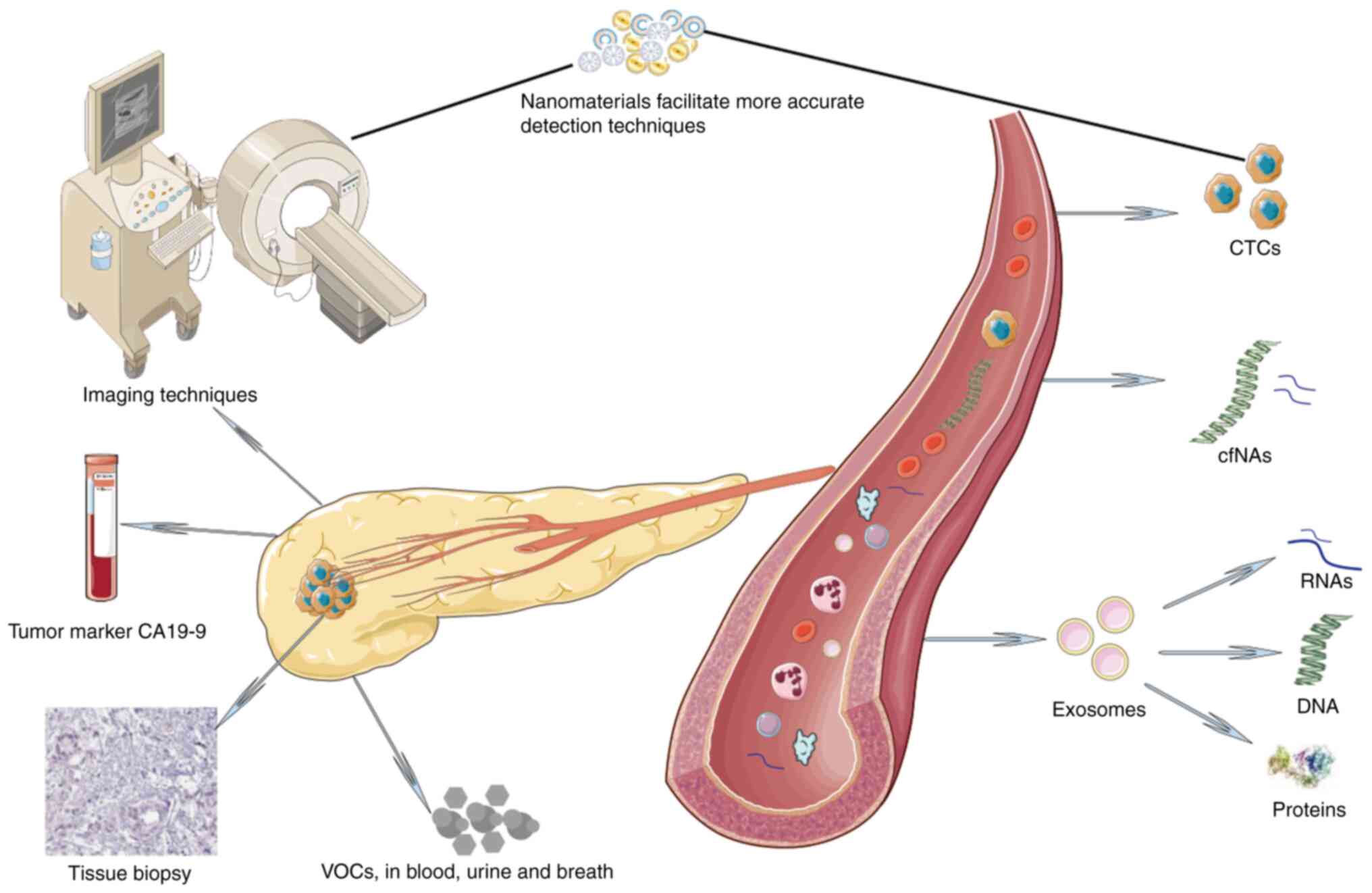
Pancreatic cancer (PC), particularly pancreatic
ductal adenocarcinoma (PDAC), continues to be one of the most fatal
cancers, marked by a poor prognosis and an elevated mortality rate.
The American Cancer Society reports that PC is the 10th most common
cancer in men and the 8th in women in terms of newly diagnosed
cases. Furthermore, it is recognized as the fourth leading cause of
mortality associated with cancer (1). Patients diagnosed with stage IV
metastatic PC exhibit a 5-year survival rate of 3%. By contrast,
patients presenting with stage I PC can expect a notably higher
5-year survival rate, reaching as much as 44% (2). Patients diagnosed with stage IA PC can
attain a 5-year disease-free survival rate of 80% (3). This disease often presents without
symptoms in its early stages, leading to advanced stage diagnoses
where therapeutic options are limited and less effective (4). Consequently, timely identification and
precise diagnosis are paramount to improving patient outcomes and
survival rates.
The array of diagnostic techniques available for PC
includes various modalities, each presenting distinct benefits and
drawbacks. Imaging techniques such as ultrasound, computed
tomography (CT), magnetic resonance imaging (MRI) and endoscopic
ultrasound (EUS) are pivotal in the initial detection and staging
of pancreatic lesions (5). These
modalities provide critical anatomical and functional information
that guides clinical decision-making. Biopsy techniques, such as
percutaneous core-needle biopsy, EUS-guided fine-needle aspiration
(FNA) and fine-needle biopsy, remain the gold standard for
definitive diagnosis. These methods allow for histopathological and
molecular analyses, providing essential information for
personalized treatment strategies (6). However, challenges such as sample
adequacy and procedural complications persist, highlighting the
need for optimization and standardization (7). Tumor markers, particularly
carbohydrate antigen 19-9 (CA19-9), play a significant role in the
biochemical diagnosis of PC. Although CA19-9 is the most widely
used serum marker, its specificity and sensitivity are suboptimal,
necessitating the exploration of additional biomarkers to enhance
diagnostic accuracy (8). Emerging
diagnostic technologies, including liquid biopsy and advanced
genomic and molecular techniques, are revolutionizing the field.
Liquid biopsy, which analyzes indicators in the blood or other body
fluids, offers a minimally invasive alternative for early detection
and real-time monitoring of disease progression (9). Previous advances have identified
several potential indicators, including circulating tumor cells
(CTCs), circulating tumor (ct)DNA and exosomes, which hold promise
for non-invasive diagnosis and monitoring (10). Additionally, genomic and molecular
profiling of pancreatic tumors can uncover actionable mutations and
guide targeted therapies, thereby enhancing precision medicine
approaches (11). The metabolic
changes within tumor cells have revealed that these cells release
specific volatile organic compounds (VOCs), which could serve as
potential indicators for the early identification of PC (12–14).
Furthermore, the incorporation of nanomaterials into pre-existing
technologies, such as sensors and biosensors, has resulted in
notable improvements in both detection sensitivity and specificity
(Fig. 1) (15).
The present review aims to discuss the latest
research on imaging techniques, tumor markers, biopsy methods and
novel diagnostic technologies, providing a holistic overview of
their clinical applications and potential for integration into
standard practice, offering insights into their efficacy,
limitations and future directions.
Ultrasound, CT, MRI and EUS represent the
cornerstone of non-invasive PC diagnostics. CT and MRI, with their
superior anatomical resolution, have proven invaluable in
pancreatic cancer staging and surgical planning. However, their
sensitivity in detecting early-stage PC remains suboptimal. EUS,
with its high-resolution images and potential for guided biopsies,
has emerged as a critical tool (16). Artificial intelligence (AI) and
machine learning (ML) algorithms are also being investigated to
enhance diagnostic precision and optimize clinical workflows by
analyzing complex data sets from imaging and molecular diagnostics
(17).
Transabdominal ultrasonography (TUS) examination is
a widely used initial imaging modality for the diagnosis of PC due
to its non-invasive nature, accessibility and cost-effectiveness.
It employs high-frequency sound waves to produce images of the
pancreas and surrounding structures. Despite its advantages, the
sensitivity and specificity of ultrasound in detecting PC are
relatively low, particularly for small lesions or pancreatic tail
tumors (17). The accuracy of
ultrasound can be significantly affected by obesity and bowel gas,
which can obscure the pancreas. Previous studies have indicated
that the presence of dilation and stenosis in the main pancreatic
duct, pancreatic cyst formation, as well as localized adipose
tissue alterations within the pancreas, as observed through imaging
techniques, are distinctive features associated with the early
stages of PDAC (18,19). Kanno et al (20) reported that the rate of tumor
identification in stage I PC was 67.3% and the rate of main
pancreatic duct dilation was 74.3% using TUS. Advancements in
ultrasound technology, such as contrast-enhanced ultrasound (CEUS)
and elastography, have improved diagnostic performance. CEUS
enhances the visualization of vascular patterns within the tumor,
aiding in the differentiation between benign and malignant lesions
(21). CEUS has high diagnostic
value in evaluating vascular invasion in patients with PDAC,
especially invasion into the celiac artery and its branches
(22). Elastography measures tissue
stiffness, providing additional information that can help in the
characterization of pancreatic masses (23).
EUS integrates endoscopy and ultrasound to provide
high-resolution images of the pancreas and surrounding structures.
EUS is valuable for detecting small pancreatic tumors and assessing
local invasion and lymph node involvement (24). It allows for FNA of lesions,
providing tissue samples for cytological examination and molecular
analysis (25). The sensitivity of
EUS in detecting PC is reported to be as high as 94%, with a
specificity of 89% (26).
Contrast-Enhanced Harmonic Imaging EUS (CEH EUS) has emerged as a
superior tool for the early detection of smaller lesions, with
studies showing 95.6% sensitivity compared to 82.7% for standard
EUS (27). EUS is also valuable in
guiding therapeutic interventions such as celiac plexus neurolysis
for pain management in patients with PC (28). However, the accuracy of EUS can be
operator-dependent, and the procedure is invasive, requiring
sedation and carrying risks such as pancreatitis and infection.
CT is considered the benchmark for the assessment
and staging of PC. It offers comprehensive cross-sectional images
of the pancreas, facilitating the evaluation of tumor size,
location and involvement of adjacent structures. Multidetector CT
with pancreatic protocol enhances the identification and
characterization of pancreatic tumors (29). The sensitivity of CT in identifying
PC is 76–92%, while the specificity is 85–95% (30). CT accurately detects PDAC at 98% for
stage III but is less effective for stage I tumors (31). CT is also valuable in evaluating the
resectability of the tumor by assessing vascular involvement, which
is crucial for surgical planning. However, small tumors and those
with isoattenuating characteristics may be missed on CT,
necessitating the use of additional imaging modalities (32).
MRI is another essential imaging modality for the
diagnosis and staging of PC. MRI offers enhanced contrast for soft
tissues compared with CT, making it particularly useful for
characterizing pancreatic lesions and detecting liver metastases
(33). MRI techniques such as
magnetic resonance cholangiopancreatography and diffusion-weighted
imaging enhance the visualization of the pancreatic ductal system
and tumor cellularity, respectively (34). Wiest et al (35) reported a sensitivity of 88–100%, a
specificity of 63.4–94%, a positive predictive value (PPV) of
71.4–96.2% and a negative (N) PV of 68.5–100% for MRI. The
sensitivity and specificity of MRI for detecting PC are comparable
to those of CT, with some studies suggesting slightly higher
sensitivity for small lesions (10,36).
Additionally, MRI does not utilize ionizing radiation, which
renders it a safer alternative for specific groups of patients.
Positron emission tomography/CT (PET/CT) and positron emission
tomography/MRI (PET/MRI) are not routinely used in the staging of
patients with PDAC, may aid in detecting suspected pancreatic
tumors (37).
In recent years, there has been a notable rise in
the application of AI and ML within the healthcare sector (38). Similar to various other
malignancies, screening, diagnosing and formulating treatment
strategies for PDAC can be enhanced by AI models and ML algorithms.
AI-driven analysis shows notable potential for enhancing the
capabilities of imaging techniques in the early identification and
characterization of PC (39). The
present study consolidates current literature and a summary of a
set of recent AI studies focused on the detection and diagnosis of
PDAC, along with details regarding the models, datasets and
evaluation metrics (40–63) (Table
I).
Percutaneous biopsy, particularly CT-guided
percutaneous FNA biopsy (FNAB), is widely used for diagnosing PC.
This technique involves the insertion of a needle through the skin
to obtain tissue samples from the pancreas, guided by imaging
techniques such as CT or ultrasound. The accuracy of CT-guided
percutaneous FNAB had a diagnostic accuracy rate of 95.1% in a
study involving 84 patients with peritoneal lesions (64). The procedure is generally safe, with
complications such as bleeding and ascite leakage occurring in a
small percentage of cases (64).
The high diagnostic yield and safety profile make percutaneous
biopsy a valuable tool in the diagnostic arsenal for PC, especially
when other less invasive methods fail to provide a definitive
diagnosis.
EUS-guided FNAB (EUS-FNAB) has emerged as a
preferred technique for obtaining tissue from pancreatic lesions.
This technique integrates endoscopy and ultrasound to facilitate
real-time imaging and precise needle placement. EUS-FNAB is
especially advantageous for diagnosing PC, as it enables the
sampling of lesions that are not easily accessible by percutaneous
methods. Lee et al (65)
have shown that EUS-FNAB has a high diagnostic accuracy, with
sensitivity and specificity rates often >90%. Additionally,
EUS-FNAB can be used to establish patient-derived PC organoids,
which serve as valuable models for research and personalized
treatment planning (32). Despite
its high accuracy, there are instances where EUS-FNAB may fail to
provide a definitive diagnosis, necessitating alternative methods
such as serial pancreatic juice aspiration cytological examination
(66).
FNAB uses a thin needle to extract cells from a
lesion for cytological examination. The procedure is often guided
by ultrasound or CT to ensure accurate needle placement. FNAB has
demonstrated efficacy in the diagnosis of PC, with study reporting
high sensitivity (93%) and specificity rates (91%) (67). However, the diagnostic yield of FNAB
can be influenced by factors such as the skill of the operator and
the quality of the obtained sample. In cases where FNAB results are
inconclusive, additional diagnostic methods or repeat biopsies may
be necessary (68). The integration
of molecular techniques, such as immunocytochemistry and genomic
profiling, can enhance the diagnostic accuracy of FNAB and provide
valuable information for personalized treatment planning (69).
Tissue biopsies, including percutaneous, endoscopic
and FNA techniques, provide definitive histopathological diagnosis.
However, the invasive nature and associated risks, such as
pancreatitis and tumor seeding, limit their widespread use.
Advances in biopsy techniques and the integration of molecular
profiling could enhance diagnostic accuracy and guide personalized
treatment strategies.
CA19-9 is recognized as the most commonly utilized
serum biomarker for PC. Despite its widespread use, CA19-9 has
limitations, such as 80% sensitivity and 75% specificity,
particularly in the early stages of PC (70). CA19-9 levels are elevated in ~80% of
patients with advanced PC, but it can also be elevated in
cholangitis, cirrhosis and other gastrointestinal malignancies,
which complicates its diagnostic utility (10). Moreover, ~5-10% of patients with PC
lack the Lewis antigen, which is necessary for CA19-9 expression,
rendering this biomarker ineffective for these individuals
(71). Its role in early detection
remains limited, highlighting the necessity for supplementary
biomarkers to enhance the precision of diagnostics. Joint detection
of carcinoembryonic antigen (CEA), CA125, CA242 and CA19-9 may
enhance the diagnostic efficiency for PC, increasing sensitivity to
90.4% and specificity to 93.8%, obviously higher than single
detection of those markers in diagnosis of pancreatic cancer
(72). Joint detection of MUC5AC
and CA19-9 demonstrated enhanced performance and increased
specificity in distinguishing PC from control groups, achieving an
area under the curve (AUC) of 0.894, sensitivity of 0.738 and
specificity of 0.886 (73).
Combination of trefoil factors (TFFs) with CA19.9 emerged as a
promising strategy for discriminating early-stage PC from benign
controls (BC) (AUCTFF1 + TFF2 + TFF3 + CA19.9=0.93) as
well as chronic pancreatitis (CP) (AUCTFF1 + TFF2 + TFF3 +
CA19.9=0.93) (74).
Combination of serum CA19-9 and serum glycoproteomics (IL.17E, B7.1
and DR6) can differentiate stage I PC from healthy controls (HCs;
AUC=0.988; 100% sensitivity at 90% specificity) (75). However, these results still need to
be further verified with large-scale, retrospective and prospective
clinical studies. Given the limitations of CA19-9, research has
focused on identifying additional biomarkers that could enhance the
early identification and diagnosis of PC. The heterogeneity of PC
and the lack of a universal biomarker underscore the necessity for
a multimodal approach in biomarker-based diagnostics.
Genomics and molecular biology techniques have
revolutionized the field of cancer diagnostics, providing
comprehensive understanding of the genetic and molecular
characteristics of tumors. In PC, these techniques have facilitated
the detection of specific genetic mutations and alterations that
drive tumorigenesis. Next-generation sequencing has been
instrumental in identifying actionable mutations in PC, guiding
targeted therapy and personalized treatment approaches (76). Molecular biology techniques such as
polymerase chain reaction (PCR) and digital droplet PCR (ddPCR)
have also been employed to detect specific genetic alterations in
PC. These techniques offer high sensitivity and specificity,
allowing for the detection of low-abundance mutations in
circulating free DNA (cfDNA) and exosomal DNA. ddPCR, in
particular, has shown promise in detecting KRAS mutations in PC,
providing valuable information for diagnosis and monitoring
(77). The combination of CTCs,
circulating free nucleic acids (cfNAs) and exosomes with advanced
genomic analysis provides a comprehensive and non-invasive approach
to PC diagnosis and monitoring (78).
Liquid biopsy is an innovative and minimally
invasive diagnostic method that has shown promise in the early
detection and monitoring of PC. This technique involves the
analysis of CTCs, cfNAs and exosomes in bodily fluids. Studies have
demonstrated the existence of CTCs associated with disease stage
and prognosis (79,80). ctDNA, which refers to the diminutive
segments of DNA that are emitted by neoplastic cells into the
circulatory system, offers another promising avenue as it can
provide information on genetic mutations, tumor burden and
treatment responses (81).
Exosomes, small extracellular vesicles containing proteins, lipids
and nucleic acids, have also been recognized as promising
biomarkers. Exosomal RNA and proteins can reflect the molecular
characteristics of the tumor and have been shown to have diagnostic
and prognostic value (82).
CTCs are malignant tumor cells present in the blood,
which can either shed directly from primary tumor cells after
undergoing epithelial-mesenchymal transition (EMT) or enter the
bloodstream through the lymphatic system to reach secondary sites.
They provide critical information regarding the genetic and
phenotypic characteristics of the tumor, aiding in the diagnosis
and monitoring of PC. Study has demonstrated that CTCs can be
detected in patients with PC with varying sensitivity and
specificity, making them a valuable tool for early diagnosis and
prognosis (83). In a suitable
environment, CTCs settle and proliferate, forming metastatic
tumors, and are considered the ‘seeds’ of malignant tumor
metastasis (84). CTCs are used as
non-invasive assessment indicators for disease progression and
prognosis in breast, colorectal and prostate cancer (85,86).
In PC, patients with PDAC with positive CTCs have a poorer overall
survival (OS) (HR, 1.23; 95% CI, 0.88–2.08; P<0.001) (87,88).
Ankeny et al (89) found
that the number of peripheral blood CTCs can serve as a biomarker
for the diagnosis and staging of PC. However, there is still
controversy regarding the use of CTCs for early detection of PDAC.
Bidard et al (90)
discovered that the detection rate of CTCs is low in the early
stages of malignant tumors, with only 11% of patients with locally
advanced PC having detectable levels of circulating CTCs. Tien
et al (91) found that 68%
of patients with PDAC had detectable CTCs in portal vein blood,
while only 40% had detectable CTCs in peripheral blood, suggesting
that portal vein blood may serve as a more effective alternative
sample. However, currently, reliable separation and detection of
CTCs remain technically challenging. On one hand, CTCs have a short
half-life in peripheral blood (1–2.4 h) and low concentration
(100,000–1,000,000/ml). On the other hand, CTC detection and
enrichment rely on the utilization of highly specific biomarkers to
attain the required levels of specificity and sensitivity.
Furthermore, the high heterogeneity and plasticity of tumor cells
complicate the selection of CTC marker detection (92).
cfNAs, including ctDNA and ctRNA, are another
component of liquid biopsy. These nucleic acids are released into
the circulatory system from apoptotic and necrotic tumor cells.
cfNAs can offer an extensive genetic characterization of the tumor,
encompassing details such as mutations, variations in copy number
and patterns of methylation. Previous advancements have improved
the sensitivity and specificity of cfNA detection, making it a
powerful tool for the early detection of PC (93). cfDNA released through tumor cell
apoptosis, necrosis or active release is termed ctDNA, which
accounts for a small portion of total cfDNA (94). ctDNA encompasses not only mutations
that mirror those found in neoplastic cells but also demonstrates
analogous epigenetic configurations, including DNA methylation,
histone modifications and chromatin remodeling, which are
consistent with gene expression and tumor characteristics (95). In the blood, ctDNA is swiftly
eliminated from the bloodstream via the activity of endonucleases
and exonucleases, in addition to renal excretion, exhibiting a
half-life that varies between several minutes to 2 h (96). Therefore, it can reflect the actual
condition of malignant tumors and the dynamic molecular changes
during tumor development. Consequently, ctDNA has been used to
understand the mutation status of malignant tumors, including PC
(97). Additionally, ctDNA can also
serve as a diagnostic method for diseases. Research has shown that
the sensitivity and specificity of ctDNA for diagnosing PDAC are 65
and 75%, respectively, while the combined sensitivity and
specificity of ctDNA, CA199 and CTCs increase to 78 and 91%,
respectively. Combining ctDNA with CA199, CEA, hepatocyte growth
factor and osteopontin can enhance the sensitivity to 64% and
specificity to 99.5% of PDAC diagnosis, respectively (98). Zill et al (99) compared ctDNA samples from 26
patients with PC with tumor tissue sequences and assessed 54 gene
mutations, finding that ctDNA mutations in genes including KRAS,
TP53, APC, FBXW7 and SMAD could accurately detect PDAC. Li et
al (100) have reported
significant variability in the frequency of detected ctDNA
mutations, such as KRAS in patients with PDAC ranging from 30 to
92%. Another option for detecting DNA mutations may be to examine
variations in DNA methylation. Li et al (101) compared differentially methylated
regions of cfDNA between patients with PC and HCs and developed a
diagnostic prediction model incorporating MAPT, SIX3, MIR663,
EPB41L3, FAM150A, TRIM73, LOC100128977 and LOC100130148, which
serve as potential non-invasive diagnostic indicators for PC. Eissa
et al (102) found that the
ctDNA ADAMTS1 and BNC1 methylation could be used as diagnostic
markers for early detection of PC with an AUC of 0.95 (sensitivity,
97.4%; specificity, 91.6%). However, ctDNA sequencing technology
requires very high sensitivity and specificity to overcome the low
concentration of ctDNA in the early stages of malignant tumors and
the potential for false positives from cfDNA present in normal
individuals (103).
miRNA is a non-coding RNA, consisting of ~21-25
nucleotides, that can affect the post-transcriptional expression of
target genes. It is crucial for various essential biological
functions, including development, proliferation and apoptosis.
Studies have shown that miRNAs are present in saliva, serum, plasma
and urine, with circulating miRNAs in the blood showing potential
roles in the diagnosis of cancer. In the diagnosis of PC,
Słotwiński and Slotwinska (104)
found that plasma miR-16 and miR-196a combined with CA199 can
better distinguish PC from HCs. In addition, Wang (105) discovered that serum miR-133a can
differentiate PC from HC (AUC, 0.893; sensitivity, 90.6%;
specificity, 87.2%). Furthermore, Wei et al (106) found that serum miR-1290 and
miR-1246 combined with CA199 can effectively distinguish PC from HC
(AUC, 0.97).
Due to the low abundance and fragility of
circulating miRNAs in the bloodstream, they are easily degraded by
RNase degradation in the bloodstream, leading to loss and/or damage
during the extraction process. With advancements in research, it
has been found that serum exosomes may serve as important carriers
for circulating miRNAs. Exosomes can package miRNAs, protecting
them from RNA enzyme digestion, and their high stability and ease
of enrichment also address the challenges of enriching circulating
miRNAs (107).
Exosomes are lipid-based vesicles released by cells
into the extracellular environment, with a diameter of 40–160 nm
and a phospholipid bilayer membrane structure. They encompass
various bioactive components derived from the source cells, such as
miRNA, mRNA, transcription factors, cytokines, growth factors and
lipids. All cells can secrete exosomes, which can be found in
various bodily fluids such as blood, urine, saliva, breast milk and
bile. Initially, exosomes were considered to be a means for cells
to release unwanted substances. However, subsequent research has
revealed their significant role in facilitating intercellular
communication and contributing to tumor advancement. The bioactive
components contained within exosomes are closely related to the
parent cells from which they originate, and thus, differences or
specific expressions of some bioactive components in exosomes may
make them potential biomarkers for the diagnosis of PC (108–145) (Table
II).
miRNAs in exosomes are relatively stable under
various physicochemical conditions due to the protective membrane
structure of exosomes, which shields them from ribonuclease
digestion (146). Zhou et
al (110) found that serum
exosomal miR-122-5p and miR-193b-3p were upregulated in PC compared
with HCs, while miR-221-3p was downregulated. Goto et al
(111) compared 32 PC, 29
intraductal papillary mucinous neoplasm (IPMN) and 22 HC samples,
and found that serum exosomal miR-191, miR-21 and miR-451a were
upregulated in both PC and IPMN. Exosomal miRNAs could distinguish
HCs from stage I and IIA PC, with miR-21 achieving a diagnostic
accuracy of 80.8%. Additionally, exosomal miRNAs from other bodily
fluids have shown potential diagnostic value for PC, such as
salivary exosomal miR-1246 and miR-4644, which could serve as
candidate biomarkers for diagnosing cholangiocarcinoma (AUC,
0.833). Yoshizawa et al (114) found that the proportion of
miR-3940-5p to miR-8069 in urinary exosomes from patients with PDAC
was elevated, achieving sensitivities and specificities of 93.0 and
78.4%, respectively, when combined with CA199 for diagnosing PDAC.
To date, multiple studies have demonstrated that exosomal miRNAs,
either individually or in conjunction, can serve as potential
biomarkers for diagnosing PC (115,116).
Long noncoding RNAs (lncRNAs) are classified as
noncoding RNAs that exceed a length of 200 nucleotides (147). Several studies have confirmed the
abnormal expression of exosomal lncRNAs in PC (148). Takahashi et al (124) analyzed serum exosomal lncRNA-HULC
in 20 cases of PDAC, 22 cases of IPMN and 21 HCs, finding that the
expression of serum exosomal lncRNA-HULC in patients with PDAC was
significantly increased compared with HCs and IPMN cases, showing
good diagnostic performance (AUC, 0.920). Yu et al (126) studied 284 cases of PDAC, 100 cases
of CP and 117 cases of HC, finding that a lncRNA group composed of
FGA, KRT19, ITIH2, HIST1H2BK, MARCH2, CLDN1, MAL2 and TIMP1 showed
high accuracy for the diagnosis of PDAC (AUC, 0.931). Circular RNA
(circRNA) is also a noncoding RNA that has been discovered in
recent years, and due to its closed-loop structure, exhibits higher
stability compared with linear RNA (149). Li et al (127) conducted sequencing analysis of
exosomal circRNA from the plasma of 8 patients with early-stage
PDAC and 8 HCs, finding 155 circRNAs that were differentially
expressed between PDAC and HC, which may be potential indicators
for the early diagnosis of PDAC. In addition, small nucleolar RNA
(snoRNA) is a non-coding RNA composed of ~60-300 nucleotides
(150). Kitagawa et al
(129) studied serum exosomes from
27 patients with stage I–II PDAC and 13 HCs, finding that SNORA74A
and SNORA25l could serve as biomarkers for early detection of PDAC
(AUC, 0.946 and 0.940, respectively). Kumar et al (132) sequenced serum exosomal mRNA and
found that the expression of MMP8, TBX3, PDX1, CTSL and SIGLEC15 in
serum exosomes obtained from PDAC samples was higher than that in
HCs. Yang et al (131)
found that the combination of circulating exosomal miR-409, CK18
mRNA, CD63 mRNA, circulating cfDNA concentration and CA19-9 had
improved diagnostic efficacy for PC than CA19-9 testing alone
(Table II).
The genomic mutations of DNA in exosomes can also
serve as biomarkers for diagnosing PC (151). Allenson et al (134) found that plasma exosomal KRAS
mutations in HCs and patients with early-stage, locally advanced
and late-stage PDAC were 7.4, 66.7, 80.0 and 85.0%, respectively,
with statistically significant differences. Furthermore, with the
accumulation of exosomal analysis data, data mining identified 575
protein-coding genes, 26 RNA genes and 1 pseudogene directly
related to PC (152). This exosome
database established through pure bioinformatics methods serves as
a valuable resource for the identification and confirmation of
novel diagnostic combinations.
The diagnostic role of exosomal membrane proteins
and the proteins contained within them in PC has also received
considerable attention. GPC-1 is a proteoglycan located on the
surface of cells that has been found to be significantly
overexpressed in exosomes from prostate cancer cells and is
considered a promising potential diagnostic marker in PDAC
(153). Melo et al
(137) extracted serum exosomes
from HCs, benign pancreatic disease (BPD) and early to late-stage
PDAC for mass spectrometry and nano-flow cytometry identification,
finding that GPC-1+ exosomes could act as a marker for
detecting early PC (AUC, 1). Buscai et al (154) discovered that combining
CD63+GPC-1+ exosomes from peripheral blood
and portal vein blood with CA19-9 improved the diagnostic efficacy
for PC, and demonstrated a positive correlation between
GPC-1− exosome levels and CTCs, which were associated
with progression-free survival(PFS) and OS of patients. However,
Lai et al (155) indicate
that GPC-1 cannot diagnose PDAC. These studies confirm that
GPC-1+ serum exosomes can act as potential diagnosis
biomarkers for PC, but further research is needed for validation.
Wei et al (141) found that
serum exosomal Ephrin type-A receptor 2 (EphA2) could diagnose PC
(AUC, 0.960). Additionally, exosomal proteins such as epidermal
growth factor receptor (EGFR), alkaline phosphatase, placental like
2 (ALPPL2), cytoskeleton-associated protein 4 (CKAP4), cellular
mesenchymal-epithelial transition factor (c-met), programmed death
ligand 1 (PD-L1), epidermal growth factor receptor pathway
substrate 8 (Eps8) and ALG-2-interacting protein X (ALIX) have also
attracted the attention of researchers regarding their role in PC
diagnosis (Table II) (140,143,144). Although further research is
required for confirmation and there are differences in the types of
proteins studied, the aforementioned studies indirectly suggest
that exosomal membranes and contained proteins could serve as
potential diagnosis biomarkers for PC.
Exosomes contain rich and complex lipid components
such as cholesterol, sphingolipids and PS (156). Sharma et al (157) found that phosphatidylserine (PS)
in plasma exosomes from patients with PC increased before
histopathological confirmation of PC, suggesting it could be used
for screening and ultra-early diagnosis of PC. Tao et al
(158) applied liquid
chromatography-mass spectrometry technology to analyze the lipid
profiles of exosomes from the serum of patients with PC and HCs,
identifying 37 differentially expressed lipid components. This
suggests that these differentially expressed lipids may serve as
potential biomarkers for diagnosing PC. However, there is currently
limited research on exosomal lipids in the diagnosis of PC, making
it difficult to evaluate their diagnostic efficacy.
The aforementioned research results indicate that
circulating exosomal contents has potential value in the diagnosis
and treatment of PC. However, due to the rich variety of exosomal
contents, current studies are small-sample single-center studies,
and there are differences in exosome extraction, content separation
and sequencing methods. Additionally, body fluids are not uniform,
and factors such as region, ethnicity and tumor staging can lead to
differences in research results. Therefore, it is imperative to
conduct large-sample multi-center studies after standardizing the
technology, with the aim of finding suitable early diagnostic
markers for PC.
In conclusion, emerging diagnostic technologies such
as liquid biopsy and advanced genomic techniques hold significant
promise for the early detection of PC. These methods offer a
non-invasive, sensitive and specific approach to diagnosing PC.
However, the integration of such sophisticated technologies into
routine clinical practice poses challenges, including cost,
accessibility and the need for specialized expertise. Further
research and clinical validation are needed to fully realize the
potential of these technologies in routine clinical practice.
VOCs are a diverse group of organic chemicals
characterized by their elevated vapor pressure at ambient
temperatures, which allows them to easily evaporate into the
atmosphere. The unique metabolic activities of cancer cells can
result in the production of specific VOCs that may serve as
indicators of malignancy. The ability to detect these compounds in
non-invasive biofluids such as breath, urine and saliva presents a
significant advantage over traditional diagnostic methods, which
often require invasive procedures such as biopsies. Research has
shown that the presence and concentration of certain VOCs can
correlate with the presence of pancreatic tumors, making them
potential candidates for early diagnostic tools. Daulton et
al (14) have shown that
certain VOCs such as 2,6-dimethyl-octane and nonanal are
significantly elevated in the urine of patients with PC compared
with HCs, suggesting their potential as biomarkers for early
detection (AUC, 0.85). Martínez-Moral et al (13) found that the serum VOC
butoxymethylbenzene may be a suitable PC biomarker candidate (AUC,
0.98). Tiankanon et al (159) found that the VOCs, acetone dimers
have the potential to be new biomarkers for PDAC detection (AUC,
0.91). A rapid, non-invasive and effective diagnostic method is
particularly suitable for use in primary healthcare settings as a
preliminary screening instrument, demonstrating significant patient
acceptability and practicality. In the assessment of hepatobiliary
conditions and PC, VOCs exhibited a sensitivity of 0.79 and a
specificity of 0.81. These findings are notably encouraging and
could offer a viable approach for the early identification and
management of cancer (160).
The emergence of nanotechnology has paved the way
for the development of sophisticated diagnostic tools, particularly
through the implementation of nanomaterials. Nanomaterials can be
engineered to target specific biomarkers linked to PC, facilitating
more accurate detection techniques that could revolutionize the
field of PC diagnostics (161).
Caputo and Caracciolo (162)
demonstrated that blood tests utilizing nanoparticles were capable
of distinguishing patients with PDAC from healthy individuals
through a comprehensive alteration in the nanoparticle-protein
corona. Electrochemical biosensors utilizing nanomaterials have
exhibited exceptional sensitivity for the detection of microRNAs
and other biomarkers pertinent to PC, presenting opportunities for
timely diagnosis (163).
Optimizing clinical pathways for PC diagnosis
involves integrating multiple diagnostic modalities to enhance
early detection and treatment planning. The combination of imaging
techniques with tumor marker analysis and liquid biopsies can
streamline the diagnostic process, reducing the time to diagnosis
and improving patient outcomes. For example, a clinical pathway
that incorporates initial imaging (CT or MRI) followed by EUS-FNA
for suspicious lesions and concurrent CA19-9 testing, can provide a
comprehensive diagnostic approach (10). Additionally, incorporating liquid
biopsy techniques, such as ctDNA, exosome and VOCs analysis, can
offer non-invasive options for monitoring disease progression and
treatment response (167), and has
become an essential instrument in improving the processes of
screening, identifying, diagnosing, treating and monitoring PDAC
(39). However, nanomaterials
facilitate more accurate detection techniques, and thus
implementing these integrated diagnostic pathways requires
multidisciplinary collaboration and continuous evaluation to ensure
they are tailored to individual patient needs and clinical settings
(168). Through the integration of
diverse diagnostic approaches and cutting-edge technologies,
clinical pathways can be refined to enhance the rates of early
detection and improve outcomes for patients with PC (10). An extensive diagnostic approach that
combines imaging techniques, biomarker assessment and molecular
characterization shows potential for enhancing early diagnosis and
patient prognosis. Subsequent investigations should aim to
concentrate on the validation of novel biomarkers, refinement of
imaging techniques and the development of standardized protocols
for liquid and molecular biopsies.
In the intricate landscape of PC diagnosis, it is
evident that while notable strides have been made, there remains a
critical need for advancements to enhance early identification and
improve patient outcomes. The current diagnostic modalities, such
as imaging techniques, tumor biomarker detection and tissue
biopsies, each offer unique strengths but also present inherent
limitations in sensitivity, specificity and clinical utility.
Ultimately, the future of PC diagnosis is rooted in a
multidisciplinary approach, leveraging advancements in
technological innovations and enhanced comprehension of tumor
biology. By addressing the current challenges and fostering
collaborative research, progress can be made toward achieving early
diagnosis and, consequently, improve prognoses for patients
afflicted by PC.
Not applicable.
This work was supported by the Zhejiang Provincial Natural
Science Foundation of China (grant no. LGC22H160012) and the
Zhejiang Provincial Health Planning Commission Fund (grant no.
2023RC304).
Not applicable.
Conceptualization, JZ; writing-original draft
preparation, JW and YG; writing-review and editing, XH and LW. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang C, Hecht EM, Soloff EV, Tiwari HA,
Bhosale PR, Dasayam A, Galgano SJ, Kambadakone A, Kulkarni NM, Le
O, et al: Imaging for early detection of pancreatic ductal
adenocarcinoma: Updates and challenges in the implementation of
screening and surveillance programs. AJR Am J Roentgenol.
223:e24311512024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackford AL, Canto MI, Klein AP, Hruban
RH and Goggins M: Recent trends in the incidence and survival of
stage 1A pancreatic cancer: A surveillance, epidemiology, and end
results analysis. J Natl Cancer Inst. 112:1162–1169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haberle L, Schramm M and Esposito I:
Preoperative diagnostics of pancreatic neoplasms. Pathologe.
42:491–500. 2021.(In German). PubMed/NCBI
|
|
5
|
Bararia A, Chakraborty P, Roy P,
Chattopadhay BK, Das A, Chatterjee A and Sikdar N: Emerging role of
non-invasive and liquid biopsy biomarkers in pancreatic cancer.
World J Gastroenterol. 29:2241–2260. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schorr F and Essig MW: Early detection of
pancreatic cancer - The role of endoscopic and transabdominal
ultrasound. Z Gastroenterol. 59:1083–1090. 2021.(In German).
PubMed/NCBI
|
|
7
|
Koziol-Bohatkiewicz P, Liberda-Matyja D
and Wrobel TP: Fast cancer imaging in pancreatic biopsies using
infrared imaging. Analyst. 149:1799–1806. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zofia Rogowska A: Ultrasound-guided
percutaneous core-needle biopsy of focal pancreatic
lesions-practical aspectss. J Ultrason. 22:117–120. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi K, Takeda Y, Ono Y, Isomoto H
and Mizukami Y: Current status of molecular diagnostic approaches
using liquid biopsy. J Gastroenterol. 58:834–847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Xu R, Wang C, Qiu J, Ren B and You
L: Early screening and diagnosis strategies of pancreatic cancer: A
comprehensive review. Cancer Commun (Lond). 41:1257–1274. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inchingolo R, Acquafredda F, Posa A, Nunes
TF, Spiliopoulos S, Panzera F and Praticò CA: Endobiliary biopsy.
World J Gastrointest Endosc. 14:291–301. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Navaneethan U, Spencer C, Zhu X, Vargo JJ,
Grove D and Dweik RA: Volatile organic compounds in bile can
distinguish pancreatic cancer from chronic pancreatitis: A
prospective observational study. Endoscopy. 53:732–736. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinez-Moral MP, Tena MT,
Martin-Carnicero A and Martinez A: Highly sensitive serum
volatolomic biomarkers for pancreatic cancer diagnosis. Clin Chim
Acta. 557:1178952024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daulton E, Wicaksono AN, Tiele A, Kocher
HM, Debernardi S, Crnogorac-Jurcevic T and Covington JA: Volatile
organic compounds (VOCs) for the non-invasive detection of
pancreatic cancer from urine. Talanta. 221:1216042021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma X, He C, Wang Y, Cao X, Jin Z, Ge Y,
Cao Z, An M and Hao L: Mechanisms and applications of
manganese-based nanomaterials in tumor diagnosis and therapy.
Biomater Res. 29:01582025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu X, Wang Z, Zhu Y, Li Z, Yan H, Zhao X
and Wang Q: Advancements in molecular imaging for the diagnosis and
treatment of pancreatic ductal adenocarcinoma. Nanoscale Adv. Apr
22–2025.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Sijithra PC, Santhi N and Ramasamy N: A
review study on early detection of pancreatic ductal adenocarcinoma
using artificial intelligence assisted diagnostic methods. Eur J
Radiol. 166:1109722023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakahodo J, Kikuyama M, Nojiri S, Chiba K,
Yoshimoto K, Kamisawa T, Horiguchi SI and Honda G: Focal
parenchymal atrophy of pancreas: An important sign of underlying
high-grade pancreatic intraepithelial neoplasia without invasive
carcinoma, i.e., carcinoma in situ. Pancreatology. 20:1689–1697.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamao K, Takenaka M, Ishikawa R, Okamoto
A, Yamazaki T, Nakai A, Omoto S, Kamata K, Minaga K, Matsumoto I,
et al: Partial pancreatic parenchymal atrophy is a new specific
finding to diagnose small pancreatic cancer (</=10 mm) including
carcinoma in situ: Comparison with localized benign main pancreatic
duct stenosis patients. Diagnostics (Basel). 10:4452020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanno A, Masamune A, Hanada K, Maguchi H,
Shimizu Y, Ueki T, Hasebe O, Ohtsuka T, Nakamura M, Takenaka M, et
al: Multicenter study of early pancreatic cancer in Japan.
Pancreatology. 18:61–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kono Y, Oishi T, Ueda Y, Matsuoka E,
Kamimura R, Tokiyoshi T, Okamoto T and Kashima S: A surgically
resected case of lung metastases and sister Mary Joseph's Nodule 24
months after operation for pancreatic cancer. Gan To Kagaku Ryoho.
46:2354–2356. 2019.(In Japanese). PubMed/NCBI
|
|
22
|
Jia WY, Gui Y, Chen XQ, Tan L, Zhang J,
Xiao MS, Chang XY, Dai MH, Guo JC, Cheng YJ, et al: Efficacy of
color Doppler ultrasound and contrast-enhanced ultrasound in
identifying vascular invasion in pancreatic ductal adenocarcinoma.
Insights Imaging. 15:1812024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonde A, Smith DA, Kikano E, Yoest JM,
Tirumani SH and Ramaiya NH: Overview of serum and tissue markers in
colorectal cancer: A primer for radiologists. Abdom Radiol (NY).
46:5521–5535. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou D, Mu D, Cheng M, Dou Y, Zhang X,
Feng Z, Qiu G, Yu H, Chen Y, Xu H, et al: Differences in lipidomics
may be potential biomarkers for early diagnosis of pancreatic
cancer. Acta Cir Bras. 35:e2020005082020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyd LNC, Ali M, Comandatore A, Garajova
I, Kam L, Puik JR, Fraga Rodrigues SM, Meijer LL, Le Large TYS,
Besselink MG, et al: Prediction model for early-stage pancreatic
cancer using routinely measured blood biomarkers. JAMA Netw Open.
6:e23311972023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo W, Ying P, Ma R, Jing Z, Ma G, Long J,
Li G and Liu Z: Liquid biopsy analysis of lipometabolic exosomes in
pancreatic cancer. Cytokine Growth Factor Rev. 73:69–77. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dahiya DS, Shah YR, Ali H, Chandan S,
Gangwani MK, Canakis A, Ramai D, Hayat U, Pinnam BSM, Iqbal A, et
al: Basic principles and role of endoscopic ultrasound in diagnosis
and differentiation of pancreatic cancer from other pancreatic
lesions: A comprehensive review of endoscopic ultrasound for
pancreatic cancer. J Clin Med. 13:25992024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JM, Mau CZ, Chen YC, Su YH, Chen HA,
Huang SY, Chang JS and Chiu CF: A case-control study in Taiwanese
cohort and meta-analysis of serum ferritin in pancreatic cancer.
Sci Rep. 11:212422021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dias ESD and Chung V: Neoadjuvant
treatment for pancreatic cancer: Controversies and advances. Cancer
Treat Res Commun. 39:1008042024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roehnisch T, Martos-Contreras MC,
Manoochehri M, Nogueira M, Bremm F, Dörrie J, Christoph J, Kunz M
and Schönharting W: Individualized neoantigen peptide immunization
of a metastatic pancreatic cancer patient: A case report of
combined tumor and liquid biopsy. Front Immunol. 15:14147372024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singhi AD, Koay EJ, Chari ST and Maitra A:
Early detection of pancreatic cancer: Opportunities and challenges.
Gastroenterology. 156:2024–2040. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishikawa-Kakiya Y, Maruyama H, Kinoshita
Y, Hayashi K, Yamamura M, Tanoue K, Nagami Y, Tanigawa T, Watanabe
T and Fujiwara Y: The usefulness of serial pancreatic juice
aspiration cytological examination for pancreatic cancer not
diagnosed by EUS-FNAB. Clin J Gastroenterol. 13:1367–1372. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chalfant H, Bonds M, Scott K, Condacse A,
Dennahy IS, Martin WT, Little C, Edil BH, McNally LR and Jain A:
Innovative imaging techniques used to evaluate
borderline-resectable pancreatic adenocarcinoma. J Surg Res.
284:42–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tantau A, Leucuta DC, Tantau M, Boţan E,
Zaharie R, Mândruţiu A and Tomuleasa IC: Inflammation, tumoral
markers and interleukin-17, −10, and −6 profiles in pancreatic
adenocarcinoma and chronic pancreatitis. Dig Dis Sci. 66:3427–3438.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiest NE, Moktan VP, Oman SP and Chirila
RM: Screening for pancreatic cancer: A review for general
clinicians. Rom J Intern Med. 58:119–128. 2020.PubMed/NCBI
|
|
36
|
Granata V, Fusco R, Setola SV, Galdiero R,
Maggialetti N, Silvestro L, De Bellis M, Di Girolamo E, Grazzini G,
Chiti G, et al: Risk assessment and pancreatic cancer: Diagnostic
management and artificial intelligence. Cancers (Basel).
15:3512023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chu LC and Fishman EK: Pancreatic ductal
adenocarcinoma staging: A narrative review of radiologic techniques
and advances. Int J Surg. 110:6052–6063. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koh DM, Papanikolaou N, Bick U, Illing R,
Kahn CE Jr, Kalpathi-Cramer J, Matos C, Martí-Bonmatí L, Miles A,
Mun SK, et al: Artificial intelligence and machine learning in
cancer imaging. Commun Med (Lond). 2:1332022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukund A, Afridi MA, Karolak A, Park MA,
Permuth JB and Rasool G: Pancreatic ductal adenocarcinoma (PDAC): A
review of recent advancements enabled by artificial intelligence.
Cancers (Basel). 16:22402024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Udristoiu AL, Cazacu IM, Gruionu LG,
Gruionu G, Iacob AV, Burtea DE, Ungureanu BS, Costache MI,
Constantin A, Popescu CF, et al: Real-time computer-aided diagnosis
of focal pancreatic masses from endoscopic ultrasound imaging based
on a hybrid convolutional and long short-term memory neural network
model. PLoS One. 16:e02517012021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tong T, Gu J, Xu D, Song L, Zhao Q, Cheng
F, Yuan Z, Tian S, Yang X, Tian J, et al: Deep learning radiomics
based on contrast-enhanced ultrasound images for assisted diagnosis
of pancreatic ductal adenocarcinoma and chronic pancreatitis. BMC
Med. 20:742022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tonozuka R, Itoi T, Nagata N, Kojima H,
Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Nagakawa Y and Mukai S:
Deep learning analysis for the detection of pancreatic cancer on
endosonographic images: A pilot study. J Hepatobiliary Pancreat
Sci. 28:95–104. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marya NB, Powers PD, Chari ST, Gleeson FC,
Leggett CL, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Majumder S,
Pearson RK, et al: Utilisation of artificial intelligence for the
development of an EUS-convolutional neural network model trained to
enhance the diagnosis of autoimmune pancreatitis. Gut.
70:1335–1344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuwahara T, Hara K, Mizuno N, Haba S,
Okuno N, Kuraishi Y, Fumihara D, Yanaidani T, Ishikawa S, Yasuda T,
et al: Artificial intelligence using deep learning analysis of
endoscopic ultrasonography images for the differential diagnosis of
pancreatic masses. Endoscopy. 55:140–149. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma H, Liu ZX, Zhang JJ, Wu FT, Xu CF, Shen
Z, Yu CH and Li YM: Construction of a convolutional neural network
classifier developed by computed tomography images for pancreatic
cancer diagnosis. World J Gastroenterol. 26:5156–5168. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu SL, Li S, Guo YT, Zhou YP, Zhang ZD,
Li S and Lu Y: Establishment and application of an artificial
intelligence diagnosis system for pancreatic cancer with a faster
region-based convolutional neural network. Chin Med J (Engl).
132:2795–2803. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Si K, Xue Y, Yu X, Zhu X, Li Q, Gong W,
Liang T and Duan S: Fully end-to-end deep-learning-based diagnosis
of pancreatic tumors. Theranostics. 11:1982–1990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qiu JJ, Yin J, Qian W, Liu JH, Huang ZX,
Yu HP, Ji L and Zeng XX: A novel multiresolution-statistical
texture analysis architecture: Radiomics-aided diagnosis of PDAC
based on plain CT images. IEEE Trans Med Imaging. 40:12–25. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qureshi TA, Gaddam S, Wachsman AM, Wang L,
Azab L, Asadpour V, Chen W, Xie Y, Wu B, Pandol SJ and Li D:
Predicting pancreatic ductal adenocarcinoma using artificial
intelligence analysis of pre-diagnostic computed tomography images.
Cancer Biomark. 33:211–217. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ebrahimian S, Singh R, Netaji A,
Madhusudhan KS, Homayounieh F, Primak A, Lades F, Saini S, Kalra MK
and Sharma S: Characterization of benign and malignant pancreatic
lesions with DECT quantitative metrics and radiomics. Acad Radiol.
29:705–713. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chu LC, Park S, Kawamoto S, Fouladi DF,
Shayesteh S, Zinreich ES, Graves JS, Horton KM, Hruban RH, Yuille
AL, et al: Utility of CT radiomics features in differentiation of
pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR
Am J Roentgenol. 213:349–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mukherjee S, Patra A, Khasawneh H,
Korfiatis P, Rajamohan N, Suman G, Majumder S, Panda A, Johnson MP,
Larson NB, et al: Radiomics-based machine-learning models can
detect pancreatic cancer on prediagnostic computed tomography scans
at a substantial lead time before clinical diagnosis.
Gastroenterology. 163:1435–1446. e32022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li J, Liu F, Fang X, Cao K, Meng Y, Zhang
H, Yu J, Feng X, Li Q, Liu Y, et al: CT radiomics features in
differentiation of focal-type autoimmune pancreatitis from
pancreatic ductal adenocarcinoma: A propensity score analysis. Acad
Radiol. 29:358–366. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ziegelmayer S, Kaissis G, Harder F,
Jungmann F, Müller T, Makowski M and Braren R: Deep convolutional
neural network-assisted feature extraction for diagnostic
discrimination and feature visualization in pancreatic ductal
adenocarcinoma (PDAC) versus autoimmune pancreatitis (AIP). J Clin
Med. 9:40132020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cao K, Xia Y, Yao J, Han X, Lambert L,
Zhang T, Tang W, Jin G, Jiang H, Fang X, et al: Large-scale
pancreatic cancer detection via non-contrast CT and deep learning.
Nat Med. 29:3033–3043. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen PT, Wu T, Wang P, Chang D, Liu KL, Wu
MS, Roth HR, Lee PC, Liao WC and Wang W: pancreatic cancer
detection on ct scans with deep learning: A nationwide
population-based study. Radiology. 306:172–182. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tayebi Arasteh S, Ziller A, Kuhl C,
Makowski M, Nebelung S, Braren R, Rueckert D, Truhn D and Kaissis
G: Preserving fairness and diagnostic accuracy in private
large-scale AI models for medical imaging. Commun Med (Lond).
4:462024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang Y, Schott D, Zhang Y, Wang Z, Nasief
H, Paulson E, Hall W, Knechtges P, Erickson B and Li XA:
Auto-segmentation of pancreatic tumor in multi-parametric MRI using
deep convolutional neural networks. Radiother Oncol. 145:193–200.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li J, Feng C, Lin X and Qian X: Utilizing
GCN and meta-learning strategy in unsupervised domain adaptation
for pancreatic cancer segmentation. IEEE J Biomed Health Inform.
26:79–89. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen X, Chen Z, Li J, Zhang YD, Lin X and
Qian X: Model-driven deep learning method for pancreatic cancer
segmentation based on spiral-transformation. IEEE Trans Med
Imaging. 41:75–87. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li S, Jiang H, Wang Z, Zhang G and Yao YD:
An effective computer aided diagnosis model for pancreas cancer on
PET/CT images. Comput Methods Programs Biomed. 165:205–214. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Z, Li M, Zuo C, Yang Z and Yang X, Ren
S, Peng Y, Sun G, Shen J, Cheng C and Yang X: Radiomics model of
dual-time 2-[(18)F]FDG PET/CT imaging to distinguish between
pancreatic ductal adenocarcinoma and autoimmune pancreatitis. Eur
Radiol. 31:6983–6991. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Y, Cheng C, Liu Z, Wang L, Pan G,
Sun G, Chang Y, Zuo C and Yang X: Radiomics analysis for the
differentiation of autoimmune pancreatitis and pancreatic ductal
adenocarcinoma in (18) F-FDG PET/CT. Med Phys. 46:4520–4530. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yu H, Zhang C, Liu S, Jiang G, Li S, Zhang
L, Wang Y and Xu W: Clinical value of CT-guided percutaneous
fine-needle aspiration biopsy for peritoneal lesions. BMC Med
Imaging. 20:1222020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee JH, Kim H, Lee SH, Ku JL, Chun JW, Seo
HY, Kim SC, Paik WH, Ryu JK, Lee SK, et al: Establishment of
patient-derived pancreatic cancer organoids from endoscopic
ultrasound-guided fine-needle aspiration biopsies. Gut Liver.
16:625–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Masuda H, Kotecha K, Maitra R, Gill AJ,
Mittal A and Samra JS: Clinical suspicion of pancreatic cancer
despite negative endoscopic ultrasound-guided fine-needle
aspiration biopsy. ANZ J Surg. 92:99–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Abe I and Lam AK: Fine-needle aspiration
under guidance of ultrasound examination of thyroid lesions.
Methods Mol Biol. 2534:29–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Smetanina SV, Slavnova EN, Smetanina OV,
Golovin ST and Eremin NV: Features of differential cytological
diagnosis of primary and metastatic liver carcinoma. Klin Lab
Diagn. 66:364–370. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Taniuchi K, Ueno M, Yokose T, Sakaguchi M,
Yoshioka R, Ogasawara M, Kosaki T, Naganuma S and Furihata M:
Upregulation of PODXL and ITGB1 in pancreatic cancer tissues
preoperatively obtained by EUS-FNAB correlates with unfavorable
prognosis of postoperative pancreatic cancer patients. PLoS One.
17:e02651722022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mason J, Lundberg E, Jonsson P, Nyström H,
Franklin O, Lundin C, Naredi P, Antti H, Sund M and Öhlund D: A
cross-sectional and longitudinal analysis of pre-diagnostic blood
plasma biomarkers for early detection of pancreatic cancer. Int J
Mol Sci. 23:129692022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pietri E, Balsano R, Coriano M, Gelsomino
F, Leonardi F, Bui S, Gnetti L, Valle RD and Garajová I: The
implication of liquid biopsies to predict chemoresistance in
pancreatic cancer. Cancer Drug Resist. 4:559–572. 2021.PubMed/NCBI
|
|
72
|
Gu YL, Lan C, Pei H, Yang SN, Liu YF and
Xiao LL: Applicative Value of Serum CA19-9, CEA, CA125 and CA242 in
diagnosis and prognosis for patients with pancreatic cancer treated
by concurrent chemoradiotherapy. Asian Pac J Cancer Prev.
16:6569–6573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang J, Wang Y, Zhao T, Li Y, Tian L,
Zhao J and Zhang J: Evaluation of serum MUC5AC in combination with
CA19-9 for the diagnosis of pancreatic cancer. World J Surg Oncol.
18:312020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jahan R, Ganguly K, Smith LM, Atri P,
Carmicheal J, Sheinin Y, Rachagani S, Natarajan G, Brand RE, Macha
MA, et al: Trefoil factor(s) and CA19.9: A promising panel for
early detection of pancreatic cancer. EBioMedicine. 42:375–385.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Aronsson L, Andersson R, Bauden M,
Andersson B, Bygott T and Ansari D: High-density and targeted
glycoproteomic profiling of serum proteins in pancreatic cancer and
intraductal papillary mucinous neoplasm. Scand J Gastroenterol.
53:1597–1603. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li X, Wang Q and Wang R: Roles of exosome
genomic DNA in colorectal cancer. Front Pharmacol. 13:9232322022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang ZY, Wang RX, Ding XQ, Zhang X, Pan XR
and Tong JH: A protocol for cancer-related mutation detection on
exosomal DNA in clinical application. Front Oncol. 10:5581062020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu Y, Zhang H, Chen N, Hao J, Jin H and
Ma X: Diagnostic value of various liquid biopsy methods for
pancreatic cancer: A systematic review and meta-analysis. Medicine
(Baltimore). 99:e185812020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu Z, Zhang Y, Zhang W, Tang D, Zhang S,
Wang L, Zou X, Ni Z, Zhang S, Lv Y and Xiang N: High-throughput
enrichment of portal venous circulating tumor cells for highly
sensitive diagnosis of CA19-9-negative pancreatic cancer patients
using inertial microfluidics. Biosens Bioelectron. 259:1164112024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen M, Liu H, Xiao Y, Liang R, Xu H, Hong
B and Qian Y: Predictive biomarkers of pancreatic cancer
metastasis: A comprehensive review. Clin Chim Acta. 569:1201762025.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Luo K, Wang X, Zhang X, Liu Z, Huang S and
Li R: The value of circulating tumor cells in the prognosis and
treatment of pancreatic cancer. Front Oncol. 12:9336452022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cui Y and Cao M: Liquid biopsy in bladder
cancer. Methods Mol Biol. 2695:111–120. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nagai M, Sho M, Akahori T, Nakagawa K and
Nakamura K: Application of liquid biopsy for surgical management of
pancreatic cancer. Ann Gastroenterol Surg. 4:216–223. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gu X, Wei S and Lv X: Circulating tumor
cells: From new biological insights to clinical practice. Signal
Transduct Target Ther. 9:2262024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Antonarakis ES, Lu C, Luber B, Wang H,
Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR,
et al: Clinical significance of androgen receptor splice variant-7
mRNA detection in circulating tumor cells of men with metastatic
castration-resistant prostate cancer treated with first- and
second-line abiraterone and enzalutamide. J Clin Oncol.
35:2149–2156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Silva ATF, Rodrigues CM, Ferreira ICC,
Santos LLD, Santos DW, Araújo TG, Canto PPL, Paiva CE, Goulart LR
and Maia YCP: A novel detection method of breast cancer through a
simple panel of biomarkers. Int J Mol Sci. 23:119832022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Earl J, Garcia-Nieto S, Martinez-Avila JC,
Montans J, Sanjuanbenito A, Rodríguez-Garrote M, Lisa E, Mendía E,
Lobo E, Malats N, et al: Circulating tumor cells (Ctc) and kras
mutant circulating free Dna (cfdna) detection in peripheral blood
as biomarkers in patients diagnosed with exocrine pancreatic
cancer. BMC Cancer. 15:7972015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang ZH, Bao YW, Zhao YJ, Wang JQ, Guo JT
and Sun SY: Circulating tumor cells as potential prognostic
biomarkers for early-stage pancreatic cancer: A systematic review
and meta-analysis. World J Clin Oncol. 14:504–517. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ankeny JS, Court CM, Hou S, Li Q, Song M,
Wu D, Chen JF, Lee T, Lin M, Sho S, et al: Circulating tumour cells
as a biomarker for diagnosis and staging in pancreatic cancer. Br J
Cancer. 114:1367–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bidard FC, Huguet F, Louvet C, Mineur L,
Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C,
et al: Circulating tumor cells in locally advanced pancreatic
adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial.
Ann Oncol. 24:2057–2061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95:e34072016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ahrens TD, Bang-Christensen SR, Jorgensen
AM, Løppke C, Spliid CB, Sand NT, Clausen TM, Salanti A and Agerbæk
MØ: The role of proteoglycans in cancer metastasis and circulating
tumor cell analysis. Front Cell Dev Biol. 8:7492020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Koo B, Jun E, Liu H, Kim EJ, Park YY, Lim
SB, Kim SC and Shin Y: A biocomposite-based rapid sampling assay
for circulating cell-free DNA in liquid biopsy samples from human
cancers. Sci Rep. 10:149322020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bi F, Wang Q, Dong Q, Wang Y, Zhang L and
Zhang J: Circulating tumor DNA in colorectal cancer: opportunities
and challenges. Am J Transl Res. 12:1044–1055. 2020.PubMed/NCBI
|
|
95
|
Gai W and Sun K: Epigenetic biomarkers in
cell-free DNA and applications in liquid biopsy. Genes (Basel).
10:322019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Oliveira KCS, Ramos IB, Silva JMC, Barra
WF, Riggins GJ, Palande V, Pinho CT, Frenkel-Morgenstern M, Santos
SEB, Assumpcao PP, et al: Current perspectives on circulating tumor
DNA, precision medicine, and personalized clinical management of
cancer. Mol Cancer Res. 18:517–528. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cescon DW, Bratman SV, Chan SM and Siu LL:
Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer.
1:276–290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Topham JT, Renouf DJ and Schaeffer DF:
Circulating tumor DNA: toward evolving the clinical paradigm of
pancreatic ductal adenocarcinoma. Ther Adv Med Oncol.
15:175883592311576512023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zill OA, Greene C, Sebisanovic D, Siew LM,
Leng J, Vu M, Hendifar AE, Wang Z, Atreya CE, Kelley RK, et al:
Cell-Free DNA next-generation sequencing in pancreatobiliary
carcinomas. Cancer Discov. 5:1040–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li H, Di Y, Li J, Jiang Y, He H, Yao L, Gu
J, Lu J, Song J, Chen S, et al: Blood-based genomic profiling of
circulating tumor DNA from patients with advanced pancreatic cancer
and its value to guide clinical treatment. J Cancer. 11:4316–4323.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li S, Wang L, Zhao Q, Wang Z, Lu S, Kang
Y, Jin G and Tian J: Genome-wide analysis of cell-free DNA
methylation profiling for the early diagnosis of pancreatic cancer.
Front Genet. 11:5960782020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Eissa MAL, Lerner L, Abdelfatah E, Shankar
N, Canner JK, Hasan NM, Yaghoobi V, Huang B, Kerner Z, Takaesu F,
et al: Promoter methylation of ADAMTS1 and BNC1 as potential
biomarkers for early detection of pancreatic cancer in blood. Clin
Epigenetics. 11:592019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hu Q, Chen L, Li K, Liu R, Sun L and Han
T: Circulating tumor DNA: Current implementation issues and future
challenges for clinical utility. Clin Chem Lab Med. 62:2094–2110.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Słotwiński R and Slotwinska SM: Diagnostic
value of selected markers and apoptotic pathways for pancreatic
cancer. Cent Eur J Immunol. 41:392–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang Z: Diagnostic performance for
declined microRNA-133a in pancreatic cancer. J Cell Biochem.
121:3882–3886. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wei J, Yang L, Wu YN and Xu J: Serum
miR-1290 and miR-1246 as Potential Diagnostic Biomarkers of Human
Pancreatic Cancer. J Cancer. 11:1325–1333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kopcho S, McDew-White M, Naushad W, Mohan
M and Okeoma CM: SIV Infection regulates compartmentalization of
circulating blood plasma mirnas within extracellular vesicles (EVs)
and extracellular condensates (ECs) and decreases EV-Associated
miRNA-128. Viruses. 15:6222023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Qin C, Li T, Lin C, Zhao B, Li Z, Zhao Y
and Wang W: The systematic role of pancreatic cancer exosomes:
Distant communication, liquid biopsy and future therapy. Cancer
Cell Int. 24:2642024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zou X, Wei J, Huang Z, Zhou X, Lu Z, Zhu W
and Miao Y: Identification of a six-miRNA panel in serum benefiting
pancreatic cancer diagnosis. Cancer Med. 8:2810–2822. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou X, Lu Z, Wang T, Huang Z, Zhu W and
Miao Y: Plasma miRNAs in diagnosis and prognosis of pancreatic
cancer: A miRNA expression analysis. Gene. 673:181–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Goto T, Fujiya M, Konishi H, Sasajima J,
Fujibayashi S, Hayashi A, Utsumi T, Sato H, Iwama T, Ijiri M, et
al: An elevated expression of serum exosomal microRNA-191, −21,
−451a of pancreatic neoplasm is considered to be efficient
diagnostic marker. BMC Cancer. 18:1162018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pu X, Ding G, Wu M, Zhou S, Jia S and Cao
L: Elevated expression of exosomal microRNA-21 as a potential
biomarker for the early diagnosis of pancreatic cancer using a
tethered cationic lipoplex nanoparticle biochip. Oncol Lett.
19:2062–2070. 2020.PubMed/NCBI
|
|
113
|
Machida T, Tomofuji T, Maruyama T, Yoneda
T, Ekuni D, Azuma T, Miyai H, Mizuno H, Kato H, Tsutsumi K, et al:
miR-1246 and miR-4644 in salivary exosome as potential biomarkers
for pancreatobiliary tract cancer. Oncol Rep. 36:2375–2381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yoshizawa N, Sugimoto K, Tameda M, Inagaki
Y, Ikejiri M, Inoue H, Usui M, Ito M and Takei Y:
miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic
biomarker for pancreatic ductal adenocarcinoma. Oncol Lett.
19:2677–2684. 2020.PubMed/NCBI
|
|
115
|
Shao H, Zhang Y, Yan J, Ban X, Fan X,
Chang X, Lu Z, Wu Y, Zong L, Mo S, et al: Upregulated
MicroRNA-483-3p is an early event in pancreatic ductal
adenocarcinoma (PDAC) and as a powerful liquid biopsy biomarker in
PDAC. Onco Targets Ther. 14:2163–2175. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang C, Wang J, Cui W, Liu Y, Zhou H, Wang
Y, Chen X, Chen X and Wang Z: Serum Exosomal miRNA-1226 as
potential biomarker of pancreatic ductal adenocarcinoma. Onco
Targets Ther. 14:1441–1451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nakamura S, Sadakari Y, Ohtsuka T, Okayama
T, Nakashima Y, Gotoh Y, Saeki K, Mori Y, Nakata K, Miyasaka Y, et
al: Pancreatic juice exosomal MicroRNAs as biomarkers for detection
of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 26:2104–2111.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen J, Yao D, Chen W, Li Z, Guo Y, Zhu F
and Hu X: Serum exosomal miR-451a acts as a candidate marker for
pancreatic cancer. Int J Biol Markers. 37:74–80. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang L, Wu J, Ye N, Li F, Zhan H, Chen S
and Xu J: Plasma-derived exosome MiR-19b acts as a diagnostic
marker for pancreatic cancer. Front Oncol. 11:7391112021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Nakamura K, Zhu Z, Roy S, Jun E, Han H,
Munoz RM, Nishiwada S, Sharma G, Cridebring D, Zenhausern F, et al:
An Exosome-based transcriptomic signature for noninvasive, early
detection of patients with pancreatic ductal adenocarcinoma: A
multicenter cohort study. Gastroenterology. 163:1252–1266. e22022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Guo S, Qin H, Liu K, Wang H, Bai S, Liu S,
Shao Z, Zhang Y, Song B, Xu X, et al: Blood small extracellular
vesicles derived miRNAs to differentiate pancreatic ductal
adenocarcinoma from chronic pancreatitis. Clin Transl Med.
11:e5202021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen J, Zhang X, Zhang G, Zhu F and Liu W:
Serum-derived exosomal miR-7977 combined with miR-451a as a
potential biomarker for pancreatic ductal adenocarcinoma. BMC
Cancer. 25:2952025. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Taniguchi T, Ideno N, Araki T, Miura S,
Yamamoto M, Nakafusa T, Higashijima N, Yamamoto T, Tamura K,
Nakamura S, et al: MicroRNA-20a in extracellular vesicles derived
from duodenal fluid is a possible biomarker for pancreatic ductal
adenocarcinoma. DEN Open. 4:e3332024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Takahashi K, Ota Y, Kogure T, Suzuki Y,
Iwamoto H, Yamakita K, Kitano Y, Fujii S, Haneda M, Patel T and Ota
T: Circulating extracellular vesicle-encapsulated HULC is a
potential biomarker for human pancreatic cancer. Cancer Sci.
111:98–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kumar SR, Kimchi ET, Manjunath Y,
Gajagowni S, Stuckel AJ and Kaifi JT: Author Correction: RNA cargos
in extracellular vesicles derived from blood serum in pancreas
associated conditions. Sci Rep. 10:99812020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu S, Li Y, Liao Z, Wang Z, Li Y, Qian L,
Zhao J, Zong H, Kang B, Zou WB, et al: Plasma extracellular vesicle
long RNA profiling identifies a diagnostic signature for the
detection of pancreatic ductal adenocarcinoma. Gut. 69:540–550.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hong L, Xu L, Jin L, Xu K, Tang W, Zhu Y,
Qiu X and Wang J: Exosomal circular RNA hsa_circ_0006220, and
hsa_circ_0001666 as biomarkers in the diagnosis of pancreatic
cancer. J Clin Lab Anal. 36:e244472022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kitagawa T, Taniuchi K, Tsuboi M,
Sakaguchi M, Kohsaki T, Okabayashi T and Saibara T: Circulating
pancreatic cancer exosomal RNAs for detection of pancreatic cancer.
Mol Oncol. 13:212–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hu J, Sheng Y, Kwak KJ, Shi J, Yu B and
Lee LJ: A signal-amplifiable biochip quantifies extracellular
vesicle-associated RNAs for early cancer detection. Nat Commun.
8:16832017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yang Z, LaRiviere MJ, Ko J, Till JE,
Christensen T, Yee SS, Black TA, Tien K, Lin A, Shen H, et al: A
multianalyte panel consisting of extracellular vesicle miRNAs and
mRNAs, cfDNA, and CA19-9 shows utility for diagnosis and staging of
pancreatic ductal adenocarcinoma. Clin Cancer Res. 26:3248–3258.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kumar SR, Kimchi ET, Manjunath Y,
Gajagowni S, Stuckel AJ and Kaifi JT: RNA cargos in extracellular
vesicles derived from blood serum in pancreas associated
conditions. Sci Rep. 10:28002020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang F, Wang C, Chen S, Wei C, Ji J, Liu
Y, Liang L, Chen Y, Li X, Zhao L, et al: Identification of
blood-derived exosomal tumor RNA signatures as noninvasive
diagnostic biomarkers for multi-cancer: A multi-phase, multi-center
study. Mol Cancer. 24:602025. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Allenson K, Castillo J, San Lucas FA,
Scelo G, Kim DU, Bernard V, Davis G, Kumar T, Katz M, Overman MJ,
et al: High prevalence of mutant KRAS in circulating
exosome-derived DNA from early-stage pancreatic cancer patients.
Ann Oncol. 28:741–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Castillo J, Bernard V, San Lucas FA,
Allenson K, Capello M, Kim DU, Gascoyne P, Mulu FC, Stephens BM,
Huang J, et al: Surfaceome profiling enables isolation of
cancer-specific exosomal cargo in liquid biopsies from pancreatic
cancer patients. Ann Oncol. 29:223–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jin H, Liu P, Wu Y, Meng X, Wu M, Han J
and Tan X: Exosomal zinc transporter ZIP4 promotes cancer growth
and is a novel diagnostic biomarker for pancreatic cancer. Cancer
Sci. 109:2946–2956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Xiao D, Dong Z, Zhen L, Xia G, Huang X,
Wang T, Guo H, Yang B, Xu C, Wu W, et al: Combined exosomal GPC1,
CD82, and Serum CA19-9 as multiplex targets: A specific, sensitive,
and reproducible detection panel for the diagnosis of pancreatic
cancer. Mol Cancer Res. 18:300–310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang KS, Im H, Hong S, Pergolini I, Del
Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, et al:
Multiparametric plasma EV profiling facilitates diagnosis of
pancreatic malignancy. Sci Transl Med. 9:eaal32262017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yang J, Zhang Y, Gao X, Yuan Y, Zhao J,
Zhou S, Wang H, Wang L, Xu G, Li X, et al: Plasma-derived exosomal
ALIX as a novel biomarker for diagnosis and classification of
pancreatic cancer. Front Oncol. 11:6283462021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wei Q, Zhang J, Li Z, Wei L and Ren L:
Serum Exo-EphA2 as a potential diagnostic biomarker for pancreatic
cancer. Pancreas. 49:1213–1219. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liang K, Liu F, Fan J, Sun D, Liu C, Lyon
CJ, Bernard DW, Li Y, Yokoi K, Katz MH, et al: Nanoplasmonic
quantification of tumor-derived extracellular vesicles in plasma
microsamples for diagnosis and treatment monitoring. Nat Biomed
Eng. 1:002112017 View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Shin HS, Jung SB, Park S, Dua P and Lee
DK: ALPPL2 Is a potential diagnostic biomarker for pancreatic
cancer-derived extracellular vesicles. Mol Ther Methods Clin Dev.
15:204–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lux A, Kahlert C, Grutzmann R and Pilarsky
C: c-Met and PD-L1 on circulating exosomes as diagnostic and
prognostic markers for pancreatic cancer. Int J Mol Sci.
20:33052019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
David P, Kouhestani D, Hansen FJ, Paul S,
Czubayko F, Karabiber A, Weisel N, Klösch B, Merkel S, Ole-Baur J,
et al: Exosomal CD40, CD25, and Serum CA19-9 as combinatory novel
liquid biopsy biomarker for the diagnosis and prognosis of patients
with pancreatic ductal adenocarcinoma. Int J Mol Sci. 26:15002025.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fan Z, Yu J, Lin J, Liu Y and Liao Y:
Exosome-specific tumor diagnosis via biomedical analysis of
exosome-containing microRNA biomarkers. Analyst. 144:5856–5865.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Long Y, Wang X, Youmans DT and Cech TR:
How do lncRNAs regulate transcription? Sci Adv. 3:eaao21102017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Pandya G, Kirtonia A, Sethi G, Pandey AK
and Garg M: The implication of long non-coding RNAs in the
diagnosis, pathogenesis and drug resistance of pancreatic ductal
adenocarcinoma and their possible therapeutic potential. Biochim
Biophys Acta Rev Cancer. 1874:1884232020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Lin H, Yu J, Gu X, Ge S and Fan X: Novel
insights into exosomal circular RNAs: Redefining intercellular
communication in cancer biology. Clin Transl Med. 11:e6362021.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chen Q and Zhou T: Emerging functional
principles of tRNA-derived small RNAs and other regulatory small
RNAs. J Biol Chem. 299:1052252023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Guo S, Wang X, Shan D, Xiao Y, Ju L, Zhang
Y, Wang G and Qian K: The detection, biological function, and
liquid biopsy application of extracellular vesicle-associated DNA.
Biomark Res. 12:1232024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Makler A and Narayanan R: Mining exosomal
genes for pancreatic cancer targets. Cancer Genomics Proteomics.
14:161–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li H, Chiang CL, Kwak KJ, Wang X, Doddi S,
Ramanathan LV, Cho SM, Hou YC, Cheng TS, Mo X, et al: Extracellular
vesicular analysis of glypican 1 mRNA and protein for pancreatic
cancer diagnosis and prognosis. Adv Sci (Weinh). 11:e23063732024.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Buscail E, Chauvet A, Quincy P, Degrandi
O, Buscail C, Lamrissi I, Moranvillier I, Caumont C, Verdon S,
Brisson A, et al: CD63-GPC1-positive exosomes coupled with CA19-9
offer good diagnostic potential for resectable pancreatic ductal
adenocarcinoma. Transl Oncol. 12:1395–1403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lai X, Wang M, McElyea SD, Sherman S,
House M and Korc M: A microRNA signature in circulating exosomes is
superior to exosomal glypican-1 levels for diagnosing pancreatic
cancer. Cancer Lett. 393:86–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Skotland T, Hessvik NP, Sandvig K and
Llorente A: Exosomal lipid composition and the role of ether lipids
and phosphoinositides in exosome biology. J Lipid Res. 60:9–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Sharma R, Huang X, Brekken RA and Schroit
AJ: Detection of phosphatidylserine-positive exosomes for the
diagnosis of early-stage malignancies. Br J Cancer. 117:545–552.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Tao L, Zhou J, Yuan C, Zhang L, Li D, Si
D, Xiu D and Zhong L: Metabolomics identifies serum and exosomes
metabolite markers of pancreatic cancer. Metabolomics. 15:862019.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Tiankanon K, Pungpipattrakul N, Sukaram T,
Chaiteerakij R and Rerknimitr R: Identification of breath volatile
organic compounds to distinguish pancreatic adenocarcinoma,
pancreatic cystic neoplasm, and patients without pancreatic
lesions. World J Gastrointest Oncol. 16:894–906. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Pelling M, Chandrapalan S, West E and
Arasaradnam RP: A systematic review and meta-analysis: Volatile
organic compound analysis in the detection of hepatobiliary and
pancreatic cancers. Cancers (Basel). 15:23082023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Nagarajan Y, Chandrasekaran N and Deepa
Parvathi V: Functionalized nanomaterials in pancreatic cancer
theranostics and molecular imaging. ChemistryOpen.
14:e2024002322025. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Caputo D and Caracciolo G:
Nanoparticle-enabled blood tests for early detection of pancreatic
ductal adenocarcinoma. Cancer Lett. 470:191–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yin X, He Z, Ge W and Zhao Z: Application
of aptamer functionalized nanomaterials in targeting therapeutics
of typical tumors. Front Bioeng Biotechnol. 11:10929012023.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Moccia M, Caratelli V, Cinti S, Pede B,
Avitabile C, Saviano M, Imbriani AL, Moscone D and Arduini F:
Paper-based electrochemical peptide nucleic acid (PNA) biosensor
for detection of miRNA-492: A pancreatic ductal adenocarcinoma
biomarker. Biosens Bioelectron. 165:1123712020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Carigga Gutierrez NM, Clainche TL, Bulin
AL, Leo S, Kadri M, Abdelhamid AGA, Pujol-Solé N, Obaid G,
Hograindleur MA, Gardette V, et al: Engineering radiocatalytic
nanoliposomes with hydrophobic gold nanoclusters for radiotherapy
enhancement. Adv Mater. 36:e24046052024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Phua VJX, Yang CT, Xia B, Yan SX, Liu J,
Aw SE, He T and Ng DCE: Nanomaterial probes for nuclear imaging.
Nanomaterials (Basel). 12:5822022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kaczor-Urbanowicz KE, Cheng J, King JC,
Sedarat A, Pandol SJ, Farrell JJ, Wong DTW and Kim Y: Reviews on
current liquid biopsy for detection and management of pancreatic
cancers. Pancreas. 49:1141–1152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Qiao Z, Ge J, He W, Xu X and He J:
Artificial intelligence algorithm-based computerized tomography
image features combined with serum tumor markers for diagnosis of
pancreatic cancer. Comput Math Methods Med. 2022:89794042022.
View Article : Google Scholar : PubMed/NCBI
|