Introduction
Lung cancer represents one of the most prevalent and
lethal malignancies worldwide. Non-small cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases (1). The advent of immune checkpoint
inhibitors (ICIs) has transformed the therapeutic landscape for
advanced NSCLC, establishing immunotherapy as a cornerstone in lung
cancer treatment (2,3). ICIs include the cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4) antibody and the
programmed cell death protein-1 (PD-1) or its ligand (PD-L1)
monoclonal antibodies. These agents disrupt the interaction between
the PD-1 receptor on T lymphocytes and the PD-L1 ligand on tumor
cells, thereby hindering tumor immune evasion and enhancing the
anti-tumor immune response (4).
CTLA-4, predominantly expressed on the surface of
activated T lymphocytes, competes with co-stimulatory receptors on
T cells, inhibiting T cell proliferation and activation during
early tumorigenesis, thus protecting tumor cells from T
cell-mediated destruction and promoting tumorigenicity. CTLA-4
antibodies effectively block the binding of CTLA-4 to ligands on
antigen-presenting cells, reducing its inhibitory effect on T cell
activation and potentially decreasing the capability of regulatory
T cells through inhabiting its interaction with macrophages,
thereby enhancing tumor cytotoxicity (5,6).
However, the use of ICIs may cause activated T cells to target
normal tissues expressing antigens shared with tumor cells, leading
to compromised immune tolerance and manifesting as immune-related
adverse effects (irAEs) (7), which
can affect the skin, digestive tract, liver, endocrine system,
lungs and other organs (8).
Dermatological adverse events are prevalent and readily
identifiable in clinical settings (9).
Emerging evidence suggests that frequent skin
adverse events can significantly impair patients' quality of life
and erode treatment adherence (10,11).
Moreover, patients with lung cancer exhibit a higher incidence of
immuno-related pneumonitis compared with patients with other tumor
types (12,13), with this condition representing a
primary cause of mortality among immunotherapy-treated patients
(14). Therefore, vigilant
monitoring for dermatological adverse effects and immuno-related
pneumonitis is essential in the immunotherapies for lung
cancer.
The present report presents a rare NSCLC case
complicated by severe dermatitis and grade 4 pneumonitis following
immunotherapy, which was successfully managed with a combination of
mycophenolate mofetil (MMF) with methylprednisolone, overcoming
steroid-dependent dermatitis and pneumonitis. The patient had
maintained a partial response for >3 years after the cessation
of immunotherapy. The study was approved by the Ethics Committee of
the Affiliated People's Hospital of Ningbo University (approval no.
2024-N-004; Ningbo, China).
Case report
A 64-year-old man was diagnosed with advanced
pulmonary adenocarcinoma with intrapulmonary metastasis (cT4N2M1a,
stage IV) at Ningbo Medical Centre Lihuili Hospital (Ningbo, China)
in January 2021. The patient then immediately underwent the
aforementioned genetic testing and testing the expression of
PD-L1.The actionable gene mutations, such as EGFR, anaplastic
lymphoma kinase, ROS proto-oncogene 1, receptor tyrosine kinase and
MET proto-oncogene, were negative detected by next-generation
sequencing. Sample processing and sequencing were performed in a
CLIA-certified and CAP-accredited laboratory (Geneseeq Technology
Inc.). DNA extraction, library preparation, and targeted capture
enrichment were carried out following the methods as previously
described with modifications (15).
Formalin-Fixed Paraffin-Embedded (FFPE) samples were
de-paraffinized with xylene, and genomic DNA was extracted using
the QIAamp DNA FFPE Tissue Kit (Qiagen GmbH). DNA was quantified by
Qubit 3.0 using the dsDNA HS Assay Kit (Thermo Fisher Scientific,
Inc.) and the quality was evaluated by a Nanodrop 2000 (Thermo
Fisher Scientific, Inc.). Libraries were prepared by KAPA Hyper
Prep kit (Kapa Biosystems; Roche Diagnostics), as previously
described (16). Briefly, 1–2 µg of
genomic DNA was sheared into ~350 bp fragments using a Covaris M220
instrument. End repair, A-tailing, and adaptor ligation of
fragmented DNA were performed using the KAPA Hyper DNA Library Prep
kit (Roche Diagnostics), followed by size selection with Agencourt
AMPure XP beads (Beckman Coulter, Inc.). DNA Libraries were then
amplified by PCR and purified using Agencourt AMPure XP beads
(Beckman Coulter, Inc.). Customized xGen lockdown probes
(Integrated DNA Technologies, Inc.) targeting NSCLC-related genes
were used for hybridization enrichment. Human cot-1 DNA (Thermo
Fisher Scientific, Inc.) and xGen Universal Blocking Oligos
(Integrated DNA Technologies, Inc.) were added as blocking
reagents. The capture reaction was performed with Dynabeads M-270
(Thermo Fisher Scientific, Inc.) and the xGen Lockdown
Hybridization and Wash kit (Integrated DNA Technologies, Inc.).
Captured libraries were subjected to PCR amplification with KAPA
HiFi HotStart ReadyMix (Kapa Biosystems; Roche Diagnostics). The
purified library was quantified using the KAPA Library
Quantification kit (Kapa Biosystems; Roche Diagnostics) and its
fragment size distribution was analyzed using a Bioanalyzer 2100.
Target enriched libraries were sequenced on the HiSeq4000 platform
(Illumina, Inc.) with 2×150 bp pair-end reads. Sequencing data were
demultiplexed by bcl2fastq (v2.19; Illumina, Inc.), analyzed by
Trimmomatic (http://www.usadellab.org/cms/index.php?page¼trimmomatic)
(17) to remove low-quality
(quality<15) or N bases. Then the data were aligned to the hg19
reference human genome with the Burrows-Wheeler Aligner (bwa-mem)
(18) and further processed using
the Picard suite (available at: http://broadinstitute.github.io/picard/) and the
Genome Analysis Toolkit (GATK) (19). SNPs and indels were called by
VarScan2 (20) and HaplotypeCaller/
UnifiedGenotyper in GATK, with the mutant allele frequency (MAF)
cutoff as 0.5%. Common variants were removed using dbSNP and the
1000 Genome project. Germline mutations were filtered out by
comparing to patient's whole blood controls. Gene fusions were
identified by FACTERA (21) and
copy number variations (CNVs) were analyzed with ADTEx (22). The log2 ratio cut-off for copy
number gain was defined as 2.0 for tissue samples. A log2 ratio
cut-off of 0.6 was used for copy number loss detection.
Allele-specific CNVs were analyzed by FACETS (23) with a 0.2 drift cut-off for unstable
joint segments.
Additionally, immunohistochemistry (IHC) of the
tumor tissue showed negative expression for PD-L1. The
methodological details of IHC were as follows: i) Sample
Preparation: FFPE sample were collected from patients. Tissue
sections with a thickness of 4 µm were cut using a microtome and
mounted on positively charged glass slides. Sections were dried
overnight at 60°C and then deparaffinized with xylene and
rehydrated through a graded alcohol series (100, 95, 80 and 70%
ethanol) for 5 min each. ii) Heat-induced epitope retrieval (HIER):
Antigen retrieval was performed by placing slides in a cooker
pressure and immersing them in EDTA-based retrieval solution (pH
8.0) for 10 min at 120°C. After cooling for 20 min at room
temperature, slides were washed twice with phosphate buffered
saline (PBS; pH 7.4) for 5 min each. iii) Blocking and antibody
incubation: Endogenous peroxidase activity was quenched by
incubating slides in 3% hydrogen peroxide in methanol for 30 min at
room temperature. Then, slides were washed twice with PBS for 5
min. Non-specific binding was blocked using a protein blocking
reagent (Coomassie Brilliant Blue G-250; 0.1% in PBS) for 15 min at
room temperature. The primary antibody, anti-PD-L1 clone 22C3
(Thermo Fisher Scientific, Inc.), was diluted at 1:50 in antibody
diluent (0.1% bovine serum albumin in PBS). Slides were incubated
with the primary antibody overnight at 4°C in a humidified chamber.
iv) Secondary antibody and detection: After incubation with the
primary antibody, slides were washed three times with PBS for 5 min
each. The secondary antibody, a horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG antibody (cat. no.
115-035-003; Jackson ImmunoResearch Laboratories, Inc.) was diluted
at 1:200 in antibody diluent. Slides were incubated with the
antibody secondary for 30 min at room temperature. Detection was
performed using a DAB substrate kit (cat. no. K066; Agilent
Technologies) according to the manufacturer's instructions. Slides
were developed for 5–10 min until brown precipitates were visible
under a light microscope. After development, slides were rinsed
with distilled water and counterstained with hematoxylin for 1 min.
Slides were then dehydrated through a graded alcohol series (70,
80, 95 and 100% ethanol) for 5 min each and cleared with xylene. v)
Scoring and analysis: Stained slides were reviewed and scored by
two independent pathologists who were blinded to the clinical
information. PD-L1 expression was assessed based on the percentage
of tumor cells showing membrane staining and the intensity of
staining. Staining intensity was scored as 0 (no staining), 1
(weak), 2 (moderate), and 3 (strong). A combined positive score
(CPS) was calculated as follows: CPS=(number of PD-L1 positive
tumor cells/total number of tumor cells) ×100. A CPS ≥1 was
considered positive for PD-L1 expression.
Subsequently, 7 days later, the patient was treated
with a pemetrexed and carboplatin chemotherapy regimen [pemetrexed
800 mg on day (d)1 and d8, and carboplatin 500 mg (area under
curve=5) on d1, with a 21-d cycle], combined with pembrolizumab 200
mg on d1, also with a 21-d cycle. On the 15th day following the
initiation of treatment, the patient experienced myelosuppression,
which delayed the subsequent chemotherapy. Chemotherapy was
terminated after this point due to the patient subsequently
experiencing a series of immune-related adverse events, such as
fever, immune-related cystitis, immune-related pneumonitis and
severe cutaneous irAEs. However, on the 26th day, the patient
developed a fever with a peak temperature of 38.4°C and exhibited
typical urinary irritation symptoms such as dysuria, urgency and
frequency accompanied by intense bladder pain. The patient was
successively administered a levofloxacin injection (0.5 g/day) and
a piperacillin tazobactam injection (4.5 g/8 h) for anti-infection
treatment, but the symptoms did not improve. Multiple urine routine
tests indicated an increase in red and white blood cell counts,
with the highest red blood cell count being 1,279/µl and the
highest white blood cell count being 3,620/µl, while the normal
ranges are <17/µl and 11/µl, respectively. The urine cultures
were negative for bacterial growth. Computed tomography urography
revealed no apparent signs of infection or tumor metastasis. The
patient was unwilling to undergo an invasive procedure, therefore a
cystoscopy was not performed.
Due to the absence of clear evidence of infection
and tumor metastasis, a diagnosis of immune-related cystitis was
likely. Starting from February 2021, the patient was administered
methylprednisolone injection at a dose of 40 mg/d. After 5 days, a
routine urine examination showed that the red and white blood cell
counts had turned negative under a high-power field, and the
patient's urinary tract irritation symptoms were markedly improved.
According to standard medical protocols, high-dose medications are
administered intravenously, while low-dose ones are given orally.
Therefore, the oral dose of methylprednisolone was sequentially
decreased to 28 mg/d. The dose was gradually reduced 8 mg per week
and the total duration of administration was ~5 weeks.
In April 2021, the patient experienced a recurrence
of fever, accompanied by coughing, dyspnea and hypoxia. The patient
was then admitted to the Affiliated People's Hospital of Ningbo
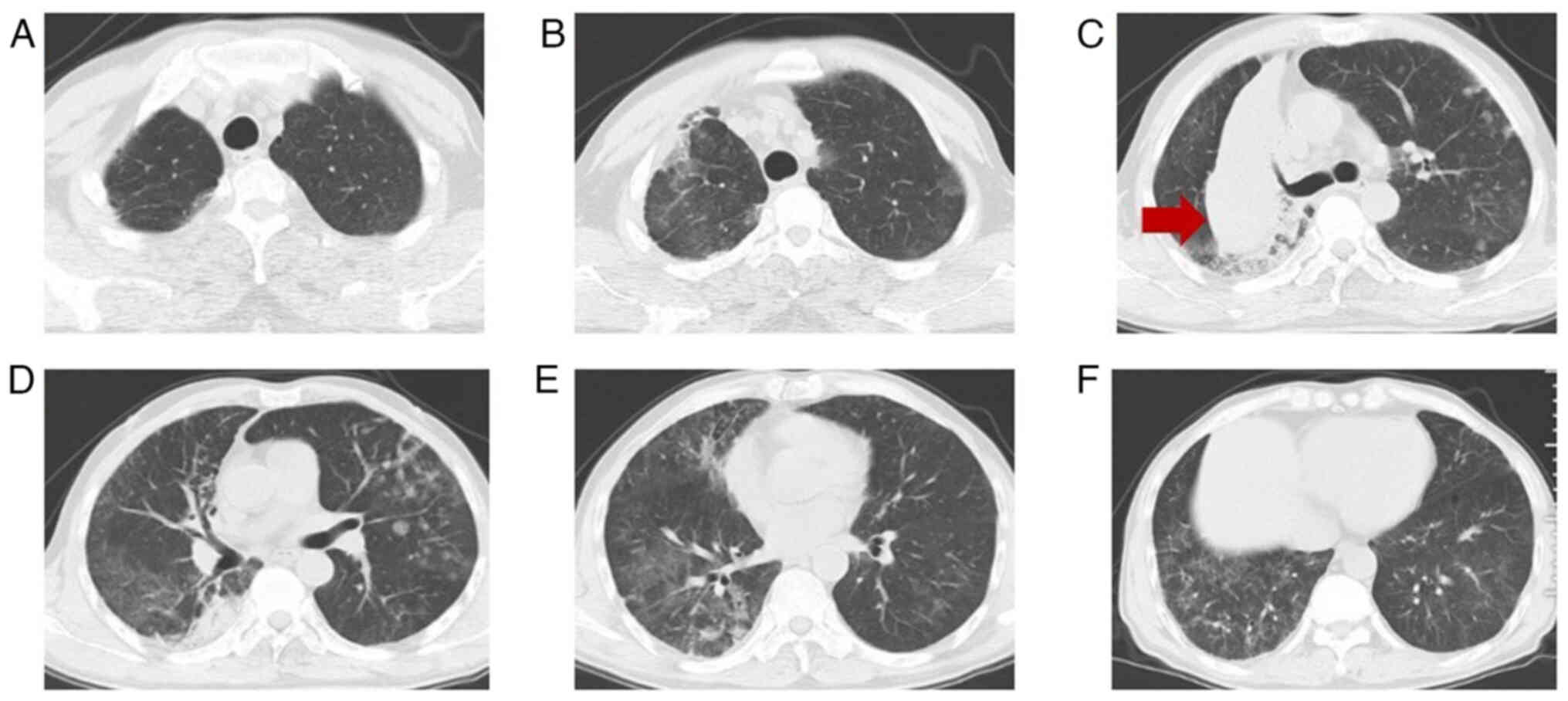
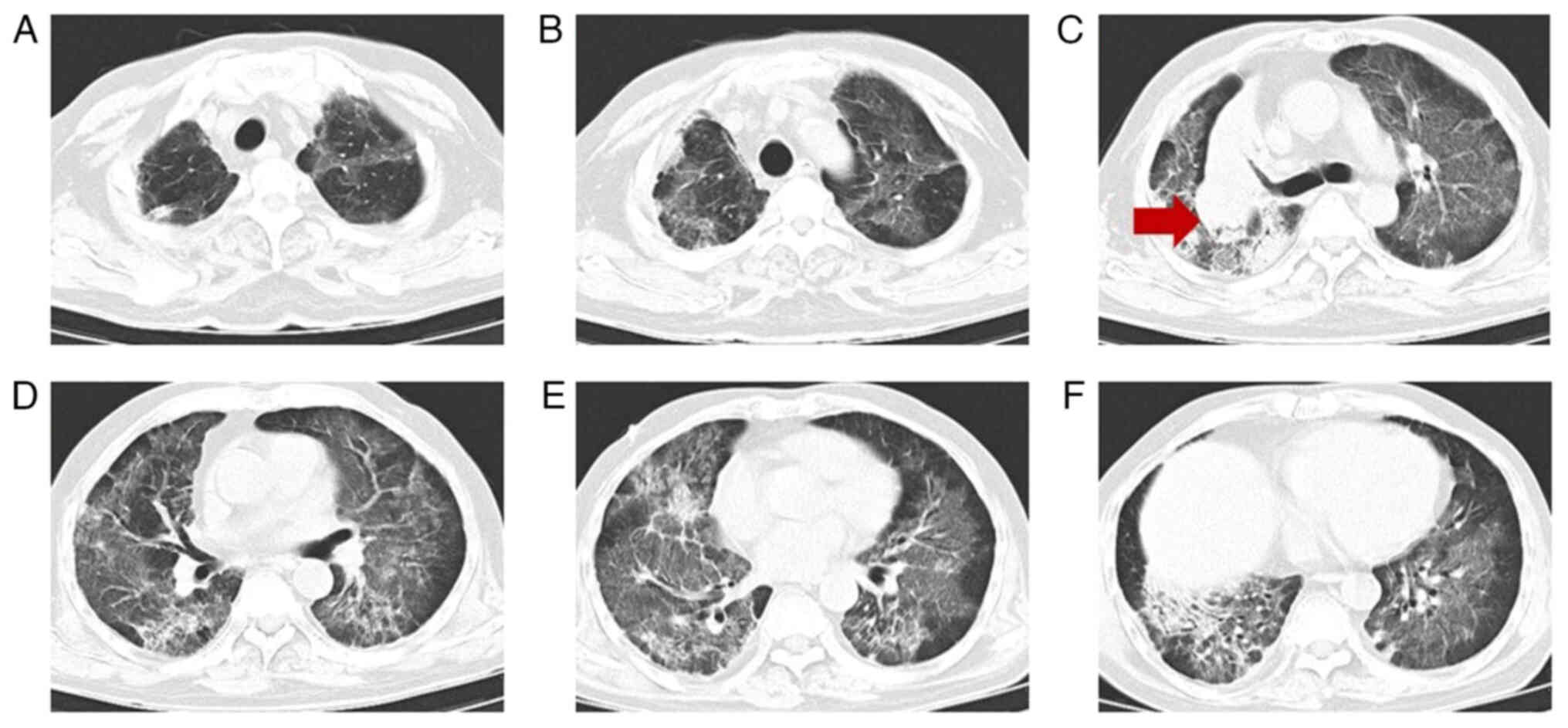
University (Ningbo, China). A chest CT scan (Fig. 1) showed multiple patchy and
grid-shaped high-density shadows with unclear borders and uneven
bilateral density in the lungs. Arterial blood gas analysis
(without oxygen inhalation) yielded the following results: pH 7.46
(normal range, 7.35–7.45), partial pressure of carbon dioxide
(PCO2) 30 mmHg (normal range, 35–45 mmHg), partial
pressure of oxygen (PO2) 56 mmHg (normal range, 80–100
mmHg) and oxygenation index 266. The non-invasive blood oxygen
saturation was 90%. A blood routine test showed white blood cells
of 8.91×109/l (normal range, 3.5–9.5×109/l),
red blood cells of 4.97×1012/l (normal range,
3.8–5.1×1012/l), hemoglobin of 122 g/l (normal range,
115–150 g/l), platelets of 213×109/l (normal range,
125–350×109/l), neutrophil count of
5.14×109/l (normal range, 1.8–6.3×109/l),
C-reactive protein (CRP) of 28.0 mg/l (normal range, <10 mg/l)
and procalcitonin of 0.24 ng/ml (normal range, 0.00–0.05 ng/ml).
Based on the medication history of the patient and the fact that
blood routine and CRP were not elevated at the onset of fever, it
was deemed probable that the patient was suffering from
immune-related pneumonitis.
Upon admission, the patient was administered an 80
mg methylprednisolone injection, supplemented by oxygen inhalation,
alongside other symptomatic and supportive measures including fever
reduction, fluid replacement and nutritional support. Concurrently,
a comprehensive suite of diagnostic tests was initiated to exclude
alternative diagnoses. These included multiple sputum cultures,
1-3-β-D glucan test, galactomannan assays, assessment of brain
natriuretic peptide, coagulation function, D-dimer and respiratory
pathogen nucleic acid detection for a range of pathogens including
Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella
pneumophila, coxsackie virus, coronavirus, echovirus, influenza
A and B viruses, respiratory syncytial virus, adenovirus and
parainfluenza virus. Additionally, electrocardiogram and
echocardiography were performed to rule out pulmonary fungal
infection, tumor progression, pulmonary embolism, cardiac events
and pleural carcinomatosis.
On the second day of treatment, the patient's
temperature normalized and there was an improvement in cough,
dyspnea and hypoxia, with non-invasive blood oxygen saturation
increasing to 96%. This indicated that the initial treatment was
effective, prompting the continuation of the prescribed therapies
for 2 weeks. However, in the following week, the patient
experienced persistent low-grade fever, dyspnea and hypoxia, which
necessitated a follow-up chest CT scan. The scan, conducted 14 days
after admission (Fig. 2), indicated
the progression of the lesion compared with earlier imaging.
The patient was classified as G3 according to the
grading of immune-related pneumonitis in the Common Terminology
Criteria for Adverse Events (CTCAE) version 5.0 (24). Moreover, in line with the European
Society of Medical Oncology (ESMO) Clinical Practice Guidelines for
the management of toxicities from immunotherapy (25), which provides recommendations for
diagnosis, treatment and follow-up, patients graded G3 or G4 are
advised to discontinue the immunotherapy permanently and be
administered with methylprednisolone at a dosage of 1–2 mg/kg/d.
The guidelines suggest initiating tapering corticosteroids after
improvement to grade <1, over 4–6 weeks for grade 2 and over 6–8
weeks for grade 3. Therefore, the dosage of methylprednisolone
injection was adjusted to 240 mg (equivalent to 4 mg/kg/d) daily.
The patient's temperature normalized, and symptoms of dyspnea and
hypoxia improved within the following 72 h. Subsequently, the
dosage of methylprednisolone injection was reduced to 160 mg daily,
then gradually decreased to 80 mg daily over 2 weeks. Concurrently,
compound sulfamethoxazole tablets (SMZ) at 1.2 g twice per week
were administrated to prevent Pneumocystis jirovecii
pneumonia, omeprazole 40 mg daily was used to prevent stress ulcers
and vitamin D 60 IU daily and calcium 300 mg daily were used to
prevent osteoporosis, along with symptomatic and supportive
treatments including fever reduction, fluid replacement and
nutritional support. At ~1 month after admission, the
administration of methylprednisolone was changed to oral due to the
patient's intention to be discharged and the oral dose of
methylprednisolone was to 56 mg daily for 2 weeks. In May 2021, a
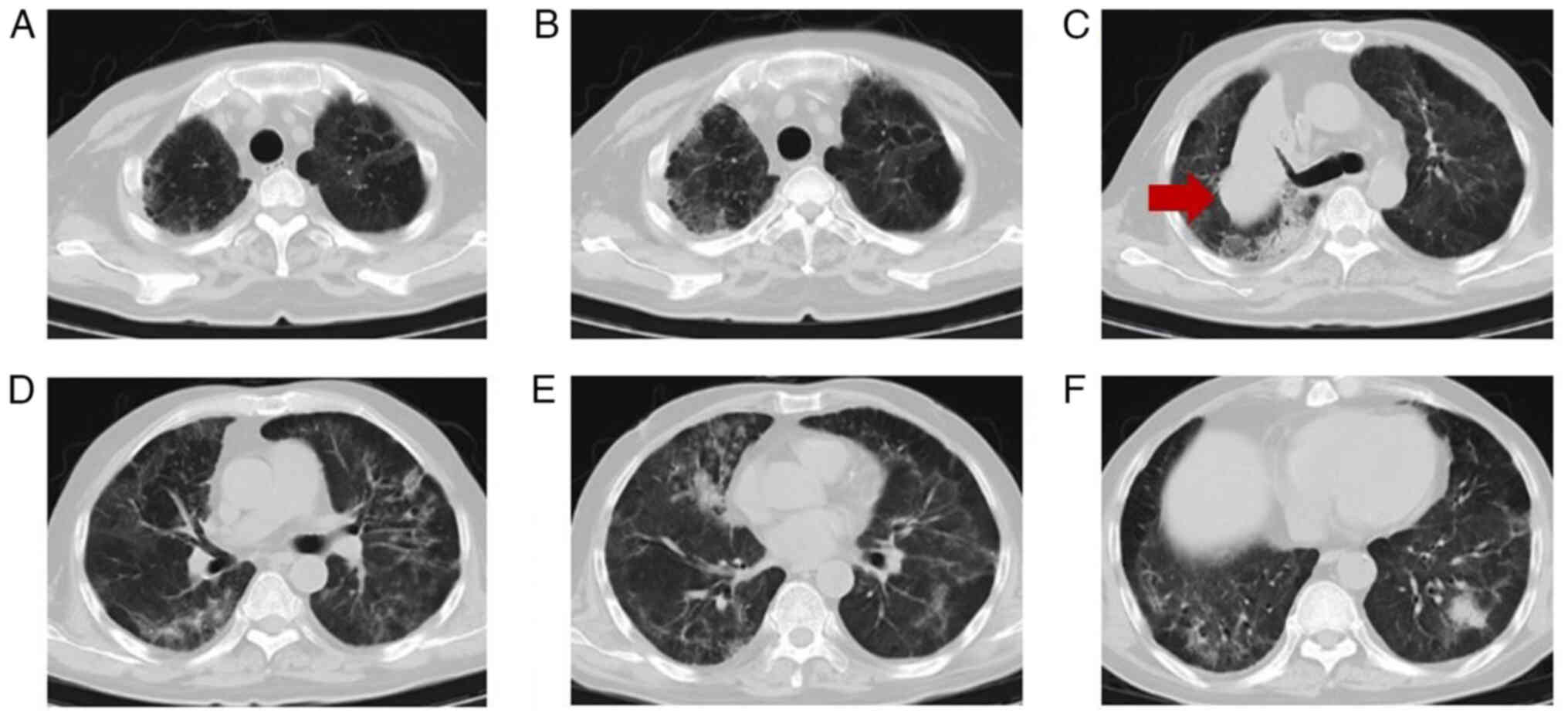
follow-up chest CT (Fig. 3)
indicated focal absorption. After a brief observation period, the
patient's symptoms did not recur, leading to their discharge from
the hospital.
In June 2021, when the oral methylprednisolone
dosage was reduced to 44 mg daily within 1 week, the patient
experienced a relapse characterized by fever, dyspnea, cough,
tachypnea and cyanosis of the lips, hands and feet. Severe
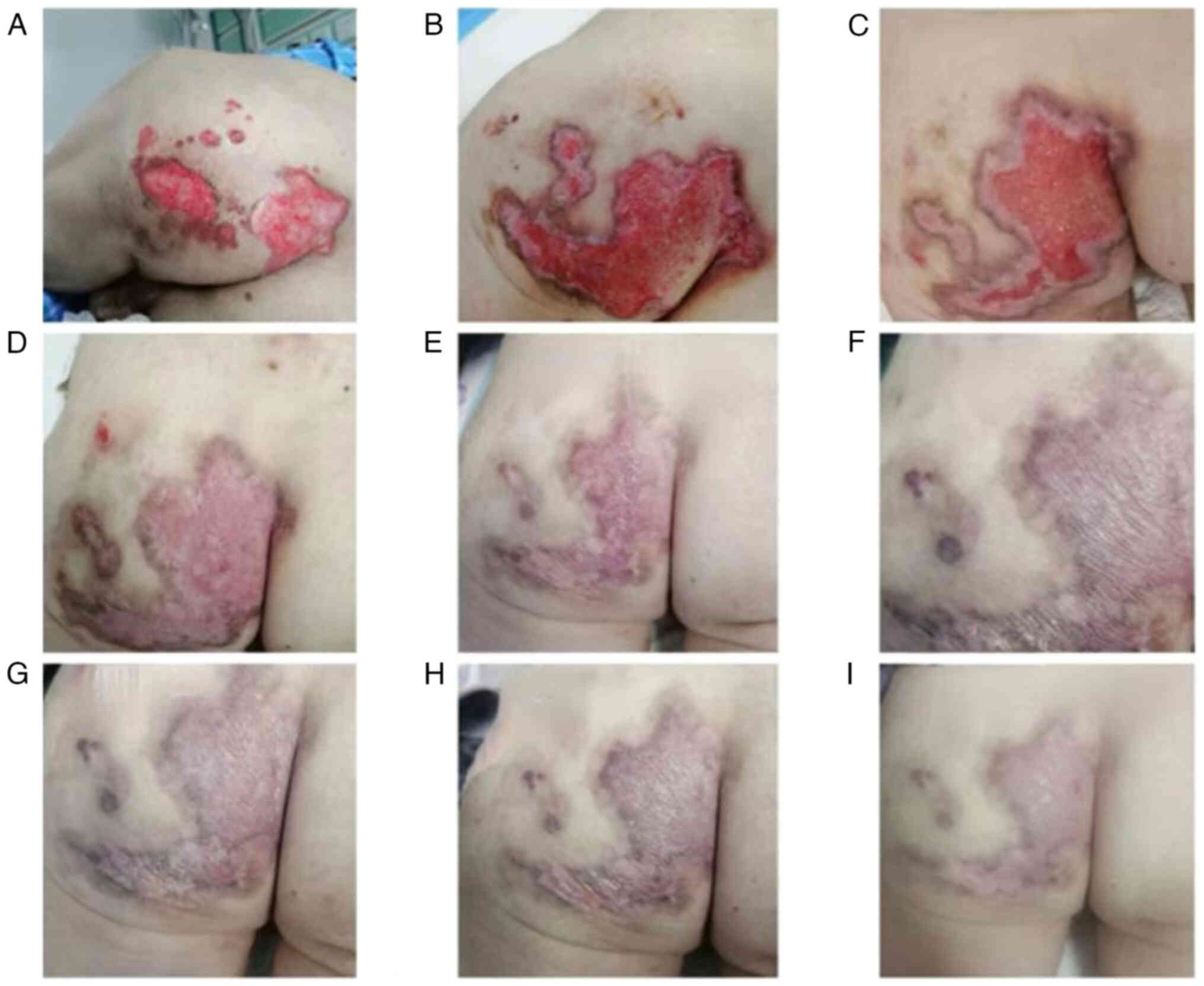
cutaneous symptoms also emerged, including canker sores and
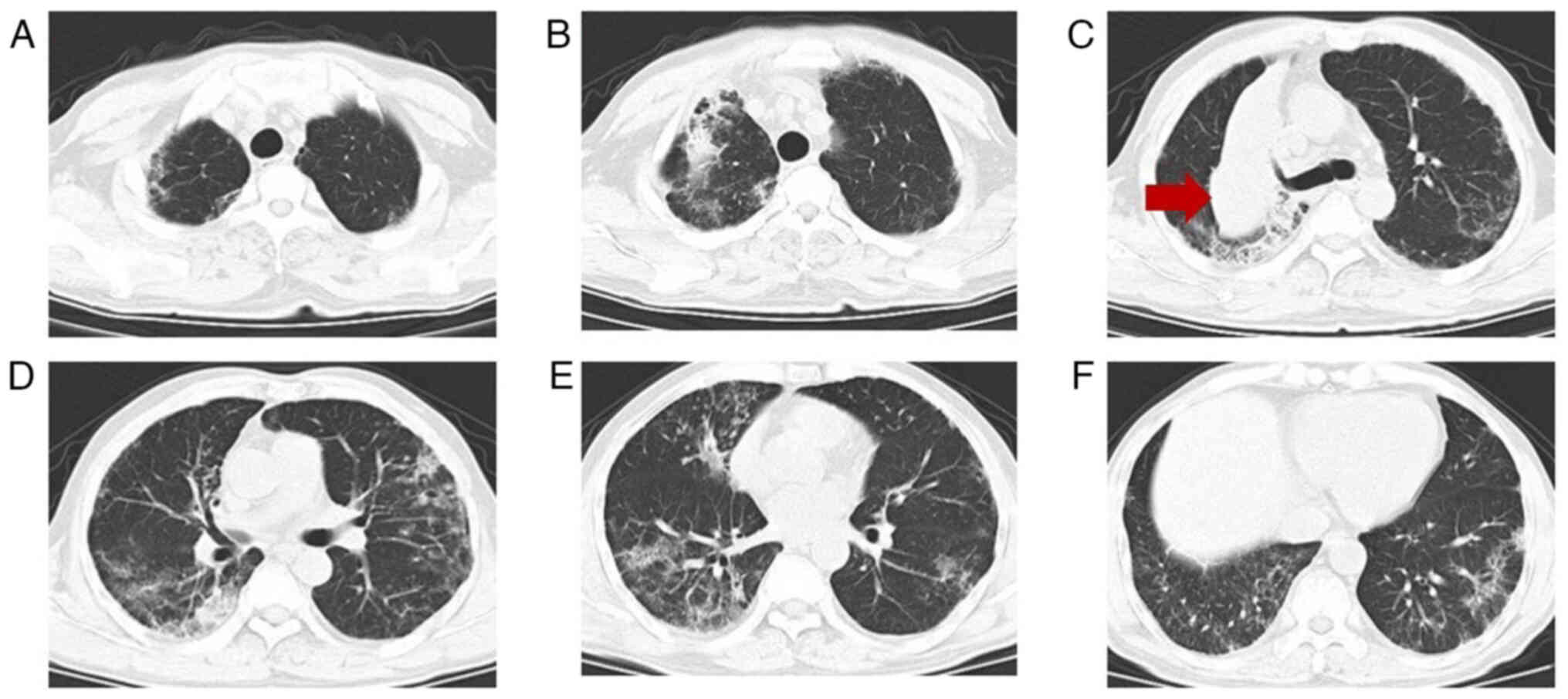
perianal ulcers (Fig. 4). A chest
CT (Fig. 5) revealed significant
lesion progression compared with the scan taken in May 2021.
Arterial blood gas analysis (performed without oxygen inhalation)
indicated a pH of 7.43, a PCO2 of 34 mmHg, a
PO2 of 36 mmHg, a blood oxygen saturation of 72% and an
oxygenation index of 171. Non-invasive oxygen saturation was
measured at 78%. The patient was diagnosed with immune-related
pneumonitis and respiratory failure and was classified as grade 4
(G4) according to the CTCAE version 5.0 (24).
Upon readmission to the Affiliated People's Hospital
of Ningbo University (Ningbo, China), the patient received a 240 mg
methylprednisolone injection (4 mg/kg/d) and was treated with
intravenous immunoglobulin 20 g/d for 5 days. Supporting therapy
was also provided through non-invasive ventilator-assisted
ventilation (oxygen concentration, 50%), which improved the
non-invasive oxygen saturation to 94%. The symptoms of tachypnea
and cyanosis were alleviated. Considering the patient's history of
tumor treatment and prolonged glucocorticoid use, co-infections,
particularly fungal infections, were a concern. Consequently,
Piperacillin-tazobactam (4.5 g daily for 2 weeks) and Caspofungin
(50 mg daily for 1 week, 1st day 70 mg) were administered. Various
tests were conducted to exclude pulmonary embolism, cardiac events
and fungal infections. Due to the patient's condition, bronchoscopy
was not performed, precluding the submission of bronchoalveolar
lavage fluid for culture and next-generation sequencing. However,
multiple sputum cultures, 1–3-β-D glucan tests, galactomannan
tests, cryptococcus capsular antigen tests and respiratory pathogen
nucleic acid detections were all negative for bacterial, fungal or
respiratory viral infections.
A dermatologist was consulted for specialized advice
to address the severe cutaneous symptoms. Due to the concurrent
presence of canker sores and perianal ulcers, the dermatologist did
not rule out Behçet's syndrome and recommended an ophthalmic
examination and skin biopsy. However, the patient did not exhibit
eye symptoms, such as uveitis, dry eyes, floater signs or vision
distortion. Further examination showed that the patient was
negative for antinuclear, anti-myeloperoxidase, anti-protease 3 and
anti-glomerular basement membrane antibodies. Notably, the reaction
to the pathergy test, which is currently the only particular
diagnostic procedure for Behçet's syndrome, was also negative. A
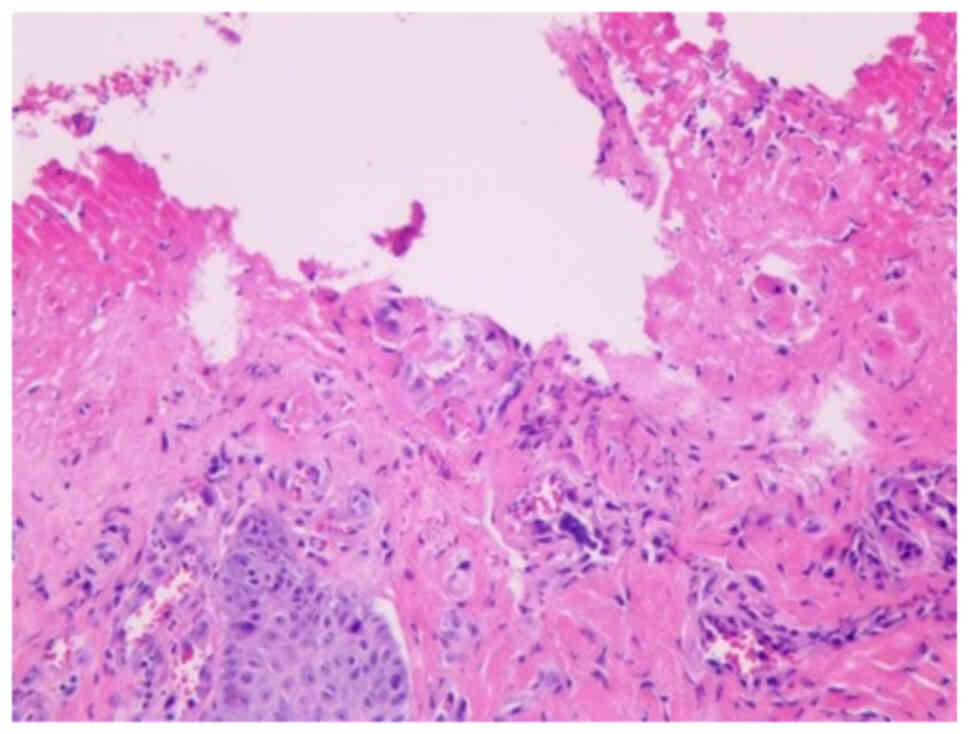
skin biopsy of the perianal ulcer (Fig.
6) revealed the absence of skin epidermis, surface ulcers and
dermal vascular hyperplasia, as well as minimal neutrophil
infiltration. The biopsy sample was fixed in 10% neutral buffered
formalin for ≥24 h at room temperature before being embedded in
paraffin and sectioned into 3 µm thick slices. Hematoxylin staining
was performed for 5–15 min at room temperature followed by
counterstaining with eosin for 1–3 min at room temperature. A light
microscope (magnification, ×50) was used to image the samples.
These findings deviate from the typical pathological
features of Behçet's syndrome, leading to the conclusion that the
canker sores and perianal ulcers were severe cutaneous irAEs. In
response, the patient was treated with thalidomide tablets (50 mg
once daily) and topical treatments, including methylprednisolone,
human epidermal growth factor (5,000 IU daily) and silver ion
antibacterial gel, applied to the perianal ulcer for 8 months.
In the following days, the patient's respiratory
symptoms, non-invasive oxygen saturation, oxygenation index and
cutaneous symptoms gradually improved. As the symptoms subsided,
the dosage of methylprednisolone was gradually decreased as
follows: 240 mg for 1 week, 160 mg for 1 week, 120 mg for 1 week,
80 mg for 1 week, 60 mg for 1 week and 40 mg for 1 week. However,
due to the patient's previous recurrence of pneumonitis during
glucocorticoid tapering, it was concluded that the disease was
corticosteroid-dependent. Therefore, the immunosuppressive drug MMF
(1 g twice daily) was introduced during glucocorticoid reduction.
Subsequently, 1 month later, the patient's canker sores had healed.
Then, 6 weeks later, the patient was free from cough, phlegm,
dyspnea and cyanosis of the lips, hands and feet. The non-invasive
oxygen saturation was >90% without oxygen inhalation. An
arterial blood gas analysis (with an oxygen concentration of 29%)
revealed a pH of 7.49, PCO2 of 35 mmHg, PO2
of 75 mmHg, blood oxygen saturation of 94% and an oxygenation index
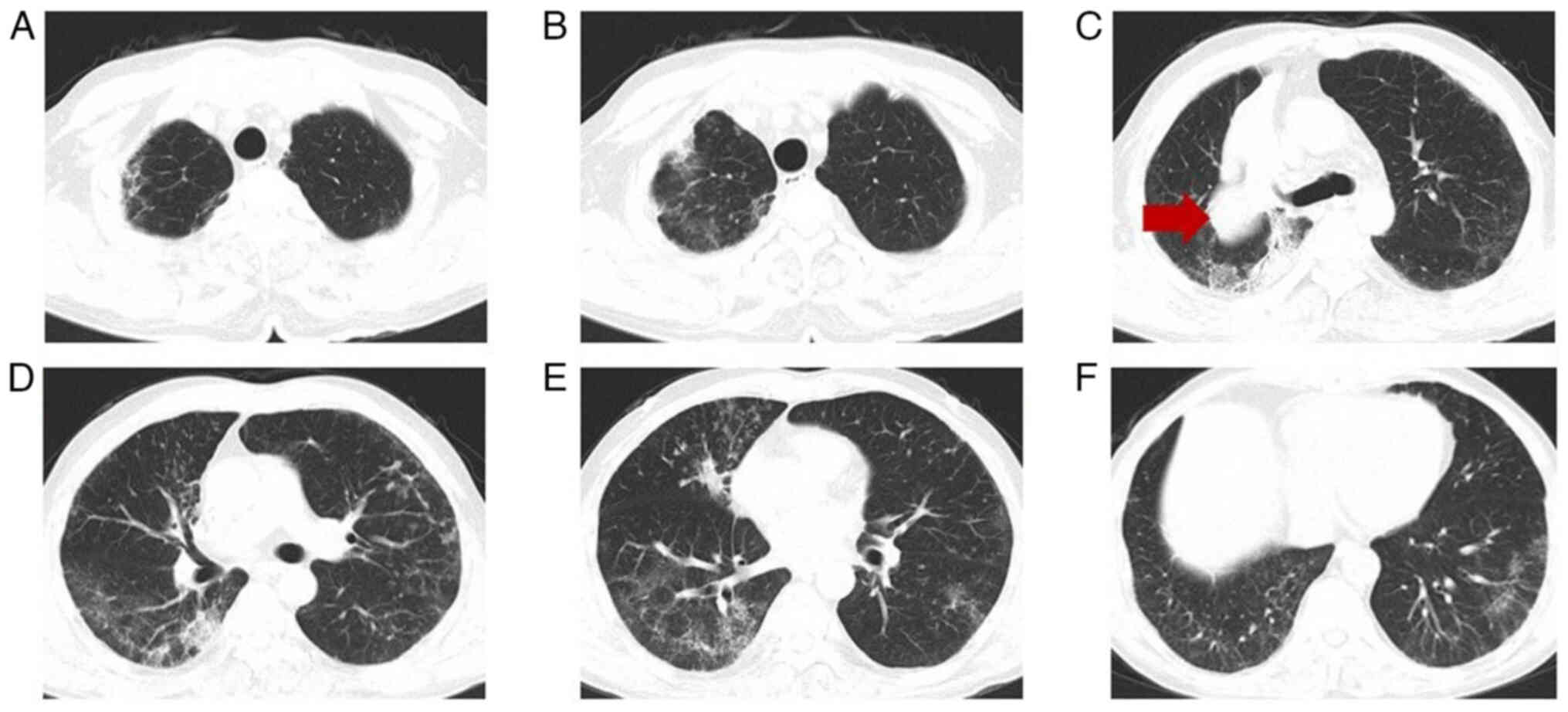
of 258. A review of the chest CT (Fig.
7) showed reduced interstitial changes in the bilateral
pulmonary fields compared with the previous scan. The perianal
ulcer did not progress further. Based on these improvements, the
patient was discharged from the hospital in July 2021 with
instructions to take methylprednisolone tablets at 32 mg once daily
and maintain the original MMF dosage. In the next months, the
dosage of methylprednisolone was gradually reduced to 12 mg daily.
Throughout methylprednisolone reduction, the perianal ulcer
gradually healed (Fig. 4), and the
respiratory symptoms did not recur.
Over the subsequent year, the methylprednisolone
tapering was conducted cautiously, with an average reduction of 4
mg every 3 to 4 months. By October 2022, the methylprednisolone
dosage was reduced to 4 mg once daily, and the MMF was decreased to
250 mg twice daily. In December 2022, a chest CT scan revealed
multiple bilateral pulmonary interstitial lesions. The patient
discontinued methylprednisolone on their own initiative, but within
a few days, they experienced moderate polyarthralgia and myalgia,
which impaired their ability to perform instrumental activities of
daily living. Consequently, the patient was referred to a
rheumatologist. A series of assessments were conducted, including
measurements of erythrocyte sedimentation rate, CRP, rheumatoid
factor, anti-cyclic citrullinated peptide and antinuclear
antibodies to rule out myositis. However, X-rays and ultrasound of
the affected joints were not performed.
Due to the medical and medication history of the
patient, they were diagnosed with immune-related inflammatory
arthritis. The patient was advised to resume methylprednisolone at
4 mg once daily and MMF 250 mg twice daily. Additional medications
prescribed included SMZ to prevent P. jirovecii pneumonia,
omeprazole 40 mg daily to prevent stress ulcers and vitamin D 60 IU
daily and calcium supplements 300 mg daily to prevent osteoporosis.
The patient continued the aforementioned treatments to September
2024.
Given the patient's notable improvement in irAEs
following effective treatments, anlotinib 12 mg daily for 2 weeks
followed by 1 week off treatment, was initiated as the second-line
treatment in June 2022. The patient maintained a partial remission,
as the tumor was >30% smaller compared with the initial tumor
size, for >3 years after discontinuing immunotherapy in January
2021. The patient returned to the hospital for follow-up visits
every 2–3 months and the last follow-up of the patient was
conducted in July 2024. In September 2024, the patient succumbed to
severe co-infection (with COVID-19 and bacterial sepsis).
Discussion
With the rapid development and cross-development of
oncology, immunology and other related disciplines, immunotherapy
has made rapid progress and has become an important antitumor
method after surgery, radiotherapy and chemotherapy. Particularly,
ICIs have achieved breakthrough progress in tumor immunotherapy.
However, with the widespread application of PD-1/PD-L1, an
increasing number of adverse effects caused by ICIs have raised
concerns (26).
PD-1/PD-L1 inhibitors function by blocking the
negative regulatory signals of T cells, alleviating
immunosuppression and enhancing the antitumor activity of T cells
(4). Simultaneously, these
inhibitors may abnormally amplify the body's autoimmune responses,
leading to an imbalance in immune tolerance (8). When these inhibitors accumulate in
non-cancerous tissues, they can induce an autoimmune-like
inflammatory response, termed irAEs. irAEs can affect multiple
organs, including the skin, digestive tract, liver, endocrine
system and lungs (4,8). In the present case, there were
concurrent occurrences of immune-related cystitis, cutaneous
lesions and pneumonitis, which is a rare phenomenon and sparsely
documented in the literature. A recent retrospective study
identified co-occurring irAEs, classifying them into seven distinct
clusters: Endocrine, cutaneous, respiratory, gastrointestinal,
hepatic, musculoskeletal and neurological (27). Notably, in the present case, the
patient exhibited irAEs that spanned the cutaneous, respiratory and
urinary systems, showing the complex and multifaceted nature of the
immune response triggered by PD-1/PD-L1 blockade.
Currently, no specific grading standards exist for
irAEs. The grading of irAEs still follows the CTCAE version 5.0
(24). The ESMO (25) and the National Comprehensive Cancer
Network (28) have published
guidelines for irAEs based on expert consensus. These guidelines
provide general treatment options for the most common irAEs and
detail the use of immunosuppressive drugs and the course of
treatment according to the severity of irAEs. The treatment
principles vary for different grades of toxic reactions. For grade
1, it is recommended to closely observe and intervene on time to
avoid further changes in toxicity. For grade 2, if necessary,
withhold ICI therapy and glucocorticoids (prednisone 0.5 to 1
mg/kg/d or equivalent per day) and resume ICI therapy upon
improvement. For grades 3 and 4, a high dose of glucocorticoids
should be given (prednisone 1 to 2 mg/kg/d or equivalent). When
symptoms subside to grade 1 or below, glucocorticoids should
gradually decrease. Whether to resume ICI therapy for grade 3
toxicity requires a complete evaluation of the patient's tumor
status and a careful weighing of the risks and benefits before
resuming ICI therapy. For grade 4 toxicity, except for endocrine
disease controlled by hormone replacement therapy, ICIs should be
permanently discontinued. For corticosteroid-dependent or
refractory or ineffective cases, other immunosuppressive drugs can
be considered, including tumor necrosis factor-α inhibitors
(infliximab), conventional synthetic disease-modifying
antirheumatic drugs (such as MMF) and anti-interleukin 6 receptor
therapies (such as tocilizumab or sarilumab).
In the present study, MMF was crucial in the steroid
tapering process. MMF functions by inhibiting the proliferation of
T and B lymphocytes, thus modulating the immune responses that lead
to irAEs. The use of MMF during glucocorticoid tapering is not
traditionally regarded as a standard treatment approach (29). However, a previous report has
demonstrated that MMF can consistently reduce the cumulative dose
of glucocorticosteroids in patients with IgA nephropathy (30). MMF is increasingly recognized as an
important option in managing severe irAEs. Recent research supports
the use of MMF in the treatment of corticosteroid-resistant irAEs
in patients with cancer, particularly as a first-line treatment for
severe hepatitis (31).
Additionally, MMF is a widely used immunosuppressive drug for
several conditions, including dermatomyositis and IgA-associated
nephropathy (32). MMF is generally
well-tolerated but can cause gastrointestinal issues such as
diarrhea and nausea in ~30% of patients; it has a lower rate of
hepato-nephrotoxicity compared with other immunosuppressants, such
as calcineurin inhibitors and azathioprine (33), which is an important advantage
(34). However, MMF is classified
as a reproductive toxin and is suspected of causing genetic
defects, so it should be used with caution in pregnant patients
(35).
The patient in the present study initially exhibited
typical symptoms of urinary irritation. After ruling out infection
and tumor metastasis, the development of cystitis was associated
with the duration of ICI use and could not be attributed to the
adverse reactions of chemotherapy drugs or the progression of the
patient's disease. Consequently, the patient was diagnosed with
immune-related cystitis. Immune-related cystitis is a rare
condition, with limited published cases and no detailed information
in the relevant management guidelines, such as the ESMO (25) and the National Comprehensive Cancer
Network (28) guidelines.
Additionally, to the best of our knowledge, there are no clear,
quantifiable indicators to assess the severity of this condition.
In the present case, the symptoms improved after discontinuing ICIs
and glucocorticoid treatment. It can be confirmed that
glucocorticoids are effective for immune-related cystitis, but
there is no consensus on the specific dosage yet. Therefore,
clinicians must pay attention to immune-related cystitis. The
experience in diagnosing and treating cystitis is valuable for
discussion and reference.
Cutaneous irAEs are the most common adverse events
related to immune ICI therapy, affecting >50% of all patients in
all grades. Although the precise pathogenesis remains unclear, the
reported mechanisms underlying these cutaneous irAEs involve the
non-specific activation of the immune system by ICIs, leading to
autoimmune-like or inflammatory conditions (36). T cell-mediated immunopathology is
central to these reactions, with varying effector cells and
cytokines depending on the clinical phenotype (37). Cutaneous irAEs manifest in the skin,
mucous membrane and skin appendages such as fingernails, toenails
and hair. They often present with rashes, pruritus and
maculopapular eruptions; some are similar to common skin diseases,
such as vitiligo, psoriasis, lichen planus and hemangioma. Although
severe cases are rare and generally do not interfere with the
continuation of treatment, it is important to monitor and manage
these symptoms (9,38). According to the CTCAE version 5.0,
the present patient experienced a severe perianal ulcer, classified
as grade 3 or grade 4. The diversity of immune-related skin
illnesses necessitates their distinction from other typical skin
diseases. Therefore, consultation with a dermatologist for
specialist advice and treatment planning is essential. Mild cases
of skin irAEs can affect patients' quality of life, increase
economic burden and reduce compliance with formal immunotherapy.
These adverse events can threaten the safety of the patients' life
in severe cases.
Immune-related pneumonitis is a relatively rare but
potentially life-threatening adverse effect of ICI therapies
(13). The pathophysiology of
immune-related pneumonitis is not fully understood; however, it is
considered to involve increased T cell activity, autoantibody
production, inflammatory cytokine levels and complement-mediated
inflammation (39). A previous
study reported an increased presence of lymphocytes in the
bronchoalveolar lavage fluid of patients with immune-related
pneumonitis, suggesting a role for T lymphocytes and macrophages in
its pathogenesis (39). The
incidence of any-grade immune-related pneumonitis in clinical
studies is ~4% for anti-PD-1 therapies, with high-grade pneumonitis
occurring at a rate of ~1% (40).
Diagnosing immune-related pneumonitis primarily
relies on clinical symptoms, laboratory findings and imaging. When
immune-related pneumonitis is suspected, a chest CT scan is
essential. The most common imaging findings include patterns
similar to organizing pneumonia, with multiple bilateral pulmonary
lesions, such as ground-glass opacities and interstitial lung
involvement (41). In the present
case, the chest CT lesions initially showed ground-glass opacities
and interstitial involvement, and >1 year later, lung
consolidation was observed. In this case, the characteristics of
immune-related pneumonitis include an early acute onset after the
application of ICIs and a relatively severe degree. After the
exclusionary examination, the condition of the patient improved
with sufficient glucocorticoid treatment. However, during the
process of glucocorticoid reduction, the symptoms recurred and were
aggravated, requiring non-invasive ventilator-assisted ventilation.
Considering the rapid reduction of glucocorticoids and
corticosteroid dependence, the use of MMF was added, leading to a
rapid reversal of the disease and achieving good prognosis.
In conclusion, the patient experienced multiple
organ adverse effects, which were effectively alleviated with
proper treatment, leading to a favorable prognosis. After
discontinuing immunotherapy, the patient has maintained a partial
response for >3 years.
IrAEs can affect various organs and exhibit notable
variability in their onset times. Due to the complexity and
diversity of irAEs, clinicians are increasingly encountering both
common and rare types. The characteristics of different diseases
and the treatment trajectories of various cancers can complicate
the outcomes of current retrospective studies. It is crucial to
enhance interdisciplinary cooperation to improve the identification
and management of irAEs. This approach is particularly important
for continuously optimizing the handling of rare adverse effects
such as immune-related cystitis and severe skin-related adverse
events. Recent research has deepened the understanding of the
relationship between irAEs and the efficacy of immunotherapy. A
previous study found that the occurrence and progression of irAEs
are associated with improved immunotherapy responses (42). Patients who develop irAEs generally
show improved treatment responses compared with those who do not
experience such adverse events (43).
Acknowledgements
Not applicable.
Funding
The present study was supported by The Ningbo Science and
Technology Project (grant no. 2017A47) and The Ningbo Natural
Science Foundation (grant nos. 2022J032 and 2023J3870).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and XC designed the study. YW was the principal
person responsible for the study and wrote the original manuscript.
ZF performed analysis and interpretation of CT imaging data. YW, HW
and WY performed a critical literature review and contributed to
the acquisition, analysis and interpretation of data. ZF and WY
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the ethical
standards of The Declaration of Helsinki and its later amendments.
Local ethical approval was obtained from the Ethics Committee of
the Affiliated People's Hospital of Ningbo University (approval no.
2024-N-004; approval date, March 2024; Ningbo, China).
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and images to be published in this
case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Passiglia F, Galvano A, Rizzo S, Incorvaia
L, Listì A, Bazan V and Russo A: Looking for the best
immune-checkpoint inhibitor in pre-treated NSCLC patients: An
indirect comparison between nivolumab, pembrolizumab and
atezolizumab. Int J Cancer. 142:1277–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wakamatsu E, Mathis D and Benoist C:
Convergent and divergent effects of co-stimulatory molecules in
conventional and regulatory CD4+ T cells. Proc Natl Acad Sci USA.
110:1023–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simpson TR, Li F, Montalvo-Ortiz W,
Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H
and Wolchok JD: Fc-dependent depletion of tumor-infiltrating
regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy
against melanoma. J Exp Med. 210:1695–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Tang L, Zhou Y, He W and Li W:
Immune checkpoint Inhibitor-associated pneumonitis in Non-small
cell lung cancer: Current understanding in characteristics,
diagnosis, and management. Front Immunol. 12:6639862021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sibaud V: Dermatologic reactions to immune
checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin
Dermatol. 19:345–361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huerth KA, Hassan S and Callender VD:
Therapeutic insights in melasma and hyperpigmentation management. J
Drugs Dermatol. 18:718–729. 2019.PubMed/NCBI
|
|
11
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE and Ha L: Fatal toxic
effects associated with immune checkpoint inhibitors: A systematic
review and Meta-analysis. JAMA Oncol. 4:1721–1728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
Meta-analysis. Front Pharmacol. 9:14302018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi FS: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
Systematic review and Meta-analysis. JAMA Oncol. 2:1607–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Zhang X, Yao T and Zhang Y and Zhang
Y: Fatal adverse events associated with immune checkpoint
inhibitors in Non-small cell lung cancer: A systematic review and
Meta-analysis. Front Med (Lausanne). 8:6270892021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong L, Ding N, Tong XL, Li J, Zhang Y,
Wang X, Xu X, Ye M, Li C, Wu X, et al: Tumor-derived DNA from
pleural effusion supernatant as a promising alternative to tumor
tissue in genomic profiling of advanced lung cancer. Theranostics.
9:5532–5541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Yang N, Ou QX, Xiang Y, Jiang T,
Wu X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for Illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Depristo MA, Banks E, Poplin R, Garimella
KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA,
Hanna M, et al: A framework for variation discovery and genotyping
using next-generation DNA sequencing data. Nature Genet.
43:491–498. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koboldt DC, Zhang Q, Larson DE, Shen D,
McLellan MD, Lin L, Miller CA, Mardis ER, Ding L and Wilson RK:
VarScan 2: Somatic mutation and copy number alteration discovery in
cancer by exome sequencing. Genome Res. 22:568–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newman AM, Bratman SV, Stehr H, Lee LJ,
Liu CL, Diehn M and Alizadeh AA: FACTERA: a practical method for
the discovery of genomic rearrangements at breakpoint resolution.
Bioinformatics. 30:3390–3393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amarasinghe KC, Li J and Halgamuge SK:
CoNVEX: Copy number variation estimation in exome sequencing data
using HMM. BMC Bioinformatics. 14 (Suppl 2):S22013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen R and Seshan VE: FACETS:
Allele-specific copy number and clonal heterogeneity analysis tool
for high-throughput DNA sequencing. Nucleic Acids Res. 44:e1312016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Institute NC: Common terminology criteria
for adverse events (CTCAE) version 5.0. Accessed October.
15:20212017.
|
|
25
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M and Peters S:
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jayathilaka B, Mian F, Franchini F,
Au-Yeung G and IJzerman M: Cancer and treatment specific incidence
rates of immune-related adverse events induced by immune checkpoint
inhibitors: A systematic review. Br J Cancer. 132:51–57. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan G, Chen W, Khattab S, Roster K, Nguyen
N, Yan B, Rajeh A, Seo J, Rashdan H, Zubiri L, et al: Multi-organ
immune-related adverse events from immune checkpoint inhibitors and
their downstream implications: A retrospective multicohort study.
Lancet Oncol. 25:1053–1069. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of Immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Broen JCA and Van Laar JM: Mycophenolate
mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology.
Nat Rev Rheumatol. 16:167–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roccatello D, Careddu A, Ferro M, Naretto
C, Quattrocchio G, Fenoglio R and Sciascia S: The steroid-sparing
effects of a mycophenolate mofetil-based regimen in the management
of immunoglobulin A nephropathy in patients with histologically
active lesions: A comparison with a control cohort receiving
conventional therapy. J Nephrol. 36:2223–2231. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daetwyler E, Wallrabenstein T, König D,
Cappelli LC, Naidoo J, Zippelius A and Läubli H:
Corticosteroid-resistant immune-related adverse events: A
systematic review. J Immunother Cancer. 12:e0074092024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhat R, Tonutti A, Timilsina S, Selmi C
and Gershwin ME: Perspectives on mycophenolate mofetil in the
management of autoimmunity. Clin Rev Allergy Immunol. 65:86–100.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Snijders R and Stoelinga A: An open-label
randomised-controlled trial of azathioprine vs. mycophenolate
mofetil for the induction of remission in treatment-naive
autoimmune hepatitis. J Hepatol. 80:576–585. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busca A, Locatelli F and Falda M: Safety
profile of mycophenolate mofetil: A response. Bone Marrow
Transplant. 27:8922001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim M, Rostas S and Gabardi S:
Mycophenolate fetal toxicity and risk evaluation and mitigation
strategies. Am J Transplant. 13:1383–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teng YS and Yu S: Molecular mechanisms of
cutaneous Immune-related adverse events (irAEs) induced by immune
checkpoint inhibitors. Curr Oncol. 30:6805–6819. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peter JG, Lehloenya R, Dlamini S, Risma K,
White KD, Konvinse KC and Phillips EJ: Severe delayed cutaneous and
systemic reactions to drugs: A global perspective on the science
and art of current practice. J Allergy Clin Immunol Pract.
5:547–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geisler AN, Phillips GS, Barrios DM, Wu J,
Leung DYM, Moy AP, Kern JA and Lacouture ME: Immune checkpoint
inhibitor-related dermatologic adverse events. J Am Acad Dermatol.
83:1255–1268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu S, Fu Y, Zhu B, Zhang B and Wang J:
Pneumonitis induced by immune checkpoint inhibitors: From clinical
data to translational investigation. Front Oncol. 10:17852020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pillai RN, Behera M, Owonikoko TK,
Kamphorst AO, Pakkala S, Belani CP, Khuri FR, Ahmed R and
Ramalingam SS: Comparison of the toxicity profile of PD-1 versus
PD-L1 inhibitors in non-small cell lung cancer: A systematic
analysis of the literature. Cancer. 124:271–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishino M, Chambers ES, Chong CR, Ramaiya
NH, Gray SW, Marcoux JP, Hatabu H, Jänne PA, Hodi FS and Awad MM:
Anti-PD-1 Inhibitor-related pneumonitis in Non-small cell lung
cancer. Cancer Immunol Res. 4:289–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin L, Liu Y, Chen C, Wei A and Li W:
Association between immune-related adverse events and immunotherapy
efficacy in non-small-cell lung cancer: A meta-analysis. Front
Pharmacol. 14:11900012023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Otsuka H, Kita Y, Ito K, Sano T, Inokuchi
J, Tomida R, Takahashi A, Matsumoto K, Kurahashi R, Ozaki Y, et al:
Immune-related adverse events in urothelial cancer patients:
Adjustment for immortal time bias. Cancer Sci. 113:3912–3921. 2022.
View Article : Google Scholar : PubMed/NCBI
|