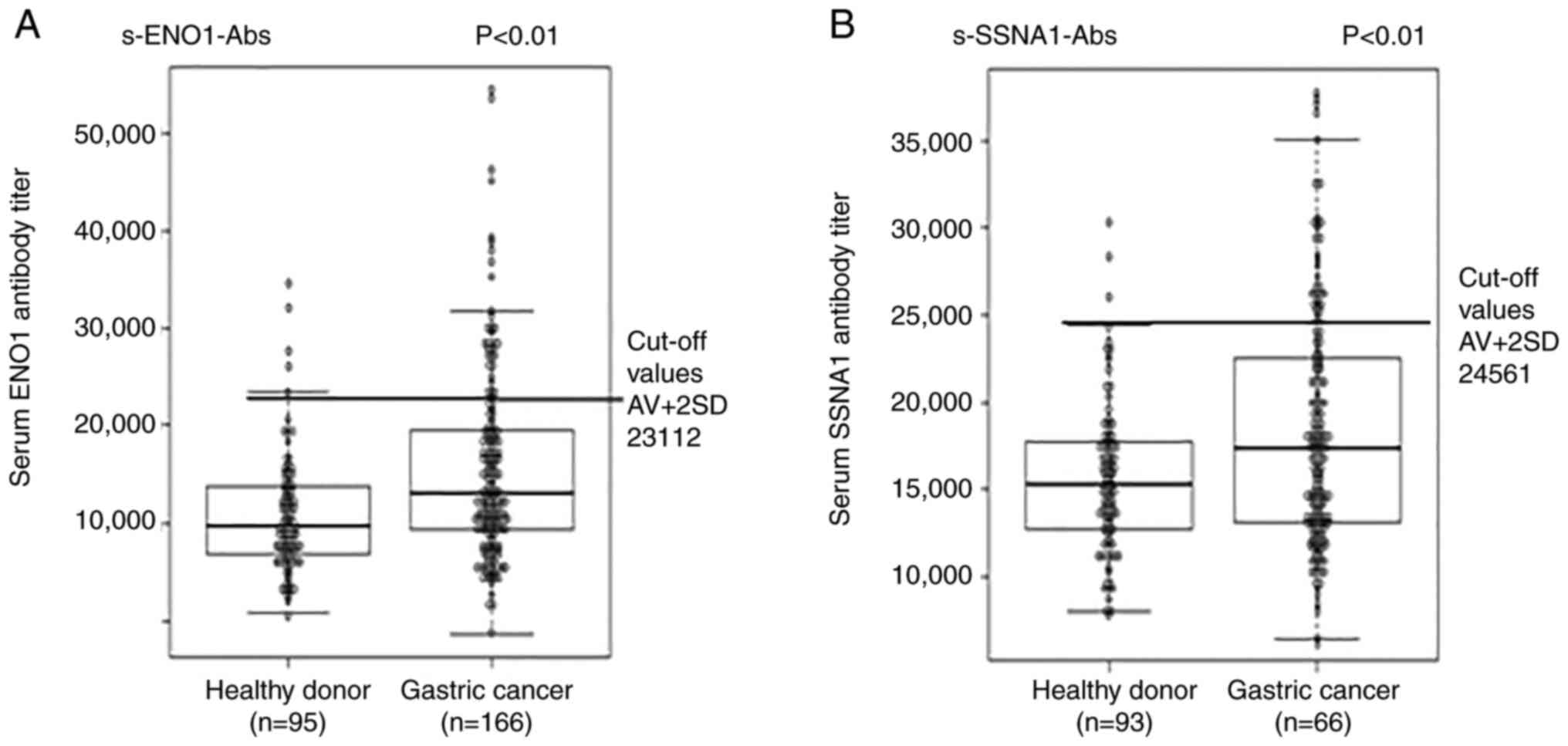
The s-ENO1-Abs and s-SSNA1-Abs titer levels were
measured using the AlphaLISA assay with GST-ENO1 and GST-SSNA1 as
antigens. s-ENO1-Ab and s-SSNA1-Ab titer levels were significantly
increased in patients with gastric cancer compared with that in
healthy donors (Fig. 1A and B;
P<0.01). The ROC curve analysis demonstrated that the areas
under the ROC curve of s-ENO1-Abs and s-SSNA1-Abs were 0.656 and
0.607, respectively, for gastric cancer (Fig. 2A and B). The sensitivity and
specificity were 39.1 and 87.3% for s-ENO1-Abs, and 37.3 and 84.9%
for s-SSNA1-Abs respectively, using cut-off values that were
determined as per the Youden index to maximize the sum of
sensitivity and specificity.
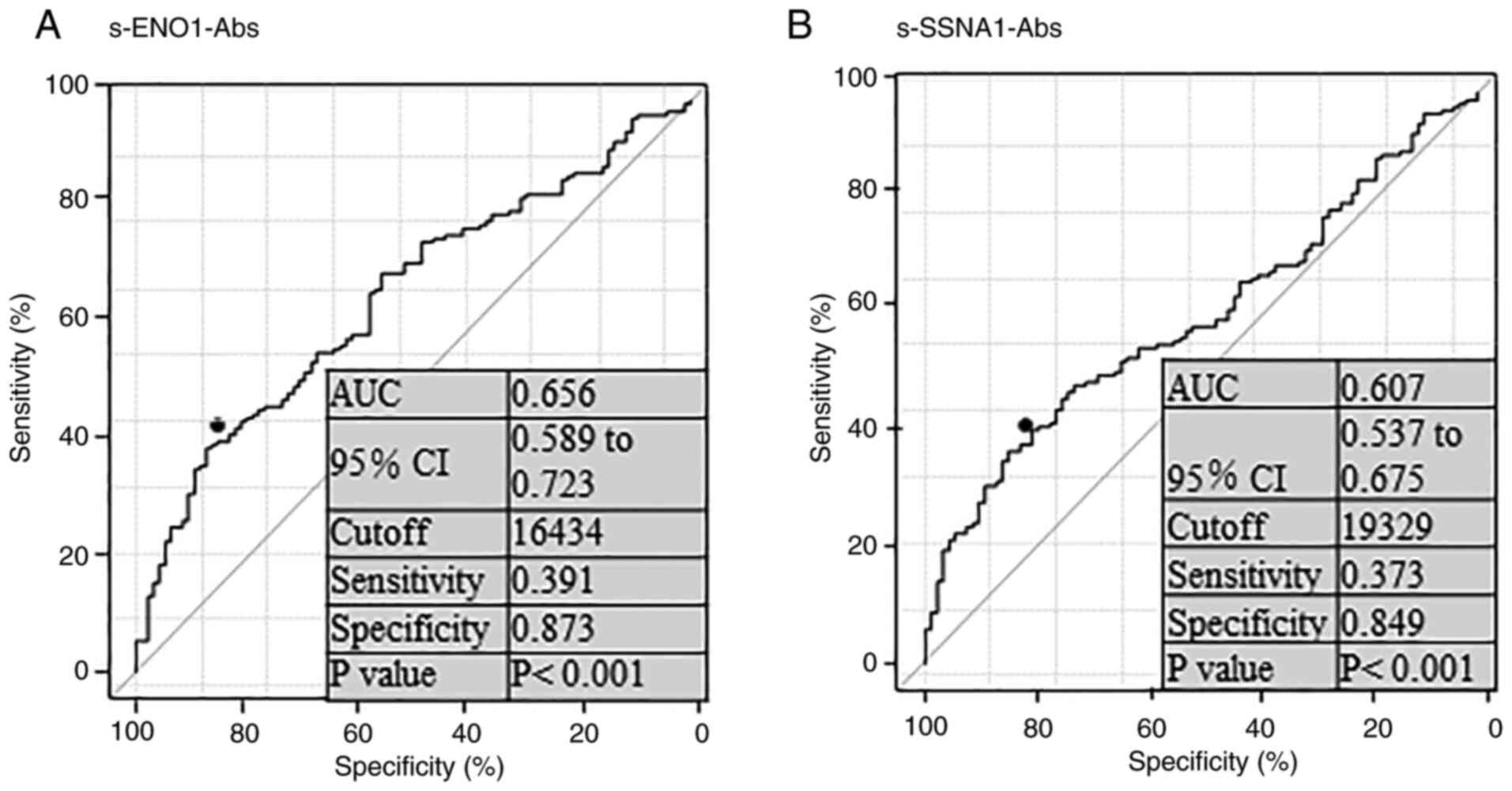 | Figure 2.Receiver operating characteristic
curve analysis for s-ENO1-Abs and s-ENO1-Abs. Data in (A)
s-ENO1-Abs and (B) s-ENO1-Abs analysis represents the AUC, 95% CI,
cut-off level, sensitivity, specificity and P-values. ENO1, enolase
1; SSNA1, Sjögren syndrome nuclear autoantigen 1; s-ENO1-Abs,
serum anti-ENO1 antibodies; s-SSNA1-Abs, serum anti-SSNA1
antibodies; CI, confidence interval; AUC, area under curve. |
The positive rate of s-ENO1-Abs was 18.7% (31/166)
and the false positive rate of healthy donors was 5.3% (5/95; one
sample measurement failed); the positive rate of s-SSNA1-Abs was
19.9% (33/166) and the false positive rate of healthy donors was
3.2% (3/93; three sample measurements failed), when the cut-off
values were set at the mean value + 2 × SD value of healthy donors
(Fig. 1). Patients with positive
s-ENO1-Abs and s-SSNA1-Abs demonstrated little overlap with
patients in the CEA-positive (+) group and exhibited different
positive patterns (Fig. 3).
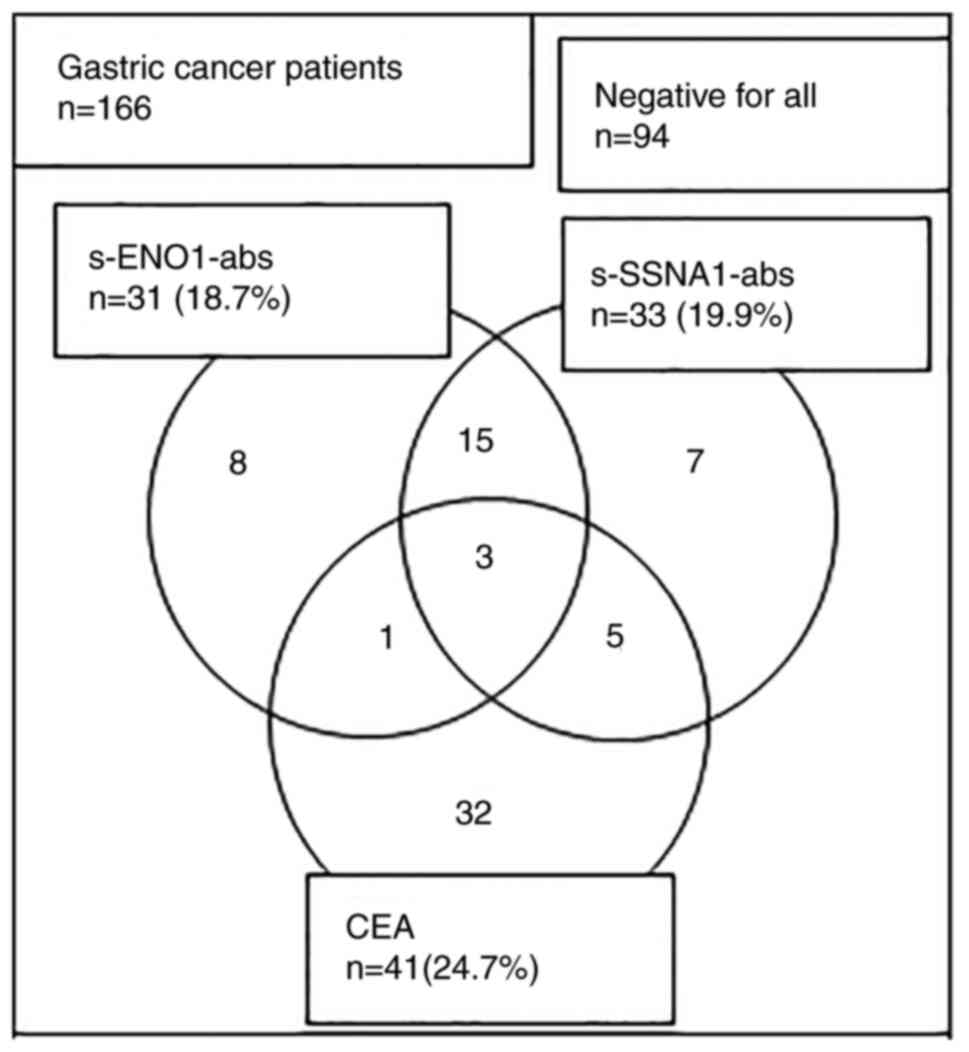 | Figure 3.Relationship among positive sample
numbers of s-ENO1-Abs, s-SSNA1-Abs and CEA in patients with gastric
cancer. Total positive numbers of s-ENO1-Abs, s-SSNA1-Abs, and CEA
were 31, 33 and 41, respectively. The numbers in the figure
represent single, double- and triple-positive sample numbers. ENO1,
enolase 1; SSNA1, Sjögren syndrome nuclear autoantigen 1;
s-ENO1-Abs, serum anti-ENO1 antibodies; s-SSNA1-Abs, serum
anti-SSNA1 antibodies; CEA, carcinoembryonic antigen. |
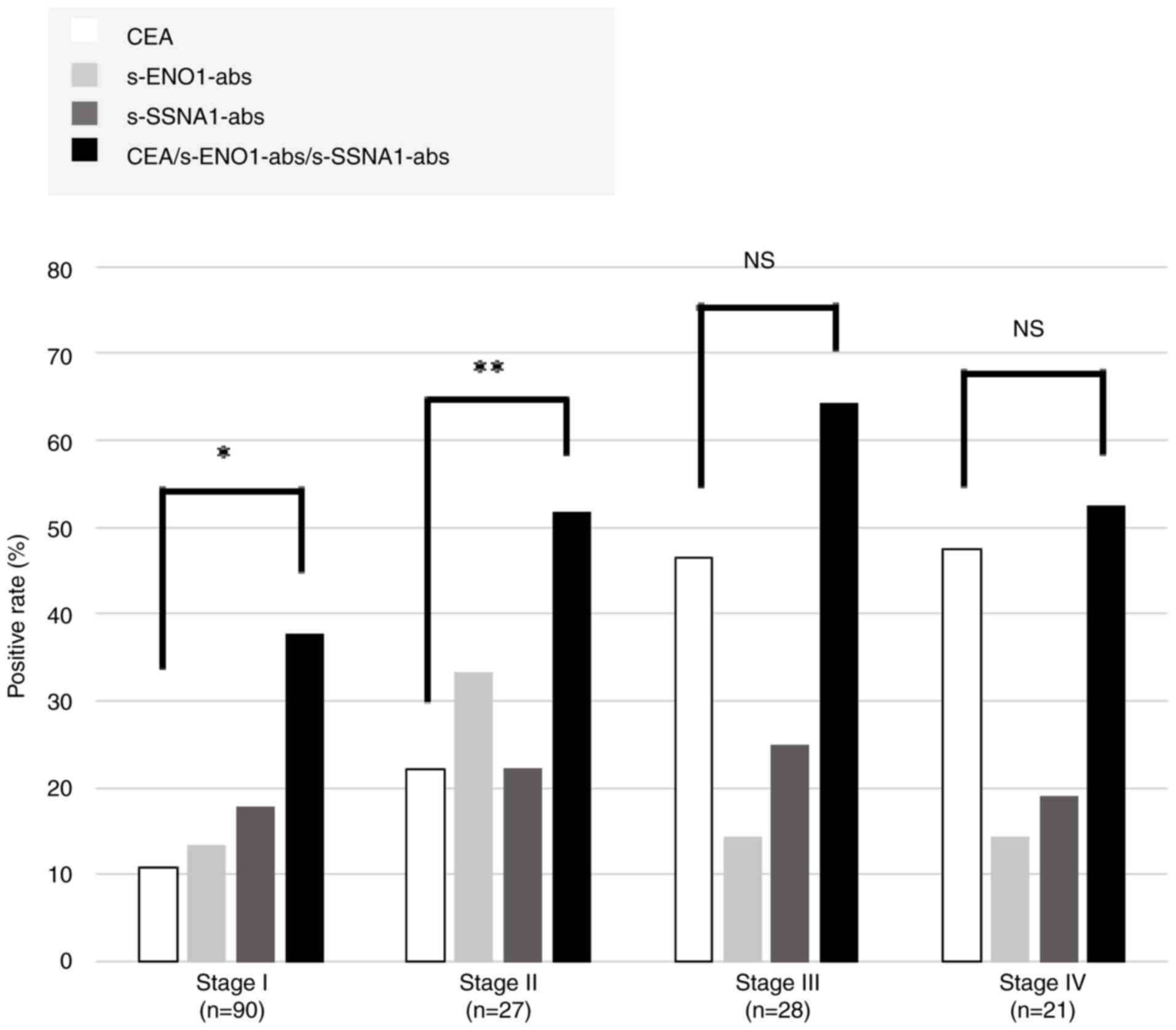
The positive rates of s-ENO1-Abs, s-SSNA1-Abs and
CEA among various tumor stages (I–IV) were compared. The positive
rates of CEA elevated as the stage progressed, whereas those of
s-ENO1-Abs and s-SSNA1-Abs were independent of the stage. The
positive rates in stages I and II increased significantly in the
combination group positive for all three markers compared with that
in CEA alone (Fig. 4; P<0.01 and
P<0.05, respectively).
Correlation of s-ENO1-Ab and
s-SSNA1-Ab levels with clinicopathological parameters
The examination of s-ENO1-Abs, s-SSNA1-Abs and CEA
in terms of clinicopathological factors indicated the s-ENO1-Ab and
s-SSNA1-Ab levels were stratified based on the cut-off values
identified using X-tile analysis (26). Fisher's exact test demonstrated a
significant association between patients with CEA(+) and tumor
depth, lymph node metastasis and disease stage (Table I). However, other
clinicopathological variables and patients with positive s-ENO1-Ab
or s-SSNA1-Ab levels exhibited no significant association.
 | Table I.Clinicopathological significance of
positive status of three biomarkers, s-ENO1-Ab, s-SSNA-Ab and
CEA. |
Table I.
Clinicopathological significance of
positive status of three biomarkers, s-ENO1-Ab, s-SSNA-Ab and
CEA.
|
Characteristics | No. of
patients | s-ENO1-Ab
>9,358b, n |
P-valuea | s-SSNA-Ab
>11,711b, n |
P-valuea | CEA >5.0 ng/ml,
n |
P-valuea |
|---|
| Sex (n=166) |
|
|
|
|
|
|
|
|
Female | 48 | 34 | 0.56 | 42 | 0.64 | 11 | 0.84 |
|
Male | 118 | 90 |
| 99 |
| 30 |
|
| Age, years
(n=166) |
|
|
|
|
|
|
|
|
≤65 | 67 | 54 | 0.20 | 55 | 0.51 | 15 | 0.59 |
|
>65 | 99 | 70 |
| 86 |
| 26 |
|
| Histological type
(n=163) |
|
|
|
|
|
|
|
|
Differentiated | 88 | 64 | 0.59 | 73 | 0.66 | 24 | 0.27 |
|
Undifferentiated | 75 | 58 |
| 65 |
| 14 |
|
| Tumor size, mm
(n=163) |
|
|
|
|
|
|
|
|
≤40 | 95 | 71 | 0.85 | 84 | 0.16 | 21 | 0.84 |
|
>40 | 57 | 44 |
| 45 |
| 14 |
|
| Tumor depth
(n=144) |
|
|
|
|
|
|
|
|
T1-2 | 78 | 59 | >0.99 | 67 | >0.99 | 11 | 0.04 |
|
T3-4 | 66 | 50 |
| 56 |
| 19 |
|
| Nodal status
(n=124) |
|
|
|
|
|
|
|
|
Negative | 75 | 51 | 0.31 | 62 | 0.61 | 7 | <0.01 |
|
Positive | 49 | 38 |
| 43 |
| 19 |
|
| Cytology
(n=118) |
|
|
|
|
|
|
|
|
Negative | 110 | 87 | 0.68 | 94 | 0.35 | 23 | 0.37 |
|
Positive | 8 | 6 |
| 6 |
| 3 |
|
| Peritoneal
dissemination (n=108) |
|
|
|
|
|
|
|
|
Negative | 101 | 79 | 0.65 | 87 | 0.28 | 15 | 0.30 |
|
Positive | 7 | 5 |
| 5 |
| 2 |
|
| Stage (n=166) |
|
|
|
|
|
|
|
|
I/II | 116 | 87 | >0.99 | 98 | >0.99 | 17 | <0.01 |
|
III/IV | 50 | 37 |
| 43 |
| 24 |
|
Combined analysis of s-ENO1-Ab and
s-SSNA1-Ab titer levels in relation to patient survival
The prognostic significance of s-ENO1-Ab and
s-SSNA1-Ab titer levels was evaluated by generating survival curves
with the Kaplan-Meier method (Fig.
5). The s-ENO1-Ab and s-SSNA1-Ab levels were categorized into
positive and negative groups according to the cut-off level from
the X-tile analysis (26). No
statistically significant difference was observed in the patients'
overall survival rates between the s-ENO1-Ab(+) and
s-ENO1-Ab-negative (−) groups; however, the s-ENO1-Ab(+) group
demonstrated a notable trend toward an improved prognosis (P=0.28;
Fig. 5A). Similarly, no
statistically significant difference was observed in the patients'
overall survival rates between the s-SSNA1-Ab(+) and s-SSNA1-Ab(−)
groups; however, the s-SSNA1-Ab(+) group demonstrated a trend
toward a more favorable prognosis (P=0.30; Fig. 5B). By contrast, a significant
difference was found in the improvement of overall survival rate
between the CEA(+) and CEA(−) groups (P<0.05; Fig. 5C).
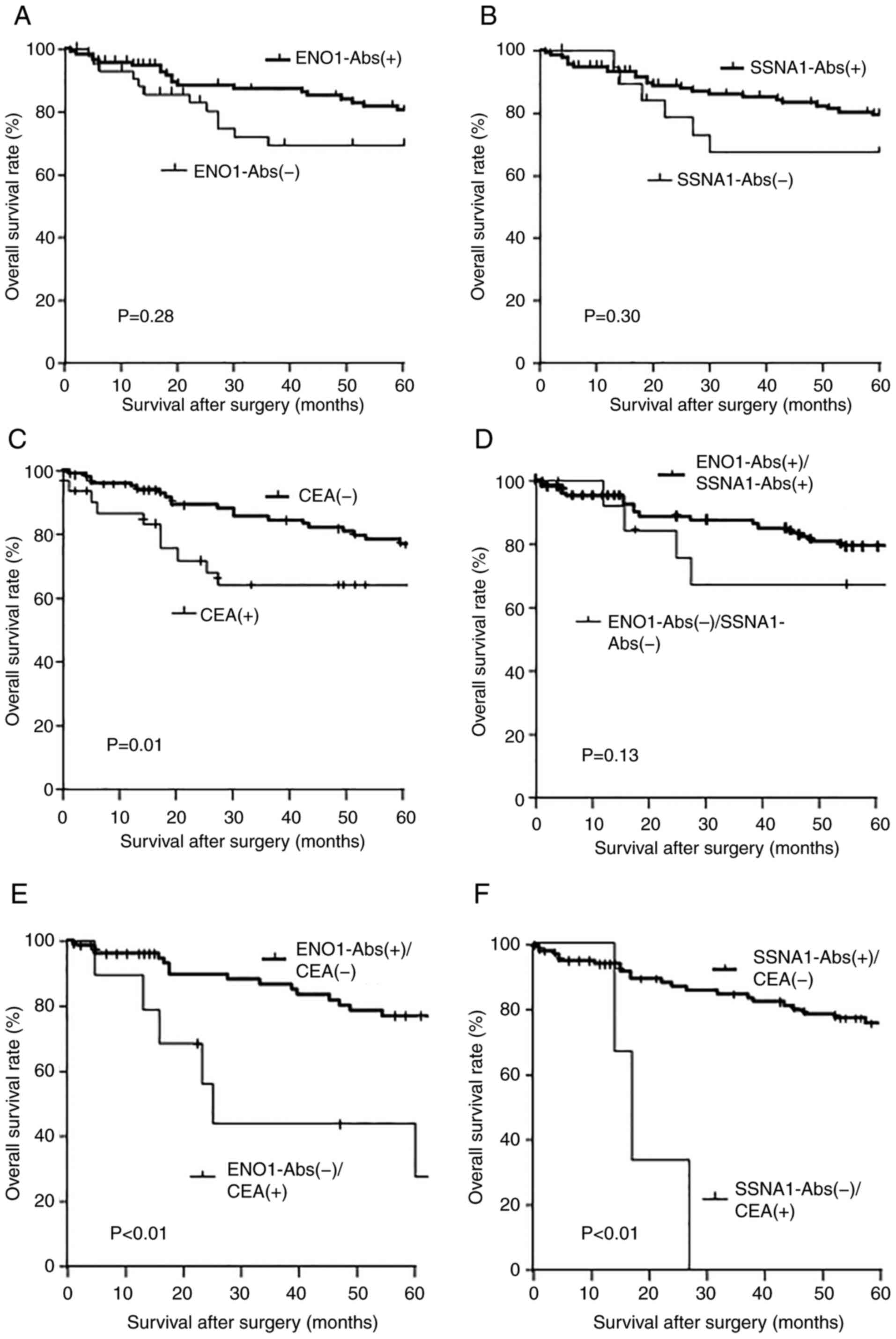 | Figure 5.Comparison of the overall survival
between the positive and negative groups of s-ENO1-Abs, s-SSNA1-Abs
and CEA alone or their combination. The overall survival of
surgically treated gastric cancer shown in Kaplan-Meyer plots of
(A) s-ENO1-Abs and (B) s-SSNA1-Abs. The levels of s-ENO1-Abs and
s-SSNA1-Abs were used to dived patients into positive and negative
groups. The s-ENO1-Abs and s-SSNA1-Abs levels were categorized into
positive and negative groups according to the cut-off level from
the X-tile analysis (s-ENO1-Abs cut-off, 9,358; s-SSNA1-Abs
cut-off, 11,711). (C) Comparison of groups with CEA >5.0 ng/ml.)
Comparison of prognosis between the group (D)
s-ENO1-Abs+/s-SSNA1-Abs+ and s-ENO1-Abs-/s-SSNA1-Abs-, (E)
s-ENO1-Abs+/CEA- and s-ENO1-Abs-/CEA+ and (F) s-SSNA1-Abs+/CEA- and
s-SSNA1-Abs-/CEA+. ENO1, enolase 1; SSNA1, Sjögren syndrome
nuclear autoantigen 1; s-ENO1-Abs, serum anti-ENO1 antibodies;
s-SSNA1-Abs, serum anti-SSNA1 antibodies; CEA, carcinoembryonic
antigen. |
The s-ENO1-Ab(+)/s-SSNA1-Ab(+) group demonstrated a
notable trend towards an improved prognosis compared with that of
the s-ENO1-Ab(−)/s-SSNA1-Ab(−) group when considering the
combination of two types of markers as prognostic factors (P=0.13;
Fig. 5D). Moreover, the
s-ENO1-Ab(+)/CEA(−) group demonstrated a significantly improved
prognosis compared with that of the s-ENO1-Ab(−)/CEA(+) group
(P<0.01; Fig. 5E). Similarly,
the s-SSNA1-Ab(+)/CEA(−) group demonstrated a significantly
improved prognosis compared with that of the s-SSNA1-Ab(−)/CEA(+)
group (P<0.01; Fig. 5F).
Univariate and multivariate analysis
of prognostic impact of clinicopathological variables including
autoantibody status
Univariate and multivariate analyses included the
overall survival and patient characteristics, including sex, age,
histological type, tumor size, tumor depth, lymph node metastasis,
laparotomy lavage cytology, peritoneal dissemination, stage,
s-ENO1-Abs, s-SSNA1-Abs and CEA levels. Both univariate and
multivariate analyses identified tumor size, tumor depth, lymph
node metastasis, laparotomy lavage cytology, peritoneal
dissemination, stage and CEA levels as significant prognostic
variables; however, s-ENO1-Abs or s-SSNA1-Abs were not significant
prognostic indicators (Table
II).
 | Table II.Univariate and multivariate analysis
of the risk factors associated with the overall survival of 166
patients with surgically treated for gastric cancer. |
Table II.
Univariate and multivariate analysis
of the risk factors associated with the overall survival of 166
patients with surgically treated for gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables |
P-valuea | Hazard ratio | 95% CI |
P-valueb |
|---|
| Sex, male vs.
female | 0.47 |
|
| 0.47 |
| Age, >65 vs. ≤65
years | 0.15 | 1.721 | 0.829–3.573 | 0.15 |
| Histological type,
differentiated vs. undifferentiated | 0.06 | 2.106 | 0.985–4.503 | 0.06 |
| Tumor size, ≤40 vs.
>40 mm | 0.03 | 2.531 | 1.095–5.850 | 0.03 |
| Tumor depth, T1-2
vs. T3-4 | <0.01 | 30.360 | 4.087–225.500 | <0.01 |
| Nodal status,
negative vs. positive | <0.01 | 3.633 | 1.536–8.594 | <0.01 |
| Cytology, negative
vs. positive | <0.01 | 5.850 | 2.105–16.260 | <0.01 |
| Peritoneal
dissemination, negative vs. positive | <0.01 | 18.370 | 5.859–57.580 | <0.01 |
| Stage, I and II vs.
III and IV | <0.01 | 6.978 | 3.286–14.820 | <0.01 |
| s-ENO1-Abs,
negative vs. positive | 0.28 |
|
| 0.28 |
| s-SSNA-Abs,
negative vs. positive | 0.30 |
|
| 0.30 |
| CEA, negative vs.
positive | 0.01 | 2.438 | 1.189–4.997 | 0.01 |
Comparison of the overall survival
rates according to the expression levels of the mRNA
Cut-off values identified by yields maximal
difference regarding survival between the two groups at the lowest
log-rank P-value were utilized to divide the participants into
high- and low-expression groups. The high ENO1 expression group
demonstrated a marked increase in overall survival rates compared
with that of the low ENO1 expression groups, but the difference was
not significant (P=0.07; Fig. 6A).
Conversely, the high SSNA1 expression group demonstrated a
significantly improved prognosis compared with that of the low
SSNA1 expression group (P<0.05; Fig.
6B). No discernible difference was observed in prognosis
between high and low CEA expression (Fig. 6C).
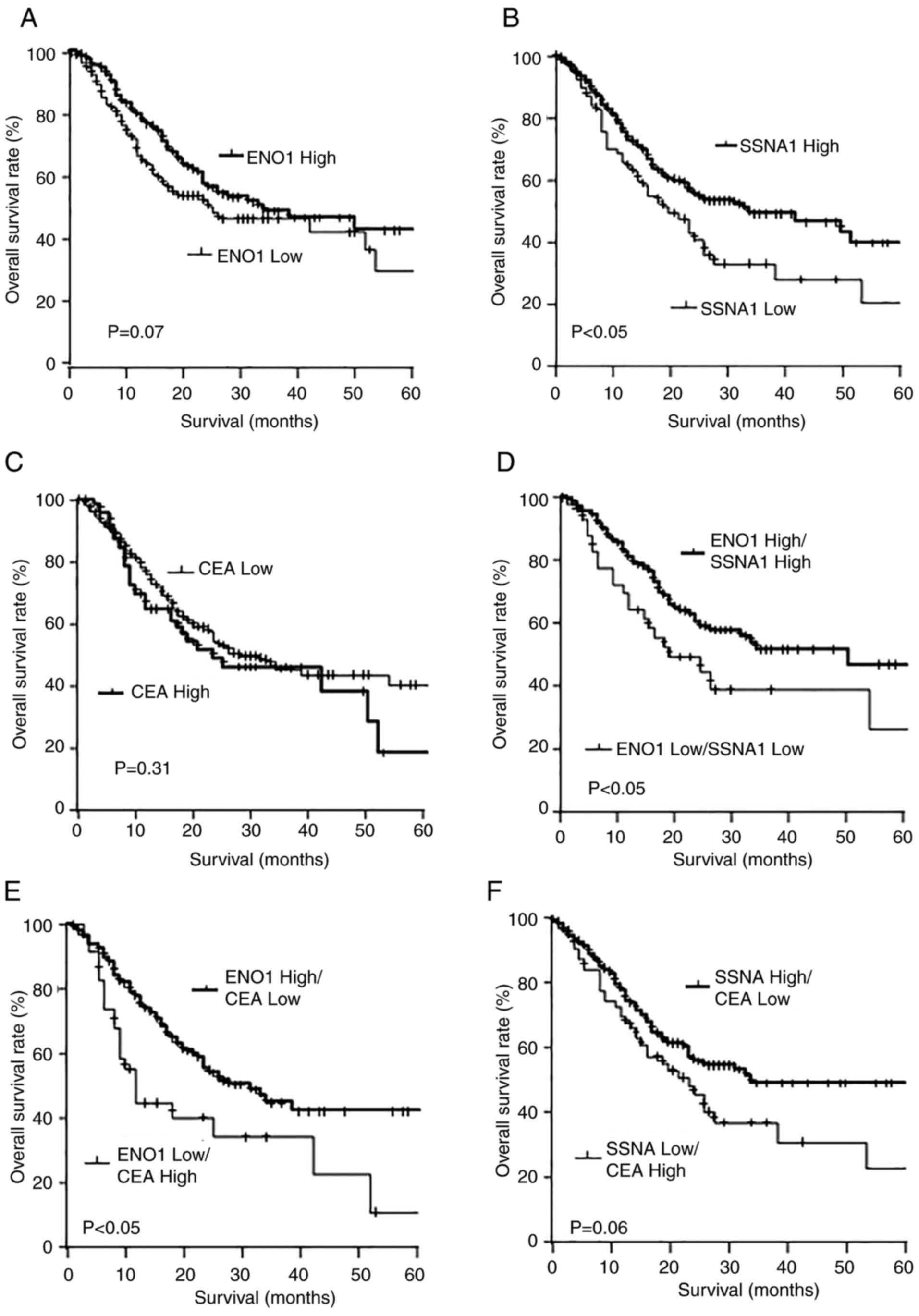 | Figure 6.Comparison of the overall survival
rates according to the mRNA expression levels. The levels of mRNA
of ENO1, SSNA1 and CEA were obtained from the public database, the
Human Protein Atlas. Overall survival of gastric cancer was
depicted with Kaplan-Meier plots of (A) ENO1, (B) SSNA1 and (C)
CEA, (D) ENO1 high/SSNA1 high and ENO1 low/SSNA1 low, (E) ENO1
high/CEA low and ENO1 low/CEA high, (F) SSNA1 high/CEA low and
SSNA1 low/CEA high. ENO1, enolase 1; SSNA1, Sjögren syndrome
nuclear autoantigen 1; s-ENO1-Abs, serum anti-ENO1 antibodies;
s-SSNA1-Abs, serum anti-SSNA1 antibodies; CEA, carcinoembryonic
antigen. |
The combined analysis demonstrated that the high
ENO1/high SSNA1 expression group demonstrated significantly
improved overall survival rates compared with that of the low
ENO1/low SSNA1 expression group (P<0.05; Fig. 6D). Furthermore, the high ENO1/low
CEA expression group exhibited significantly improved overall
survival rates compared with that of the low ENO1/high CEA
expression group (P<0.05; Fig.
6E). No significant difference was observed in the overall
survival rates between the high SSNA1/low CEA expression and the
low SSNA1/high CEA expression groups (P=0.06; Fig. 6F).
Discussion
Our previous studies identified ENO1 and SSNA1,
based on SEREX screening, as antigens recognized by serum IgG
antibodies in patients with esophageal cancer (14,20).
The present study demonstrated that patients with gastric cancer
exhibited significantly increased s-ENO1-Ab and s-SSNA1-Ab levels
compared with that of the healthy donors.
Autoantibody development frequently accompanies the
high antigen expression in tumor tissue (18,20,23,24).
The comparison of overall survival rates according to mRNA
expression levels mirrors the results obtained for autoantibodies.
These are consistent with the suggestion that autoantibodies
increase against antigen leakage due to high expression in cancer
tissues and tissue destruction accompanying cancer progression
(28).
The present study demonstrated that risk factors for
overall survival in 166 cases were associated with tumor size,
tumor depth, lymph node status, cytology and peritoneal
dissemination but not with s-ENO1-Abs or s-SSNA1-Abs (Table II). CEA levels were associated with
tumor depth, lymph node status and stages (Table I) as previously reported (29,30),
whereas s-ENO1-Abs and s-SSNA1-Abs were not. Patients that were
CEA(+) demonstrated a poorer prognosis compared with those that
were CEA(−). The positivity and negativity of s-ENO1-Abs or
s-SSNA1-Abs demonstrated no significant difference in the overall
survival rates, but the combination of CEA(+) and s-ENO1-Ab(+) or
s-SSNA1-Ab(+) exhibited more significant associations compared with
CEA alone. Thus, s-ENO1-Abs and s-SSNA1-Abs may be involved in the
overall survival by presenting some prognosis-associated aspects
other than tumor status, lymph node status or metastasis.
ENO1 protein is known to localize not only in the
mitochondria membrane but also in the cell surface to mediate
intracellular signaling such as PI3K/AKT, AMPK/mTOR and
Wnt/β-catenin pathways (31,32).
Serum anti-ENO1 autoantibodies block the signaling via direct
binding to cell surface ENO1 if these signaling molecules are
involved in gastric cancer proliferation/progression (33,34).
Hence, the autoantibodies actively affect the cancer development.
Treatment using anti-ENO1 monoclonal antibodies has been proposed
for lung cancer (32). These are
consistent with the present findings that s-ENO1-Ab(+) patients
demonstrated more favorable prognoses compared with s-ENO1-Ab(−)
patients in combination with CEA positivity. CEA levels, a major
tumor marker for gastric cancer, have been reported to correlate
with TNM stage (35).
By contrast, autoantibodies measure IgG antibodies
against mutant protein antigens, and may not correlate with tumor
mass. The antibody markers may decrease in the late stages of
cancer (36). Although s-p53-Abs,
which is used clinically to measure this IgG antibody, has been
reported to not correlate with survival rates in gastric cancer
(18), although a large-scale
multi-center cohort study is necessary to verify this as the sample
size was small. ENO1 protein binds to HGFR and activates its signal
transduction. By contrast, HGFR serves an important role in the
prognosis of gastric cancer (37).
ENO1 is highly expressed as a glycolytic enzyme, and as a result,
ENO1 antibodies increase through the destruction of cancer tissue.
As the stage progresses, HGFR signals are further required, but
ENO1 antibodies bind to ENO1 protein on the cell surface and block
the signals. Therefore, this may explain the slightly improved
prognosis in the s-ENO1-Ab(+) group demonstrated in the present
study. ENO1 antibodies do not increase further in advanced cancer.
ENO1 is upregulated in gastric cancer cells to obtain energy, which
results in the production of antibodies due to tissue destruction
(38,39). The autoantibodies produced inhibit
the HGFR signaling of gastric cancer cells, which may improve
prognosis. This feedback phenomenon is important; since the HGFR
signaling promotes the proliferation of cancer cells themselves
rather than invasion and metastasis (40,41),
therefore, suppressing this signaling could ultimately lead to
improved prognosis.
SSNA1, which is localized in the centrosome and
presumed to be involved in cell division by regulating microtubule
polymerization, currently has limited studies on its association
with gastrointestinal cancer (42,43).
SSNA1 is reported to promote metastasis in hepatocellular carcinoma
(44). Additionally, SSNA1 mRNA
expression levels demonstrate a more favorable positive trend
toward overall survival in liver cancer (44). The functional relationship between
s-SSNA1-Abs and SSNA1 protein was not confirmed in the present
study, although s-SSNA1-Ab positivity exhibited a similar tendency
for an improved prognosis as s-ENO1-Ab positivity.
The present study had several limitations. First,
the association between protein expression levels in the resected
specimens and the two serum antibody levels in the same patients
was not assessed. Second, to the best of our knowledge, this was
the first study to investigate the association between the two
serum antibodies levels and gastric cancer; thus, a cut-off value
for the two serum antibodies was identified using a test cohort of
all patients. Due to the small sample size and single-center nature
of the study, a large multi-institutional cohort is required for
assessment.
Serum antibodies are less expensive than tissue mRNA
assays (45,46), making them a useful marker.
Additionally, s-ENO1-Abs and s-SSNA1-Abs may be utilized as
diagnostic and prognostic biomarkers for gastric cancer and may
indicate future research directions for innovative approaches to
target and treat gastric cancer.
Acknowledgements
The authors would like to thank Ms. Seiko Otsuka,
Ms. Masae Suzuki, Ms. Chiho Kusaka and Ms. Satoko Ishibashi (all
from Toho University Graduate School of Medicine, Tokyo, Japan) for
preparing patient data.
Funding
The present study was supported by the Project for Cancer
Research and Therapeutic Evolution from the Japan Agency for
Medical Research and Development, AMED (grant no.
21cm0106403h0006), research grants from the Japan Science and
Technology Agency (JST Exploratory Research; grant no. 14657335)
and Grants-in-Aid for Scientific Research from the Japan Society
for the Promotion of Science (grant nos. 15K10117, 16K10520,
21K08695, 20K16396, 20K17953 and 22K07273).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SY, MI, TH and HS conceived and designed the present
study. TS, YO, MS and FS analyzed the data. TH developed the
AlphaLISA™ system. HT, SY, YO and FS acquired serum samples. SYL,
BSZ, YY and TM analyzed patient data and drafted the manuscript. HS
and SY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki, and approved by the
Ethics Committee of Toho University, Graduate School of Medicine
(approval nos.
A18103_A17052_A16035_A16001_26095_25024_24038_22047_22047; Tokyo,
Japan) and the retrospective analysis of patients' collected blood
samples and medical records was approved by the Ethics Committee of
Faculty of Medicine, Toho University (approval no.
A22038_A21089_A19030) and Toho University Omori Medical Center
(approval nos. M22211 and M23174 21320 21039 20200 20196 19056
18002; Tokyo, Japan). Ethics Committee of Chiba University Graduate
School of Medicine (approval nos. 2018-320, 2020-1129, 2022-623,
2023-836; Chiba, Japan) and Port Square Kashiwado Clinic, Kashiwado
Memorial Foundation (approval no. 2012-001) were also approved.
Sera was collected from patients who had provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ENO1
|
enolase 1
|
|
SSNA1
|
Sjögren syndrome nuclear autoantigen
1
|
|
s-ENO1-Abs
|
serum anti-ENO1 antibodies
|
|
s-SSNA1-Abs
|
serum anti-SSNA1 antibodies
|
References
|
1
|
Mamun TI, Younus S and Rahman MH: Gastric
cancer-Epidemiology, modifiable and non-modifiable risk factors,
challenges and opportunities: An updated review. Cancer Treat Res
Commun. 41:1008452024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 2021.(Epub ahead of print).
View Article : Google Scholar
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin Z, Jiang W and Wang L: Biomarkers for
gastric cancer: Progression in early diagnosis and prognosis
(Review). Oncol Lett. 9:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nie Y, Zhao W, Lu L and Zhou F: Predictive
biomarkers and new developments of immunotherapy in gastric cancer:
A 2023 update. Am J Cancer Res. 13:3169–3184. 2023.PubMed/NCBI
|
|
6
|
Sasajima N, Sumazaki M, Oshima Y, Ito M,
Yajima S, Takizawa H, Wang H, Li SY, Zhang BS, Yoshida Y, et al:
Stage-specific alteration and prognostic relationship of serum
fumarate hydratase autoantibodies in gastric cancer. Int J Mol Sci.
25:54702024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heo CK, Bahk YY and Cho EW:
Tumor-associated autoantibodies as diagnostic and prognostic
biomarkers. BMB Rep. 45:677–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montero-Calle A, Garranzo-Asensio M,
Moreno-Casbas MT, Campuzano S and Barderas R: Autoantibodies in
cancer: A systematic review of their clinical role in the most
prevalent cancers. Front Immunol. 15:14556022024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green HN: An immunological concept of
cancer: A preliminary report. Br Med J. 11:1374–1380. 1954.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong L, Coe SP, Stromberg AJ, Khattar NH,
Jett JR and Hirschowitz EA: Profiling tumor-associated antibodies
for early detection of non-small cell lung cancer. J Thorac Oncol.
1:513–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoshino I, Nagata M, Takiguchi N, Nabeya
Y, Ikeda A, Yokoi S, Kuwajima A, Tagawa M, Matsushita K, Satoshi Y
and Hideaki S: Panel of autoantibodies against multiple
tumor-associated antigens for detecting gastric cancer. Cancer Sci.
108:308–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu YW, Peng YH, Xu LY, Xie JJ and Li EM:
Autoantibodies: Potential clinical applications in early detection
of esophageal squamous cell carcinoma and esophagogastric junction
adenocarcinoma. World J Gastroenterol. 25:5049–5068. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima K, Shimada H, Ochiai T,
Kuboshima M, Kuroiwa N, Okazumi S, Matsubara H, Nomura F, Takiguchi
M and Hiwasa T: Serological identification of TROP2 by recombinant
cDNA expression cloning using sera of patients with esophageal
squamous cell carcinoma. Int J Cancer. 112:1029–1035. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada H, Nakashima K, Ochiai T, Nabeya
Y, Takiguchi M, Nomura F and Hiwasa T: Serological identification
of tumor antigens of esophageal squamous cell carcinoma. Int J
Oncol. 26:77–86. 2005.PubMed/NCBI
|
|
15
|
Niloofa R, De Zoysa MI and Seneviratne LS:
Autoantibodies in the diagnosis, prognosis, and prediction of
colorectal cancer. J Cancer Res Ther. 17:819–833. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai L, Tsay JC, Li J, Yie TA, Munger JS,
Pass H, Rom WN, Zhang Y, Tan EM and Zhang JY: Autoantibodies
against tumor-associated antigens in the early detection of lung
cancer. Lung Cancer. 99:172–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oshima Y, Suzuki T, Yajima S, Nanami T,
Shiratori F, Funahashi K and Shimada H: Serum p53 antibody: Useful
for detecting gastric cancer but not for predicting prognosis after
surgery. Surg Today. 50:1402–1408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang BS, Zhang XM, Ito M, Yajima S,
Yoshida K, Ohno M, Nishi E, Wang H, Li SY, Kubota M, et al: JMJD6
autoantibodies as a potential biomarker for inflammation-related
diseases. Int J Mol Sci. 25:49352024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuboshima M, Shimada H, Liu TL, Nakashima
K, Nomura F, Takiguchi M, Hiwasa T and Ochiai T: Identification of
a novel SEREX antigen, SLC2A1/GLUT1, in esophageal squamous cell
carcinoma. Int J Oncol. 28:463–468. 2006.PubMed/NCBI
|
|
21
|
Kobayashi S, Hiwasa T, Ishige T, Kano M,
Hoshino T, Rahmutulla B, Seimiya M, Shimada H, Nomura F, Matsubara
H, et al: Anti-FIRΔexon2 autoantibody as a novel indicator for
better overall survival in gastric cancer. Cancer Sci. 112:847–858.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beaudet L, Rodriguez-Suarez R, Venne MH,
Caron M, Bédard J, Brechler V, Parent S and Bielefeld-Sévigny M:
AlphaLISA immunoassays: The no-wash alternative to ELISAs for
research and drug discovery. Nat Methods. 5:an8–an9. 2008.
View Article : Google Scholar
|
|
23
|
Hiwasa T, Wang H, Goto K, Mine S, Machida
T, Kobayashi E, Yoshida Y, Adachi A, Matsutani T, Sata M, et al:
Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as
predictive risk markers for acute ischemic stroke. BMC Med.
19:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito M, Yajima S, Suzuki T, Oshima Y,
Nanami T, Sumazaki M, Shiratori F, Takizawa H, Li SY, Zhang BS, et
al: Combination of high anti-SKI and low anti-TMED5 antibody levels
is preferable prognostic factor in esophageal carcinoma. Cancer
Sci. 115:2209–2219. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the Task Force
of the Japanese gastric cancer association. Gastric Cancer.
17:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
June CH, Warshauer JT and Bluestone JA: Is
autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med.
23:540–547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tachibana M, Takemoto Y, Nakashima Y,
Kinugasa S, Kotoh T, Dhar DK, Kohno H and Nagasue N: Serum
carcinoembryonic antigen as a prognostic factor in resectable
gastric cancer. J Am Coll Surg. 187:64–68. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JH, Jun KH, Jung H, Park IS and Chin
HM: Prognostic value of preoperative serum levels of five tumor
markers (carcinoembryonic antigen, CA19-9, alpha-fetoprotein,
CA72-4, and CA125) in gastric cancer. Hepatogastroenterology.
61:863–869. 2014.PubMed/NCBI
|
|
31
|
Qiao G, Wu A, Chen X, Tian Y and Lin X:
Enolase 1, a moonlighting protein, as a potential target for cancer
treatment. Int J Biol Sci. 17:3981–3992. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li HJ, Ke FY, Lin CC, Lu MY, Kuo YH, Wang
YP, Liang KH, Lin SC, Chang YH, Chen HY, et al: ENO1 promotes lung
cancer metastasis via HGFR and WNT signaling-driven
epithelial-to-mesenchymal transition. Cancer Res. 81:4094–4109.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cappello P, Tonoli E, Curto R, Giordano D,
Giovarelli M and Novelli F: Anti-α-enolase antibody limits the
invasion of myeloid-derived suppressor cells and attenuates their
restraining effector T cell response. Oncoimmunology.
5:e11129402015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang N, Qiao H, Hao J, Deng C, Zhou N,
Yang L, Zeng M and Guan Q: RNA-binding protein ENO1 promotes the
tumor progression of gastric cancer by binding to and regulating
gastric cancer-related genes. J Gastrointest Oncol. 14:585–598.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng K, Yang L, Hu B, Wu H, Zhu H and Tang
C: The prognostic significance of pretreatment serum CEA levels in
gastric cancer: A meta-analysis including 14651 patients. PLoS One.
10:e01241512015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Evans RL, Pottala JV, Nagata S and Egland
KA: Longitudinal autoantibody responses against tumor-associated
antigens decrease in breast cancer patients according to treatment
modality. BMC Cancer. 18:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Xi W, Ji J, Cai Q, Zhao Q, Jiang
J, Zhou C, Shi M, Zhang H, Zhu Z and Zhang J: The prognostic value
of HGF-c-MET signaling pathway in gastric cancer: A study based on
TCGA and GEO databases. Int J Med Sci. 17:1946–1955. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiao H, Wang Y, Zhu B, Jiang L, Yuan W,
Zhou Y and Guan Q: Enolase1 overexpression regulates the growth of
gastric cancer cells and predicts poor survival. J Cell Biochem.
120:18714–18723. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang T, Shu X, Zhang HW, Sun LX, Yu L, Liu
J, Sun LC, Yang ZH and Ran YL: Enolase 1 regulates stem cell-like
properties in gastric cancer cells by stimulating glycolysis. Cell
Death Dis. 11:8702020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cecchi F, Rabe DC and Bottaro DP:
Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer.
46:1260–1270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koh SA and Lee KH: HGF-mediated S100A11
overexpression enhances proliferation and invasion of gastric
cancer. Am J Transl Res. 10:3385–3394. 2018.PubMed/NCBI
|
|
42
|
Lawrence EJ, Arpag G, Arnaiz C and Zanic
M: SSNA1 stabilizes dynamic microtubules and detects microtubule
damage. Elife. 10:e672822021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Basnet N, Nedozralova H, Crevenna AH,
Bodakuntla S, Schlichthaerle T, Taschner M, Cardone G, Janke C,
Jungmann R, Magiera MM, et al: Direct induction of microtubule
branching by microtubule nucleation factor SSNA1. Nat Cell Biol.
20:1172–1180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu LW and Hu X: SSNA1 promotes
hepatocellular carcinoma metastasis via STAT3/EMT induction.
Anticancer Res. 43:3479–3486. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Platchek M, Lu Q, Tran H and Xie W:
Comparative analysis of multiple immunoassays for cytokine
profiling in drug discovery. SLAS Discov. 25:1197–1213. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ye X, Xiong W, Xu X, Zeng J, Xie H, Li B,
He B, Chen L and Mo Q: Cost-benefit analysis of serological and
nucleic acid testing for hepatitis B virus in blood donors in
southern China. BMC Infect Dis. 24:9092024. View Article : Google Scholar : PubMed/NCBI
|