Introduction
According to National Cancer Center of China, there
were 4.82 million new cancer cases in China in 2022, including
370,000 new cases of liver cancer, making it the fourth most common
cancer in China (1). Currently, the
treatment of liver cancer primarily focuses on perioperative
treatment, surgery and systemic drug therapy (2); however, liver cancer is often
diagnosed at an advanced stage or the physical condition of the
patient may preclude surgical intervention (3). Despite advances in chemotherapy and
the development of targeted therapy and immunotherapy, toxic side
effects remain a challenge (3).
Taxanes have potential in antitumor activity and
serve an important role in cancer prevention and treatment
(4). Since its isolation from
Ornithogalum saundersiae in 1992, orsaponin (OSW-1) has
demonstrated potent antitumor activity in various cancer models
(5,6). However, its mechanism of action
remains incompletely elucidated and the compound is challenging to
synthesize, maintaining its status in the preclinical research
phase (7). OSW-1 may be associated
with mitochondrial function and trigger apoptosis via mitochondrial
signaling pathways (8). Previous
studies have reported that mitochondrial dysfunction is associated
with apoptosis in hepatocellular carcinoma (HCC) cells (9,10).
Uncoupling protein 2 (UCP2) regulates the production of reactive
oxygen species (ROS) in mitochondria, thus influencing
mitochondrial dysfunction (11).
UCP2 mediates resistance to gemcitabine-induced apoptosis in HCC
cell lines (12). A previous study
demonstrated that OSW-1 induces necrotic apoptosis in HCC cells,
primarily via the mitochondrial pathway (13). Given UCP2 role in
mitochondrial-mediated chemoresistance and OSW-1′s mitochondrial
targeting mechanism, investigating potential interactions between
UCP2 and OSW-1 could elucidate determinants of therapeutic
efficacy, though direct evidence remains unconfirmed.
The present study aimed to explore the cytotoxicity
of OSW-1 in HCC cells and elucidate its potential antitumor
mechanisms.
Materials and methods
Gene Expression Profiling Interactive
Analysis (GEPIA)
The updated version of GEPIA2 was utilized to
analyze the differential expression of UCP2 in liver cancer,
perform survival analysis, and assess its expression across
different clinical stages (gepia2.cancer-pku.cn/#analysis). GEPIA2,
an upgraded version of GEPIA, integrates RNA-seq data from 9,736
tumors (The Cancer Genome Atlas, TCGA) and 8,587 normal
(Gene-Tissue Expression, GTEx) samples processed through the UCSC
Xena standardized pipeline (http://xena.ucsc.edu).
Cell lines and culture
Human THLE-2 and Hep3B cell lines (Shanghai Fuheng
Biotechnology Co., Ltd.), derived from normal human hepatocytes and
HCC cells, respectively, were cultured in RPMI-1640 and Eagle's
minimum essential medium supplemented with 10% fetal bovine serum
and 1% penicillin and streptomycin [all (Shanghai Xiaopeng
Biotechnology Co., Ltd.)]. Cells were maintained at 37°C in a
humidified incubator with 5% CO2.
OSW-1
OSW-1 (HY-101213, CAS:145075-81-6) was purchased
from MedChem Express. It was dissolved in dimethyl sulfoxide
(Beijing Solarbio Science & Technology Co., Ltd.) to a
concentration of 100 µg/ml and stored at −20°C.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was monitored using a CCK-8 assay
(Beijing Solarbio Science & Technology Co., Ltd.). The
aforementioned cell lines in the logarithmic growth phase were
seeded in a 96-well plate (1×104/well) for 24 h. After
treatment, CCK-8 reagent was added, followed by incubation at 37°C
for 2 hours. Absorbance at 450 nm was measured using a microplate
reader (Synergy Mx; BioTek; Agilent Technologies, Inc.). Data
analysis was performed using SPSS (version 24.0; IBM Corp.) and
samples were analyzed in quintuplicate.
Mitochondrial membrane potential
assay
Hep3B cells in the logarithmic growth phase were
incubated with OSW-1 at concentrations of 0.0, 12.5, 25.0 and 50.0
ng/ml in a 6-well plate (5×104/well) at a constant 37 °C
for 24 h. JC-1 staining solution (Beyotime Institute of
Biotechnology) was added and incubated at 37°C for 25 min in the
dark. Cells were then washed twice with JC-1 staining buffer.
Images were captured using an inverted fluorescence microscope
(Discover Echo Inc.) and analyzed with ImageJ software (Version
1.54h, National Institutes of Health). Each sample was tested in
triplicate.
Cell cycle assay
The cell cycle was assessed using a cell cycle and
apoptosis detection kit (cat. no. C1052; Beyotime Institute of
Biotechnology). Following treatment aforementioned, single-cell
suspensions were prepared and stained according to the
manufacturer's instructions. Flow cytometry (FACSCalibur; BD
Biosciences) was used to analyze (FlowJo; 10.6.2; BD) the cell
cycle and triplicate experiments were performed.
ROS assay
According to the manufacturer's instructions (ROS
Assay Kit; Beyotime Institute of Biotechnology), cells were loaded
with 10 µM DCFH-DA (diluted 1:1,000 in extracellular solution) and
incubated at 37°C for 20 min in the dark. After washing three times
with extracellular solution to remove residual probe, ROS levels
were assessed using a short-term stimulation method. Based on
preliminary tests showing no detectable changes in ROS levels at
lower concentrations, the drug concentration was increased 10-fold
for experimental treatments. For positive controls, cells were
stimulated with Rosup (diluted 1:1,000 in PBS) at 37°C for 20–30
min to induce ROS elevation. After digesting the Hep3B cells and
preparing a single-cell suspension at a concentration of
1×106/ml, dichlorodihydrofluorescein diacetate was added
and the cells were incubated at 37°C. The probe was invested and
mixed every 3–5 min to allow full contact with the cells. The
non-probe and Rosup positive control groups were used as the
reference. The Rosup group was stimulated for 20 min and the OSW-1
group for 2 h. Flow cytometry was used to observe changes in ROS
levels and each sample was analyzed in triplicate.
Reverse transcription-quantitative
(RT-q)PCR
Hep3B cells were treated with OSW-1 as
aforementioned. RNA was extracted using the TRNzol Universal method
(DP424; Tiangen Biotech Co., Ltd.). Reverse transcription was
performed with the FastKing cDNA First Strand Synthesis Kit (KR116;
Tiangen Biotech Co., Ltd.) under the following conditions: 42°C for
15 min followed by 95°C for 3 min. Quantitative PCR was performed
using a Thermo 7300 Plus PCR instrument (Thermo Fisher Scientific)
with the SuperReal Fluorescence Quantitative PreMix Enhanced kit
(FP205; Tiangen Biotech Co., Ltd.). Thermocycling conditions
consisted of an initial denaturation at 95°C for 15 min (1 cycle),
followed by 40 cycles of 95°C for 10 sec and 60°C for 26 sec. Gene
expression levels were analyzed by the 2−ΔΔCq method
(14). Quantification was performed
according to the manufacturer's protocol in the kit manual. The
primer sequences were as follows: UCP2 forward,
5′-GGAGGTGGTCGGAGATACCAA-3′ and reverse,
5′-ACAATGGCATTACGAGCAACAT-3′ and GAPDH forward,
5′-TCAAGGCTGAGAACGGGAAG-3′ and reverse,
5′-TGGACTCCACGACGTACTCA-3′.
Western blotting (WB)
Cells were treated as aforementioned and proteins
were extracted using RIPA cell lysate (Beijing Solarbio Science
& Technology Co., Ltd.) mixed with PMSF. A BCA protein assay
kit (Beyotime Institute of Biotechnology) was used for protein
quantification. The prepared protein samples (40 ug/lane) were
separated by 12% SDS-PAGE (Beijing Solarbio Science &
Technology Co., Ltd.) and transferred to nitrocellulose membranes
(MilliporeSigma). The membrane was blocked in 5% skim milk at room
temperature for 1 h and incubated overnight at 4°C with the
following primary antibodies: BAX (1:1,000 dilution, catalogue no.
WL01637; Wanleibio Co., Ltd.), Bcl-2 (1:1,000, WL01556; Wanleibio
Co., Ltd.), Cleaved Caspase-3 (1:1,000, WL01992; Wanleibio Co.,
Ltd.), Caspase-3 (1:1,000, WL04004; Wanleibio Co., Ltd.), UCP2
(1:1,000, 11081-1-AP; Proteintech), and β-actin (1:1,000, AF7018;
Affbiotech). The next day, the membrane was incubated with a
fluorescent secondary antibody (IRDye 800CW goat anti-Rabbit;
1:20,000; LI-COR Biosciences) for room temperature 1 h in the dark,
followed by visualization using a dual near-infrared fluorescent
molecular imaging system. ImageJ was used to analyze the gray
values of the bands.
Statistical analysis
All data are presented as the mean ± SD from three
independent experimental repeats. All data were processed using
SPSS 24.0 and analyzed using one-way analysis of variance followed
by post hoc LSD test. Graphs were generated using the GraphPad
Prism 6 software (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
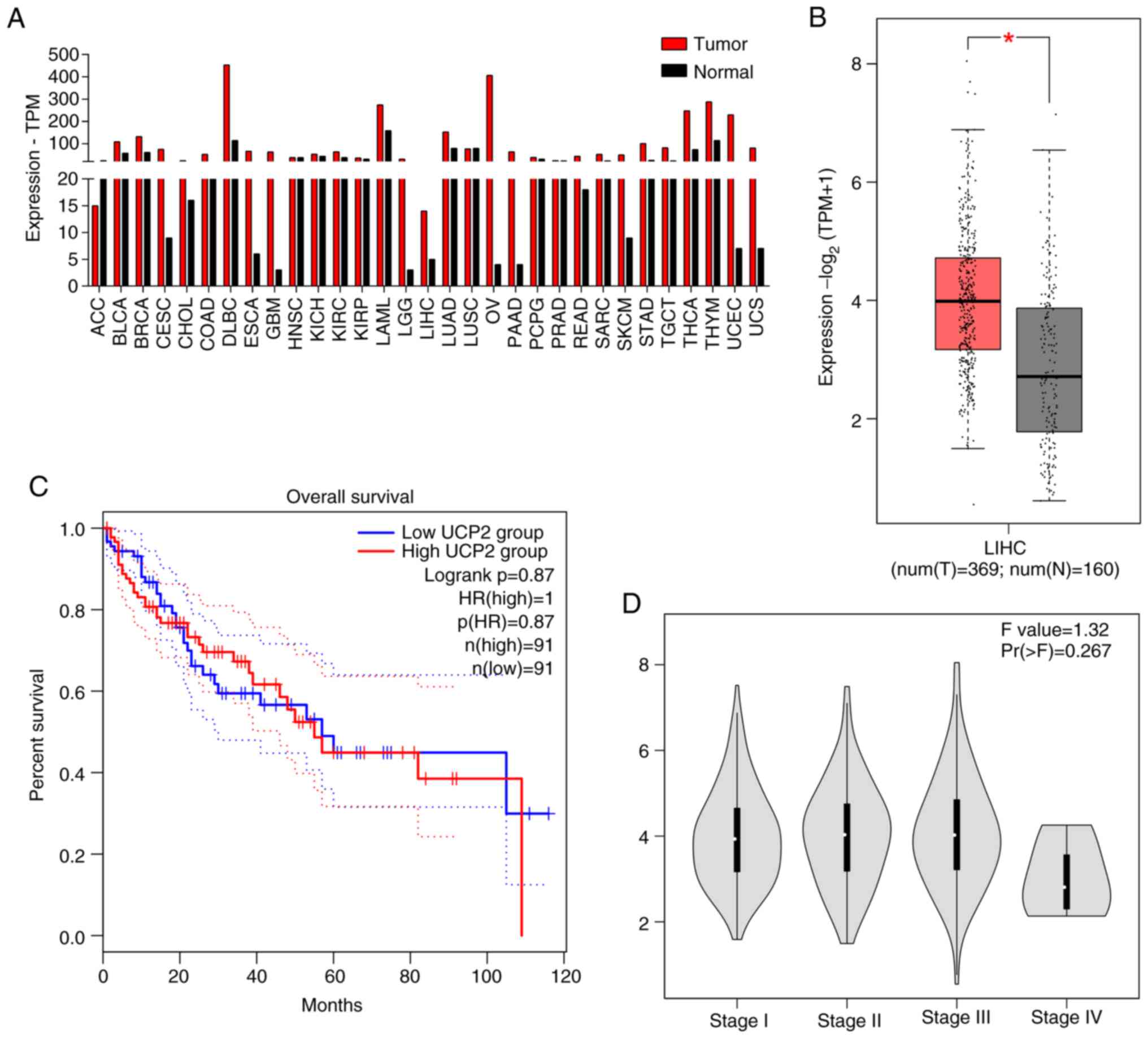
UCP2 is upregulated in liver
cancer
The GEPIA database was used to assess differences in
UCP2 expression between cancerous and normal human tissue. UCP2
expression was significantly higher in HCC compared with paired
normal tissue (Fig. 1A). Compared
with normal tissue in TCGA and GTEx data, the expression of UCP2 in
liver cancer was significantly higher compared with that in normal
tissues (P<0.05; Fig. 1B).
Moreover, patients with high UCP2 expression demonstrated shorter
overall survival compared with those with lower expression
(Fig. 1C). No significant
association was found between UCP2 expression and the clinical
stage of HCC (Fig. 1D).
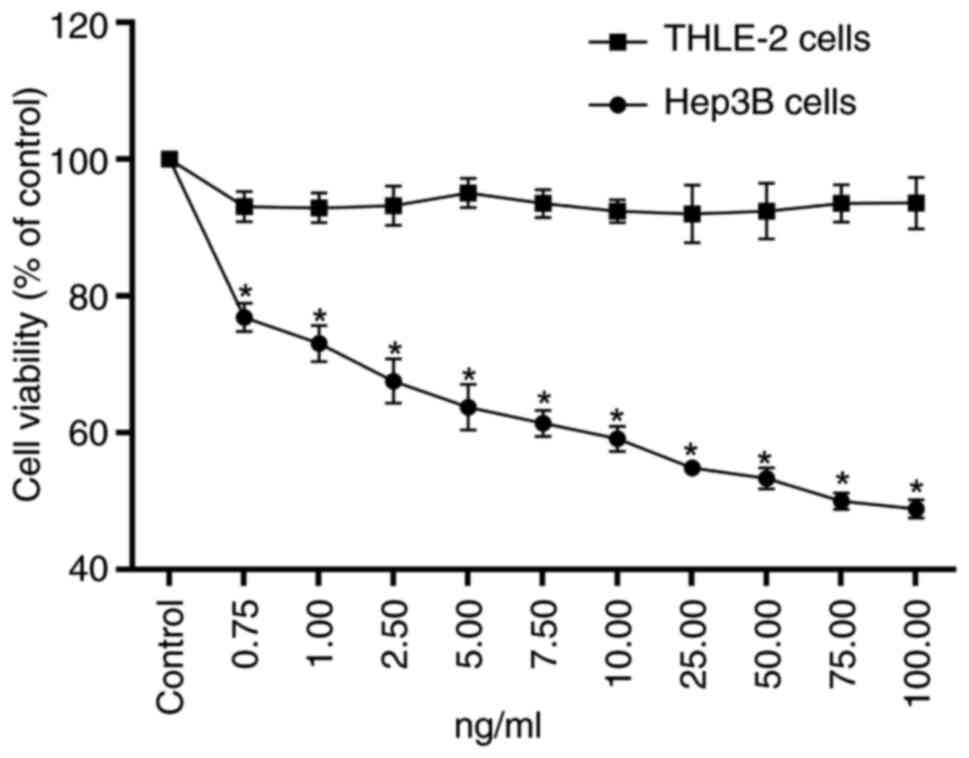
OSW-1 inhibits the proliferation of
Hep3B cells
To evaluate the ability of OSW-1 to inhibit cell
survival, a CCK-8 assay was used. OSW-1 had a significant cytotoxic
effect on Hep3B cells; however, the viability of THLE-2 cells was
significantly higher compared with Hep3B cells at the same
concentration (P<0.05; Fig. 2).
The half-maximal inhibitory concentration of OSW-1 against Hep3B
cells was 73.97 ng/ml.
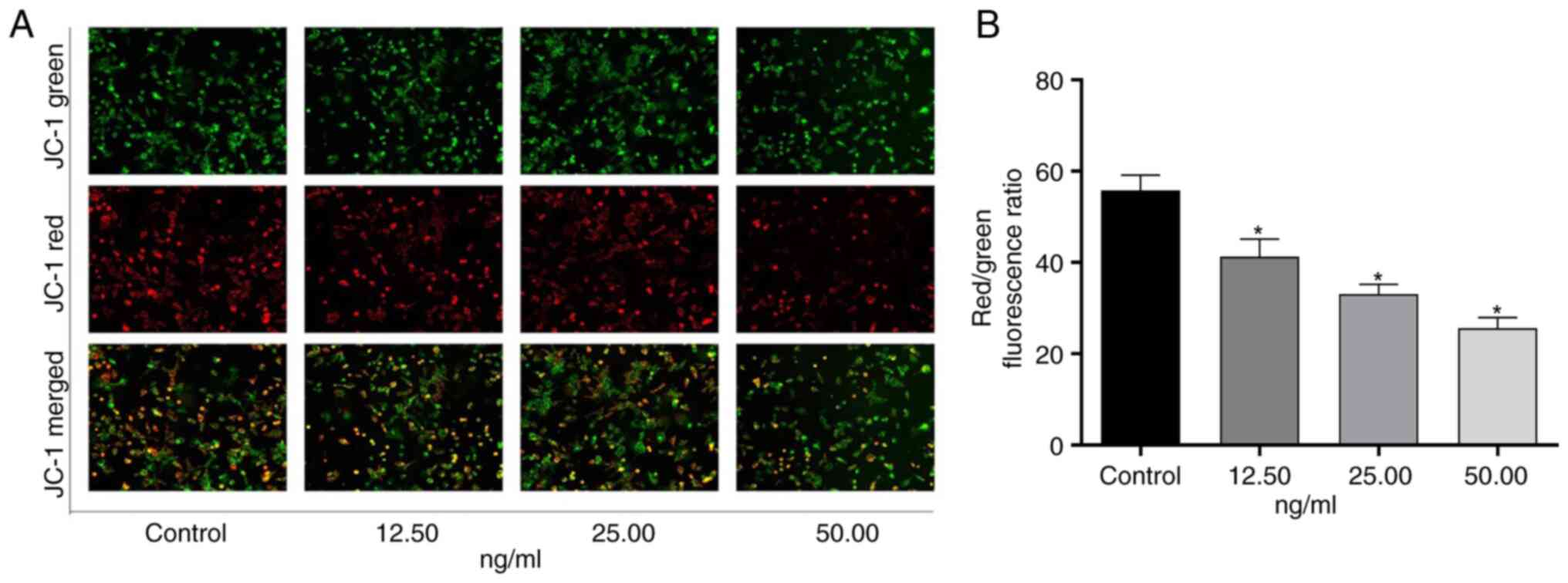
OSW-1 decreases the mitochondrial
membrane potential in Hep3B cells
A decline in mitochondrial membrane potential is a
hallmark of early apoptosis. To measure this, the
membrane-permeable cationic dye JC-1 was used as a fluorescent
probe. A decrease in mitochondrial membrane potential is indicated
by the transition of JC-1 from red to green (15). OSW-1 significantly decreased the
mitochondrial membrane potential in Hep3B cells in a dose-dependent
manner compared with untreated controls (P<0.05; Fig. 3).
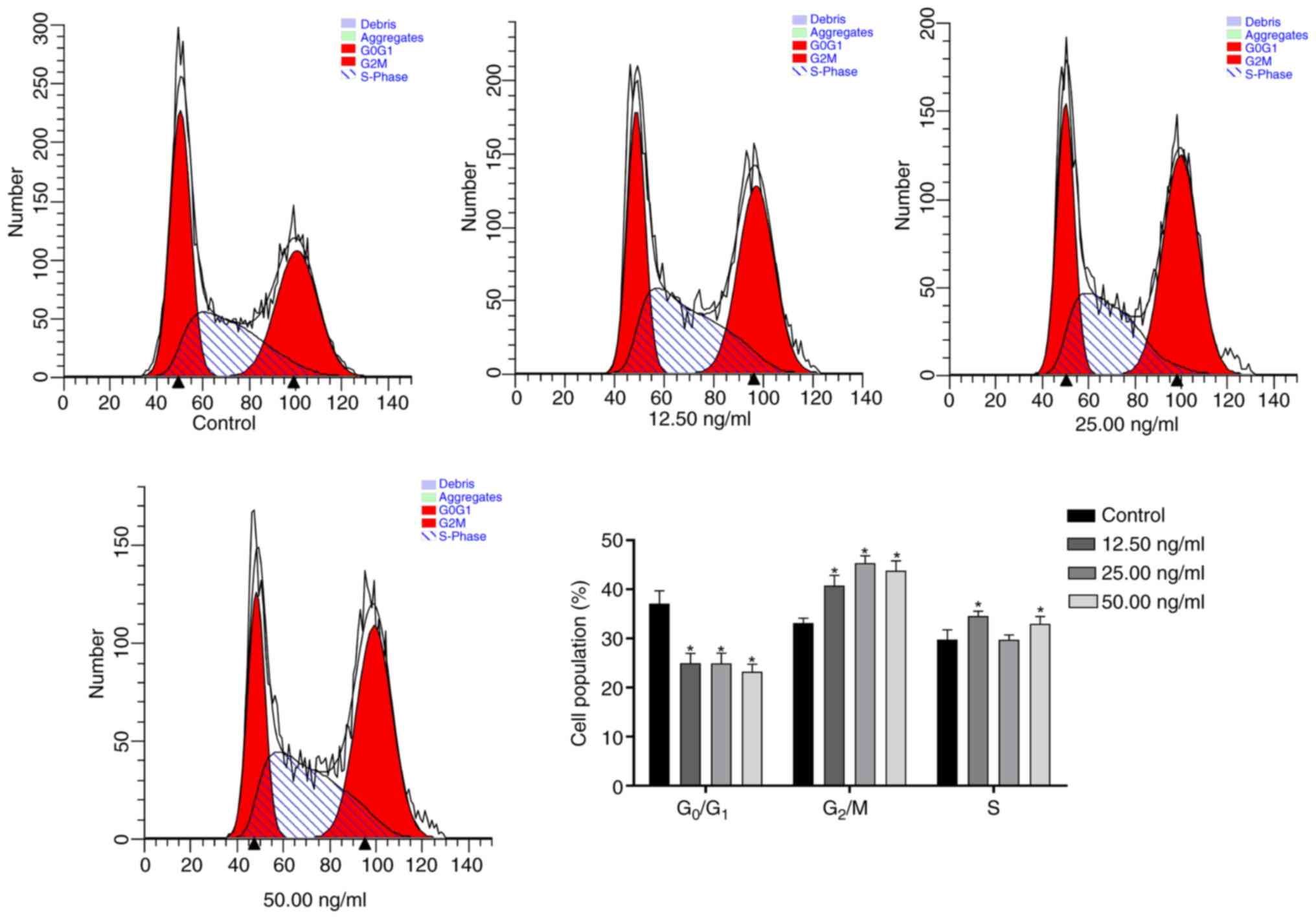
OSW-1 mediates Hep3B cell cycle
arrest
OSW-1 induced significant G2/M phase arrest and
partial S phase accumulation in Hep3B cells, concurrent with
reduced G0/G1 population. (P<0.05; Fig. 4).
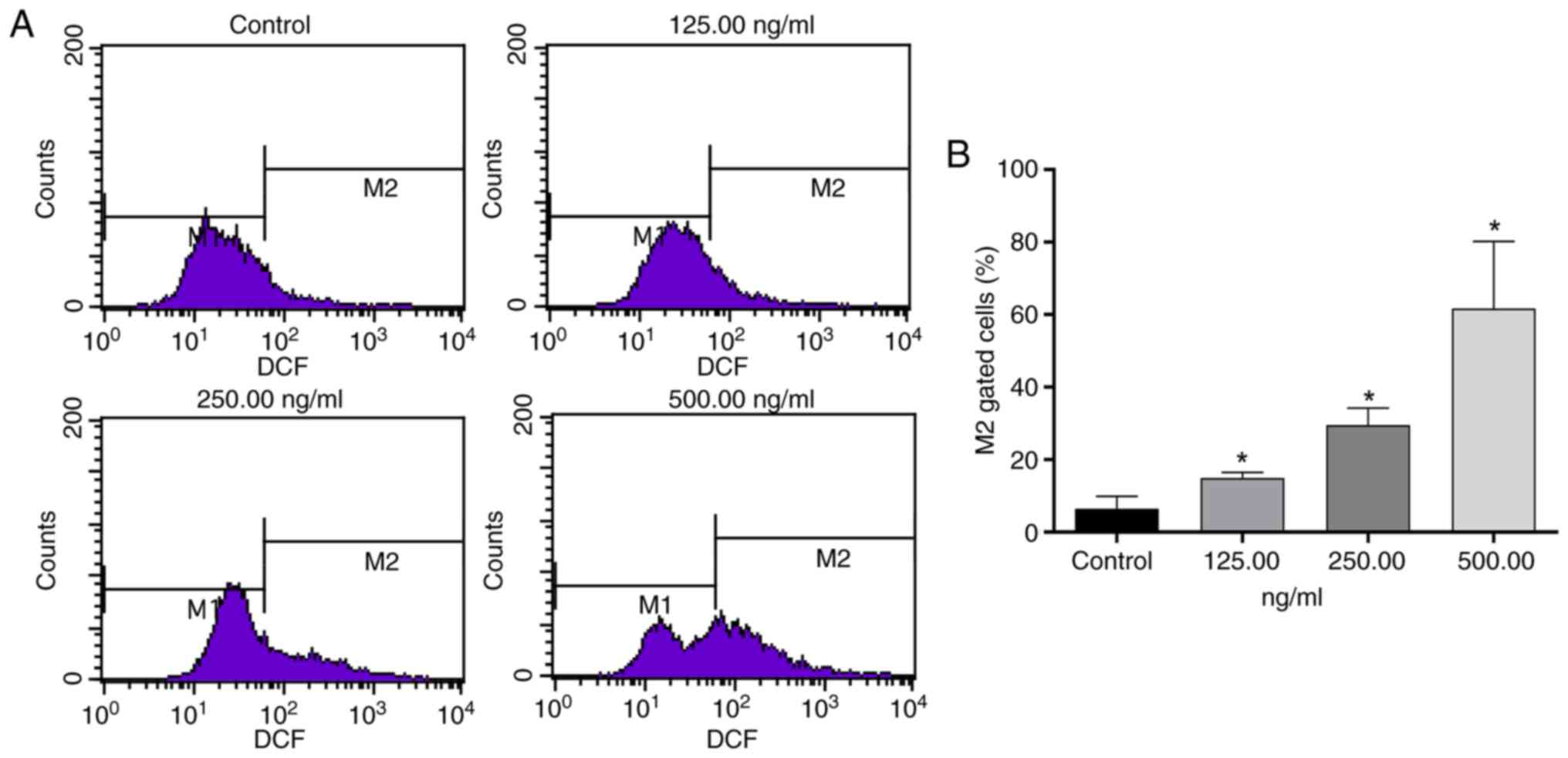
Effect of OSW-1 on ROS in Hep3B
cells
OSW-1 markedly increased intracellular ROS levels
(Fig. 5A). Compared with the
control group, 125, 250, and 500 ng/ml OSW-1 treatments induced
significant increases in M2 gated cells (%) (P<0.05;
Fig. 5B).
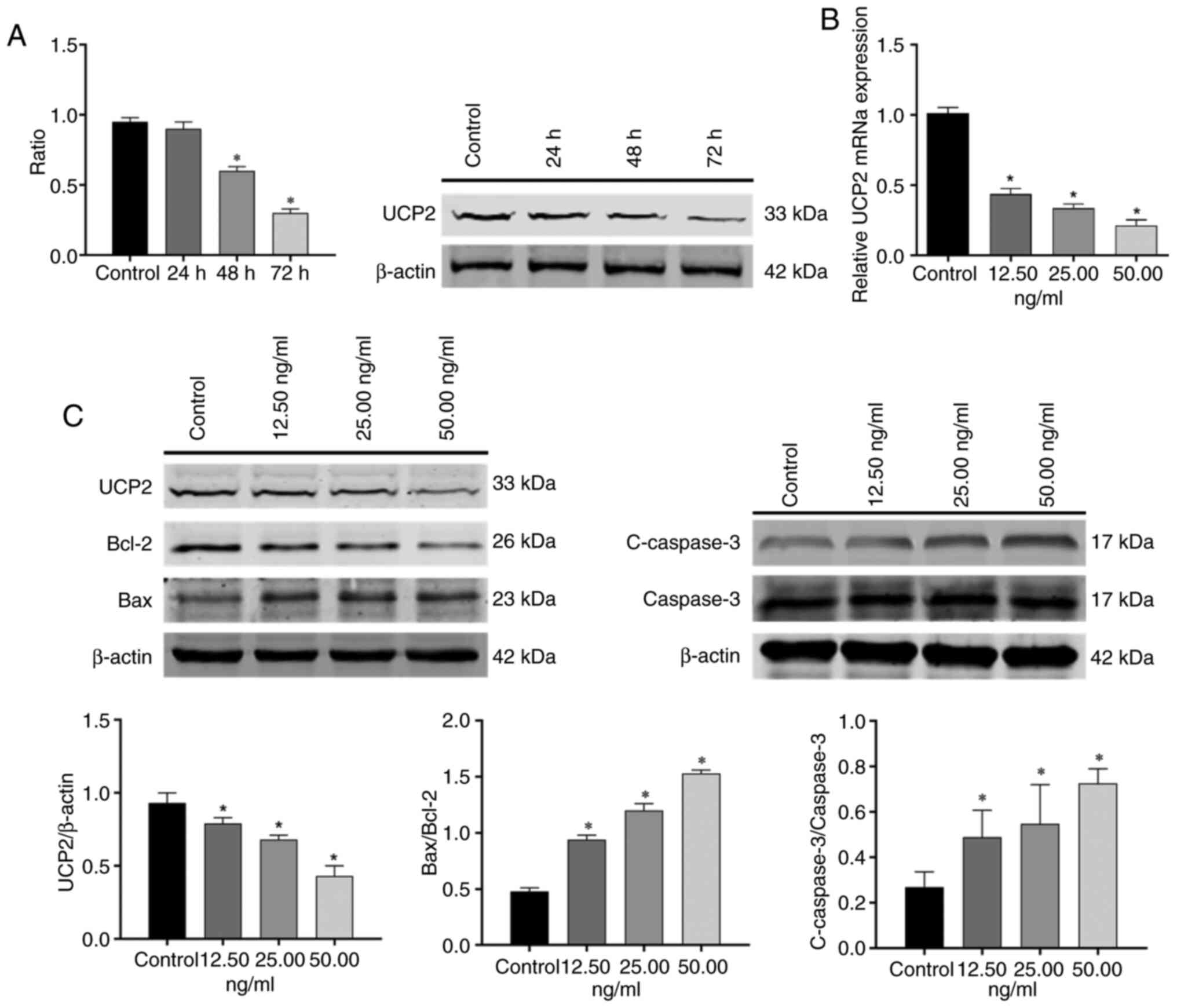
OSW-1 induces apoptosis in Hep3B cells
via UCP2
To investigate how OSW-1 induces apoptosis in Hep3B
cells, WB and RT-qPCR were performed to assess UCP2 protein and
mRNA expression, respectively. RT-qPCR analysis demonstrated that
the expression of UCP2 mRNA decreased in a dose-dependent manner
with increasing OSW-1 concentration. Compared with the control
group, 12.5, 25.00, and 50.00 ng/ml OSW-1 reduced UCP2 mRNA
(P<0.05; Fig. 6B).
Similarly, WB (Fig. 6C)
demonstrated that the expression of UCP2 decreased with an increase
in OSW-1 concentration (P<0.05). The protein expression
of UCP2 decreased with longer treatment times at 12.5 ng/ml OSW-1
(Fig. 6A) and the 48- and 72-h
groups were significantly decreased compared with the untreated
control group (P<0.05). Compared with the control, Bcl-2
was downregulated, Bax was upregulated and the Bax/Bcl-2 ratio was
significantly increased with OSW-1 treatment. In addition,
cleaved-caspase3/caspase3 levels were significantly increased
compared with the control (P<0.05).
Discussion
OSW-1 was first isolated from the bulbs of O.
saundersiae by Kubo et al (16) in 1992. Researchers have studied
OSW-1 in-depth and have reported notable anticancer activity across
several cancer types (17,18). OSW-1 is considered a promising
anticancer drug due to its low toxicity in non-malignant cells;
however, its clinical application remains limited due to its
unclear anticancer mechanisms and low yield (19). In the present study, the
cytotoxicity of OSW-1 in HCC cells and normal hepatocytes was
assessed. OSW-1 exhibited low toxicity in normal hepatocytes and
significant toxicity in HCC cells.
There is an association between mitochondrial
dysfunction and liver cancer (20).
Numerous studies have reported that UCP2 affects mitochondrial
dysfunction and ROS production (21,22).
Additionally, miR-214 suppresses HCC progression and reverses
chemoresistance by targeting UCP2 expression to regulate ROS
homeostasis and apoptosis. (23).
In the present study, using GEPIA database analysis, the expression
of UCP2 in HCC was significantly higher compared with normal
tissue. Therefore, UCP2 may serve an important role in liver cancer
progression. The lack of significance between the clinical stages
may be due to a small sample size.
Apoptosis is one of the primary mechanisms by which
anticancer drugs exert their effects. Iguchi et al (24) demonstrated that OSW-1 induces
apoptosis in HL-60 cells via a mitochondria-independent signaling
pathway, while Wu et al (25) reported that OSW-1 suppresses
triple-negative breast cancer growth and metastasis by inducing
Ca2+-dependent mitochondrial apoptosis and
cytoprotective autophagy via PI3K/Akt-mTOR pathway inhibition,
while synergizing with chemotherapy agents. Therefore, it was
hypothesized that apoptosis may be a key mechanism of OSW-1-induced
tumor cell death. Apoptosis-associated proteins were evaluated;
OSW-1 downregulated the expression of Bcl-2, upregulated the
expression of Bax, and increased Bax/Bcl-2 and
cleaved-caspase3/caspase3 ratios, indicating, apoptosis in Hep3B
cells treated with OSW-1 was markedly increased.
UCP2 can resist inflammation and apoptosis in mice
with sepsis-associated encephalopathy by affecting mitochondrial
dysfunction and ROS accumulation (26). The UCP2-associated mitochondrial
pathway is involved in orthoxylin-induced apoptosis in human colon
cancer cells (27). Therefore,
OSW-1 may target and inhibit highly expressed UCP2 in HCC,
disrupting its regulation of mitochondrial ROS homeostasis, leading
to ROS accumulation and mitochondrial dysfunction. In the present
study, the expression of UCP2 decreased with an increase in OSW-1
concentration and time, demonstrating an association between UCP2
and OSW-1. Here, membrane potential decreased with an increase in
the concentration of OSW-1. This unexpected reduction in membrane
potential, despite decreased UCP2 expression, suggests that OSW-1
may exert additional effects on mitochondrial ion homeostasis.
UCP2, a proton transporter, is an inner mitochondrial membrane
carrier protein that can induce proton leakage and dissipate the
proton gradient, which may be an important mechanism for
controlling mitochondrial ROS production by adjusting the
mitochondrial membrane potential (28–31).
Previous studies have demonstrated that changes in
the cell cycle can affect the occurrence and process of apoptosis
and that cell cycle arrest and apoptosis serve antitumor roles
(32,33). Abusaliya et al (34) demonstrated that prunetrin induces
cell cycle arrest and intrinsic apoptosis in Hep3B cells via
inhibition of Akt/mTOR and activation of p38/MAPK signaling. In the
present study, flow cytometry demonstrated that the proportion of
Hep3B cells treated with OSW-1 was significantly increased in the
G2/M phase. This was similar to the findings of Liu
et al (35), who reported
that silencing of UCP2 sensitizes HeLa cells to radiation-induced
DNA damage, leading to increased apoptosis, G2/M cell
cycle arrest and increased mitochondrial ROS production. UCP2 can
inhibit the production of mitochondrial ROS, thereby alleviating
oxidative stress-induced apoptosis (36,37).
In the present study, the concentration of ROS increased with
increasing OSW-1 concentration. Furthermore, UCP2 expression
decreased with increasing OSW-1 concentration. UCP2 is a molecular
sensor and inhibitor of mitochondrial ROS production and serves an
important role in regulating apoptosis in different cell systems
(38).
The present study has some limitations, such as
exclusive use of Hep3B cells. HCC cell lines vary in genetic makeup
and biological behavior, so the present findings may not apply to
other cell lines. This restricts full understanding of effects of
OSW-1 on HCC. Future research should involve multiple cell lines to
confirm if the effects are consistent. Additionally, the specific
molecular pathways by which OSW-1 affects UCP2 remain unclear,
requiring further investigation. Notably, the present study did not
account for patient comorbidities or treatment differences, which
may confound the association between UCP2 expression and survival
outcomes. Comorbid conditions may independently alter mitochondrial
ROS dynamics, while prior therapy might modulate UCP2 levels,
potentially obscuring the impact of UCP2 expression on prognosis
and survival. To address this, future studies should integrate
clinical metadata into survival analyses, stratify cohorts by
treatment history and employ comorbidity-mimicking models to
determine how these factors influence OSW-1 efficacy and
UCP2-associated mechanisms. Finally, the present study did not
address the potential side effects of OSW-1 in normal tissue, which
is key for its therapeutic application in cancer treatment.
In conclusion, OSW-1 promotes apoptosis in Hep3B
cells, potentially via the modulation of UCP2 expression. These
findings provide insight into the potential of OSW-1 as a
therapeutic agent for liver cancer. Future studies should explore
the effects of OSW-1 across a range of HCC cell lines and in
vivo models to validate its therapeutic potential.
Additionally, investigating the molecular mechanisms of the
interaction between OSW-1 and UCP2 and its potential side effects
on normal tissue is essential for clinical application.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 8216140392).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KS performed data analysis and wrote the manuscript.
XJ and KS designed the study and confirm the authenticity of all
the raw data. Both authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Yanbian University. The
present study complied with the principles of the Declaration of
Helsinki and the Ethical Review of Biomedical Research Involving
Human Beings.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UCP2
|
uncoupling protein 2
|
|
OSW-1
|
orsaponin
|
|
CCK-8
|
Cell Counting Kit-8
|
|
WB
|
western blot
|
|
RT-q
|
reverse transcription-quantitative
|
|
GEPIA
|
Gene Expression Profiling Interactive
Analysis
|
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Meng F and Mao J: Progress of
natural sesquiterpenoids in the treatment of hepatocellular
carcinoma. Front Oncol. 14:14452222024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang B, Hao X, Yan J, Li X, Zhao M and Han
T: A bibliometric analysis of immune-related adverse events in
cancer patients and a meta-analysis of immune-related adverse
events in patients with hepatocellular carcinoma. J Transl Int Med.
12:225–243. 2024.PubMed/NCBI
|
|
4
|
Hadkar VM, Mohanty C and Selvaraj CI:
Biopolymeric nanocarriers in cancer therapy: Unleashing the potency
of bioactive anticancer compounds for enhancing drug delivery. RSC
Adv. 14:25149–25173. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao Y, Chen Y, Yao T, Li C, Li S and Wang
N: Anticancer effects of OSW-1 on colorectal cancer cells via the
ROS/NLRP3/caspase-1 pyroptosis signaling pathway. Int
Immunopharmacol. 148:1140542025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhan Z, Liu Z, Zhang C, Gao H, Lai J, Chen
Y and Huang H: Anticancer effects of OSW-1 on glioma cells via
regulation of the PI3K/AKT signal pathway: A network pharmacology
approach and experimental validation in vitro and in vivo. Front
Pharmacol. 13:9671412022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan Z, Liu Z, Lai J, Zhang C, Chen Y and
Huang H: Anticancer effects and mechanisms of OSW-1 isolated from
Ornithogalum saundersiae: A review. Front Oncol.
11:7477182021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Prieto C, Riaz Ahmed KB, Chen Z,
Zhou Y, Hammoudi N, Kang Y, Lou C, Mei Y, Jin Z and Huang P:
Effective killing of leukemia cells by the natural product OSW-1
through disruption of cellular calcium homeostasis. J Biol Chem.
288:3240–3250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HY, Nga HT, Tian J and Yi HS:
Mitochondrial metabolic signatures in hepatocellular carcinoma.
Cells. 10:19012021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng L, Zhu L, Fu S, Li Y and Hu K:
Mitochondrial dysfunction-molecular mechanisms and potential
treatment approaches of hepatocellular carcinoma. Mol Cell Biochem.
480:2131–2142. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szewczyk A, Jarmuszkiewicz W, Koziel A,
Sobieraj I, Nobik W, Lukasiak A, Skup A, Bednarczyk P, Drabarek B,
Dymkowska D, et al: Mitochondrial mechanisms of endothelial
dysfunction. Pharmacol Rep. 67:704–710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu G, Liu J, Xu K and Dong J: Uncoupling
protein 2 mediates resistance to gemcitabine-induced apoptosis in
hepatocellular carcinoma cell lines. Biosci Rep. 35:e002312015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin J, Jin X, Qian C, Ruan Y and Jiang H:
Signaling network of OSW-1-induced apoptosis and necroptosis in
hepatocellular carcinoma. Mol Med Rep. 7:1646–1650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 22:e4302012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubo S, Mimaki Y, Terao M, Sashida Y,
Nikaido T and Ohmoto T: Acylated cholestane glycosides from the
bulbs of Ornithogalum saundersiae. Phytochemistry.
31:3969–3973. 1992. View Article : Google Scholar
|
|
17
|
Ding X, Li Y, Li J and Yin Y: OSW-1
inhibits tumor growth and metastasis by NFATc2 on triple-negative
breast cancer. Cancer Med. 9:5558–5569. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Li CY, Yao TF, Kang XD and Guo HS:
OSW-1 triggers necroptosis in colorectal cancer cells through the
RIPK1/RIPK3/MLKL signaling pathway facilitated by the
RIPK1-p62/SQSTM1 complex. World J Gastroenterol. 30:2155–2174.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Fang F, Fan K, Zhang Y, Zhang J,
Guo H, Yu P and Ma J: Effective cytotoxic activity of OSW-1 on
colon cancer by inducing apoptosis in vitro and in vivo. Oncol Rep.
37:3509–3519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Zhao Y, Yu M, Qin J, Ye B and
Wang Q: Mitochondrial dysfunction and chronic liver disease. Curr
Issues Mol Biol. 44:3156–3165. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serviddio G, Bellanti F, Tamborra R, Rollo
T, Capitanio N, Romano AD, Sastre J, Vendemiale G and Altomare E:
Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and
increases susceptibility of non-alcoholic steatohepatitis (NASH)
liver to ischaemia-reperfusion injury. Gut. 57:957–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nesci S and Rubattu SL: UCP2, a member of
the mitochondrial uncoupling proteins: An overview from
physiological to pathological roles. Biomedicines. 12:13072024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu G, Wang J, Xu K and Dong J: Dynamic
regulation of uncoupling protein 2 expression by microRNA-214 in
hepatocellular carcinoma. Biosci Rep. 36:e003352016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iguchi T, Kuroda M, Naito R, Watanabe T,
Matsuo Y, Yokosuka A and Mimaki Y: Cholestane glycosides from
Ornithogalum saundersiae bulbs and the induction of
apoptosis in HL-60 cells by OSW-1 through a
mitochondrial-independent signaling pathway. J Nat Med. 73:131–145.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu M, Huang Q, Liao M, Wu X, Xi H, Ma H,
Li S, Zhang Y and Xia Y: OSW-1 induces apoptosis and
cyto-protective autophagy, and synergizes with chemotherapy on
triple negative breast cancer metastasis. Cell Oncol (Dordr).
45:1255–1275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao P, Li X, Yang Q, Lu Y, Wang G, Yang
H, Dong J and Zhang H: Malvidin alleviates mitochondrial
dysfunction and ROS accumulation through activating AMPK-α/UCP2
axis, thereby resisting inflammation and apoptosis in SAE mice.
Front Pharmacol. 13:10388022023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao C, Wei L, Dai Q, Zhou Y, Yin Q, Li Z,
Xiao Y, Guo Q and Lu N: UCP2-related mitochondrial pathway
participates in oroxylin A-induced apoptosis in human colon cancer
cells. J Cell Physiol. 230:1054–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu J, Shi L, Lin W, Lu B and Zhao Y: UCP2
promotes proliferation and chemoresistance through regulating the
NF-κB/β-catenin axis and mitochondrial ROS in gallbladder cancer.
Biochem Pharmacol. 172:1137452020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Khoury TG, Bahr GM and Echtay KS:
Muramyl-dipeptide-induced mitochondrial proton leak in macrophages
is associated with upregulation of uncoupling protein 2 and the
production of reactive oxygen and reactive nitrogen species. FEBS
J. 278:3054–3064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El Hoss S, Bahr GM and Echtay KS:
Lopimune-induced mitochondrial toxicity is attenuated by increased
uncoupling protein-2 level in treated mouse hepatocytes. Biochem J.
468:401–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kukat A, Dogan SA, Edgar D, Mourier A,
Jacoby C, Maiti P, Mauer J, Becker C, Senft K, Wibom R, et al: Loss
of UCP2 attenuates mitochondrial dysfunction without altering ROS
production and uncoupling activity. PLoS Genet. 10:e10043852014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morihara H, Yamada T, Tona Y, Akasaka M,
Okuyama H, Chatani N, Shinonome S, Ueyama A and Kuwabara K:
Anti-CTLA-4 treatment suppresses hepatocellular carcinoma growth
through Th1-mediated cell cycle arrest and apoptosis. PLoS One.
19:e03059842024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ni K, Li ZL, Hu ZY and Hong L: Antitumor
effect of apcin on endometrial carcinoma via p21-mediated cell
cycle arrest and apoptosis. Curr Med Sci. 44:623–632. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abusaliya A, Jeong SH, Bhosale PB, Kim HH,
Park MY, Kim E, Won CK, Park KI, Heo JD, Kim HW, et al: Mechanistic
action of cell cycle arrest and intrinsic apoptosis via inhibiting
Akt/mTOR and activation of p38-MAPK signaling pathways in hep3B
liver cancer cells by prunetrin-a flavonoid with therapeutic
potential. Nutrients. 15:34072023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu CH, Huang ZH, Dong XY, Zhang XQ, Li
YH, Zhao G, Sun BS and Shen YN: Inhibition of uncoupling protein 2
enhances the radiosensitivity of cervical cancer cells by promoting
the production of reactive oxygen specie. Oxid Med Cell Longev.
2011:18417832021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu C, Zhang X, Wei W, Zhang N, Wu H, Ma Z,
Li L, Deng W and Tang Q: Matrine attenuates oxidative stress and
cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via
maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 9:690–701. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lou J, Wang Y, Wang X and Jiang Y:
Uncoupling protein 2 regulates palmitic acid-induced hepatoma cell
autophagy. Biomed Res Int. 2014:8104012014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Derdak Z, Garcia TA and Baffy G: Detection
of uncoupling protein-2 (UCP2) as a mitochondrial modulator of
apoptosis. Methods Mol Biol. 559:205–217. 2009. View Article : Google Scholar : PubMed/NCBI
|