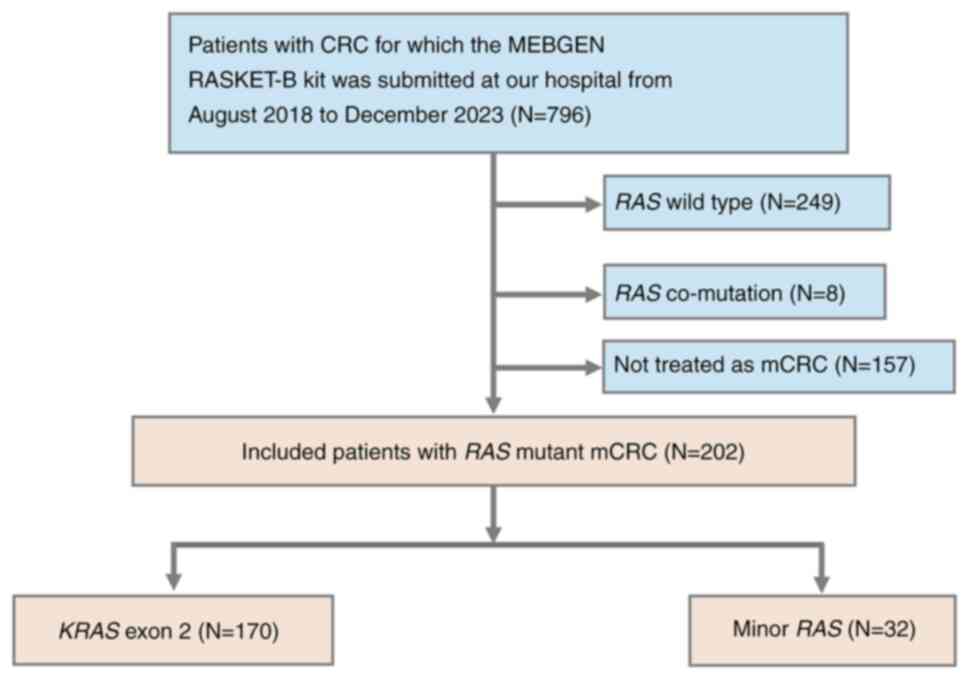
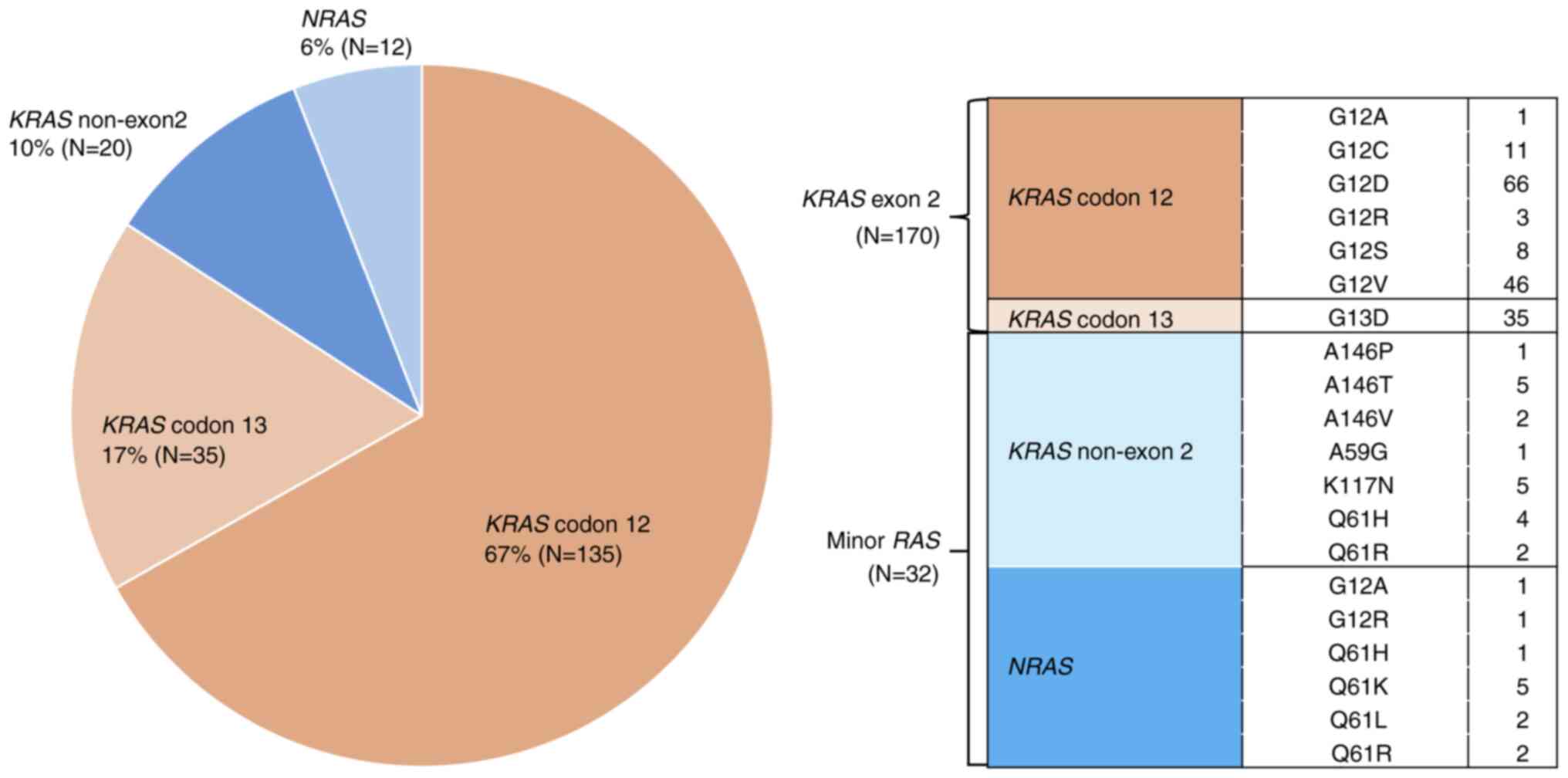
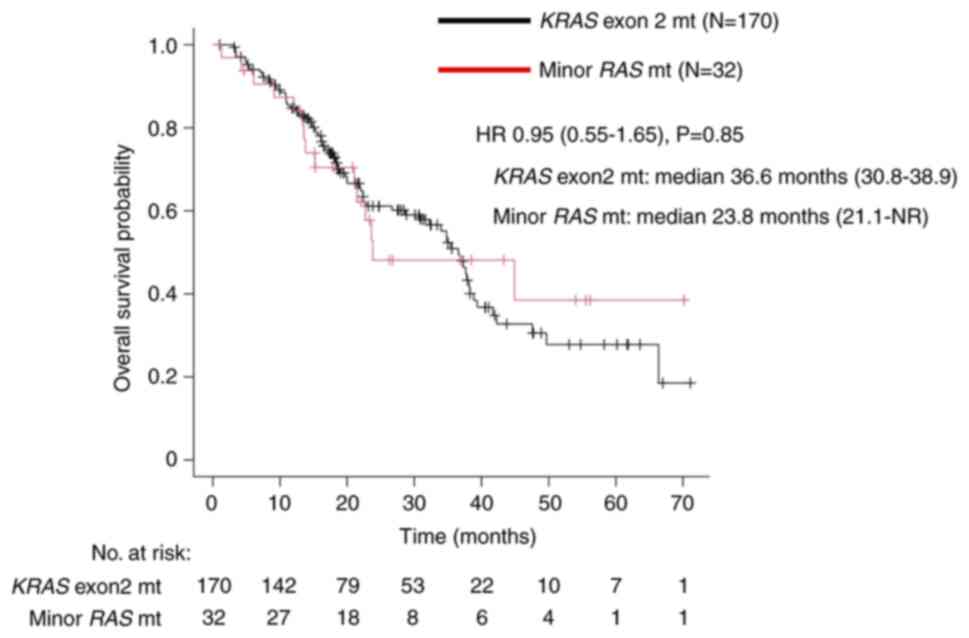
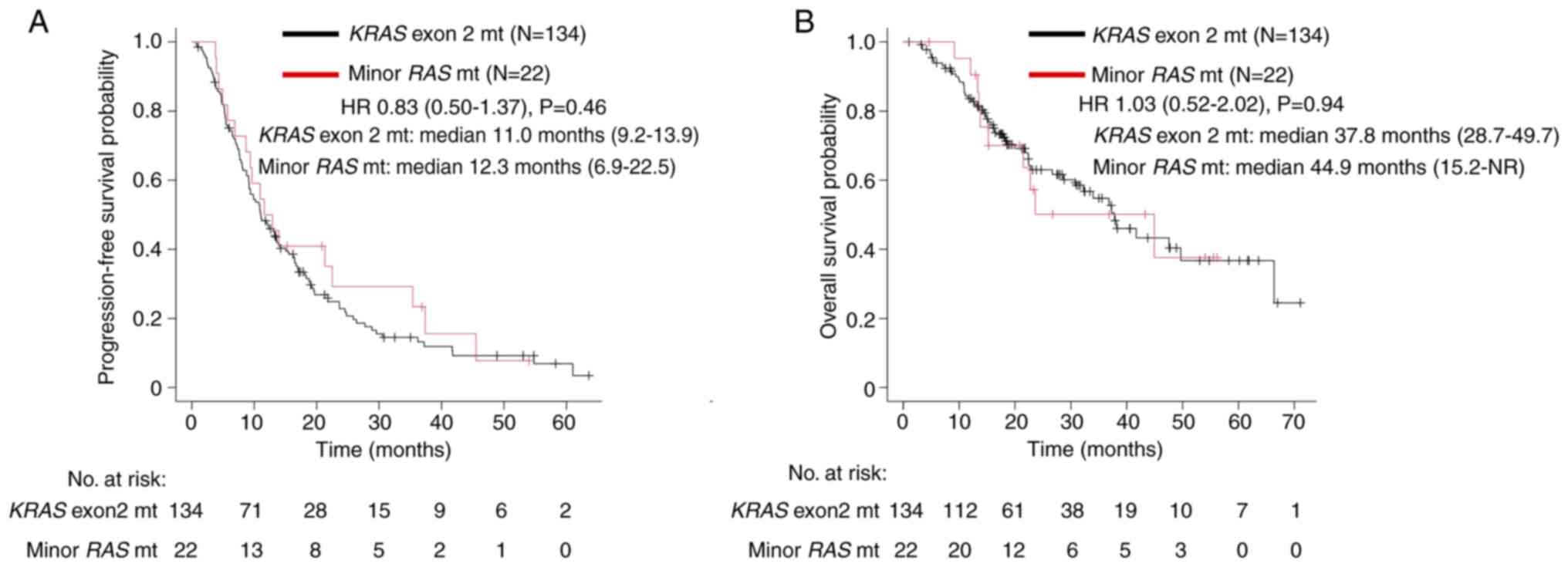
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amado RG, Wolf M, Peeters M, Van Cutsem E,
Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et
al: Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 41:3278–3286.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahabreh IJ, Terasawa T, Castaldi PJ and
Trikalinos TA: Systematic review: Anti-epidermal growth factor
receptor treatment effect modification by KRAS mutations in
advanced colorectal cancer. Ann Intern Med. 154:37–49. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lièvre A, Bachet JB, Le Corre D, Boige V,
Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, et al:
KRAS mutation status is predictive of response to cetuximab therapy
in colorectal cancer. Cancer Res. 66:3992–3995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schirripa M, Cohen SA, Battaglin F and
Lenz HJ: Biomarker-driven and molecular targeted therapies for
colorectal cancers. Semin Oncol. 45:124–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of first-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe J, Muro K, Shitara K, Yamazaki K,
Shiozawa M, Ohori H, Takashima A, Yokota M, Makiyama A, Akazawa N,
et al: Panitumumab vs bevacizumab added to standard first-line
chemotherapy and overall survival among patients with RAS
wild-type, left-sided metastatic colorectal cancer: A randomized
clinical trial. JAMA. 329:1271–1282. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones JC, Renfro LA, Al-Shamsi HO, Schrock
AB, Rankin A, Zhang BY, Kasi PM, Voss JS, Leal AD, Sun J, et al:
Non-V600 BRAF mutations define a clinically distinct
molecular subtype of metastatic colorectal cancer. J Clin Oncol.
35:2624–2630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bokemeyer C, Köhne CH, Ciardiello F, Lenz
HJ, Heinemann V, Klinkhardt U, Beier F, Duecker K, van Krieken JH
and Tejpar S: FOLFOX4 plus cetuximab treatment and RAS mutations in
colorectal cancer. Eur J Cancer. 51:1243–1252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawazoe A, Shitara K, Fukuoka S, Kuboki Y,
Bando H, Okamoto W, Kojima T, Fuse N, Yamanaka T, Doi T, et al: A
retrospective observational study of clinicopathological features
of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with
metastatic colorectal cancer. BMC Cancer. 15:2582015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randomised phase 3 MRC COIN trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peeters M, Oliner KS, Price TJ, Cervantes
A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, et
al: Analysis of KRAS/NRAS mutations in a phase III study of
panitumumab with FOLFIRI compared with FOLFIRI alone as second-line
treatment for metastatic colorectal cancer. Clin Cancer Res.
21:5469–5479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorich MJ, Wiese MD, Rowland A,
Kichenadasse G, McKinnon RA and Karapetis CS: Extended RAS
mutations and anti-EGFR monoclonal antibody survival benefit in
metastatic colorectal cancer: A meta-analysis of randomized,
controlled trials. Ann Oncol. 26:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Cutsem E, Lenz HJ, Köhne CH, Heinemann
V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken
JH and Ciardiello F: Fluorouracil, leucovorin, and irinotecan plus
cetuximab treatment and RAS mutations in colorectal cancer. J Clin
Oncol. 33:692–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taniguchi H, Okamoto W, Muro K, Akagi K,
Hara H, Nishina T, Kajiwara T, Denda T, Hironaka S, Kudo T, et al:
Clinical validation of newly developed multiplex kit using Luminex
xMAP technology for detecting simultaneous RAS and BRAF mutations
in colorectal cancer: Results of the RASKET-B study. Neoplasia.
20:1219–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshino T, Muro K, Yamaguchi K, Nishina T,
Denda T, Kudo T, Okamoto W, Taniguchi H, Akagi K, Kajiwara T, et
al: Clinical validation of a multiplex kit for RAS mutations in
colorectal cancer: Results of the RASKET (RAS KEy testing)
prospective, multicenter study. EBioMedicine. 2:317–323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
World Medical Association, . World medical
association declaration of Helsinki. Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikoma T, Shimokawa M, Kotaka M, Matsumoto
T, Nagai H, Boku S, Shibata N, Yasui H and Satake H: Clinical and
prognostic features of patients with detailed RAS/BRAF-mutant
colorectal cancer in Japan. BMC Cancer. 21:5182021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takane K, Akagi K, Fukuyo M, Yagi K,
Takayama T and Kaneda A: DNA methylation epigenotype and clinical
features of NRAS-mutation(+) colorectal cancer. Cancer Med.
6:1023–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogura T, Kakuta M, Yatsuoka T, Nishimura
Y, Sakamoto H, Yamaguchi K, Tanabe M, Tanaka Y and Akagi K:
Clinicopathological characteristics and prognostic impact of
colorectal cancers with NRAS mutations. Oncol Rep. 32:50–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yaeger R, Weiss J, Pelster MS, Spira AI,
Barve M, Ou SI, Leal TA, Bekaii-Saab TS, Paweletz CP, Heavey GA, et
al: Adagrasib with or without cetuximab in colorectal cancer with
mutated KRAS G12C. N Engl J Med. 388:44–54. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fakih MG, Salvatore L, Esaki T, Modest DP,
Lopez-Bravo DP, Taieb J, Karamouzis MV, Ruiz-Garcia E, Kim TW,
Kuboki Y, et al: Sotorasib plus panitumumab in refractory
colorectal cancer with mutated KRAS G12C. N Engl J Med.
389:2125–2139. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holderfield M, Lee BJ, Jiang J, Tomlinson
A, Seamon KJ, Mira A, Patrucco E, Goodhart G, Dilly J, Gindin Y, et
al: Concurrent inhibition of oncogenic and wild-type RAS-GTP for
cancer therapy. Nature. 629:919–926. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Watanabe T, Yoshino T, Uetake H, Yamazaki
K, Ishiguro M, Kurokawa T, Saijo N, Ohashi Y and Sugihara K: KRAS
mutational status in Japanese patients with colorectal cancer:
Results from a nationwide, multicenter, cross-sectional study. Jpn
J Clin Oncol. 43:706–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA,
Masugi Y, Shi Y, Song M, da Silva A, Gu M, et al: Fusobacterium
nucleatum in colorectal carcinoma tissue according to tumor
location. Clin Transl Gastroenterol. 7:e2002016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Imamura Y, Lochhead P, Yamauchi M, Kuchiba
A, Qian ZR, Liao X, Nishihara R, Jung S, Wu K, Nosho K, et al:
Analyses of clinicopathological, molecular, and prognostic
associations of KRAS codon 61 and codon 146 mutations in colorectal
cancer: Cohort study and literature review. Mol Cancer. 13:1352014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ouchi K, Takahashi S, Yamada Y, Tsuji S,
Tatsuno K, Takahashi H, Takahashi N, Takahashi M, Shimodaira H,
Aburatani H and Ishioka C: DNA methylation status as a biomarker of
anti-epidermal growth factor receptor treatment for metastatic
colorectal cancer. Cancer Sci. 106:1722–1729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamauchi M, Lochhead P, Morikawa T,
Huttenhower C, Chan AT, Giovannucci E, Fuchs C and Ogino S:
Colorectal cancer: A tale of two sides or a continuum? Gut.
61:794–797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamauchi M, Morikawa T, Kuchiba A, Imamura
Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower
C, et al: Assessment of colorectal cancer molecular features along
bowel subsites challenges the conception of distinct dichotomy of
proximal versus distal colorectum. Gut. 61:847–854. 2012.
View Article : Google Scholar : PubMed/NCBI
|