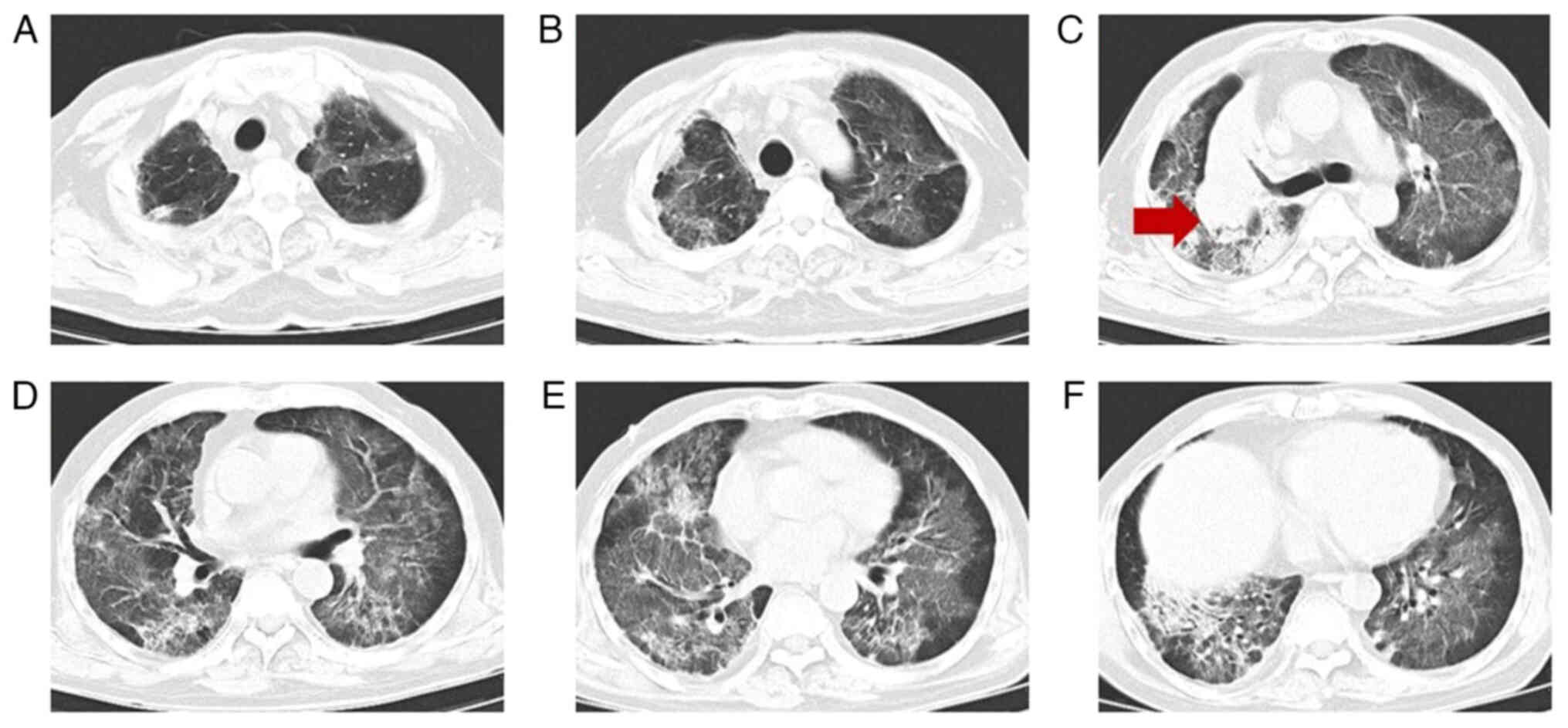
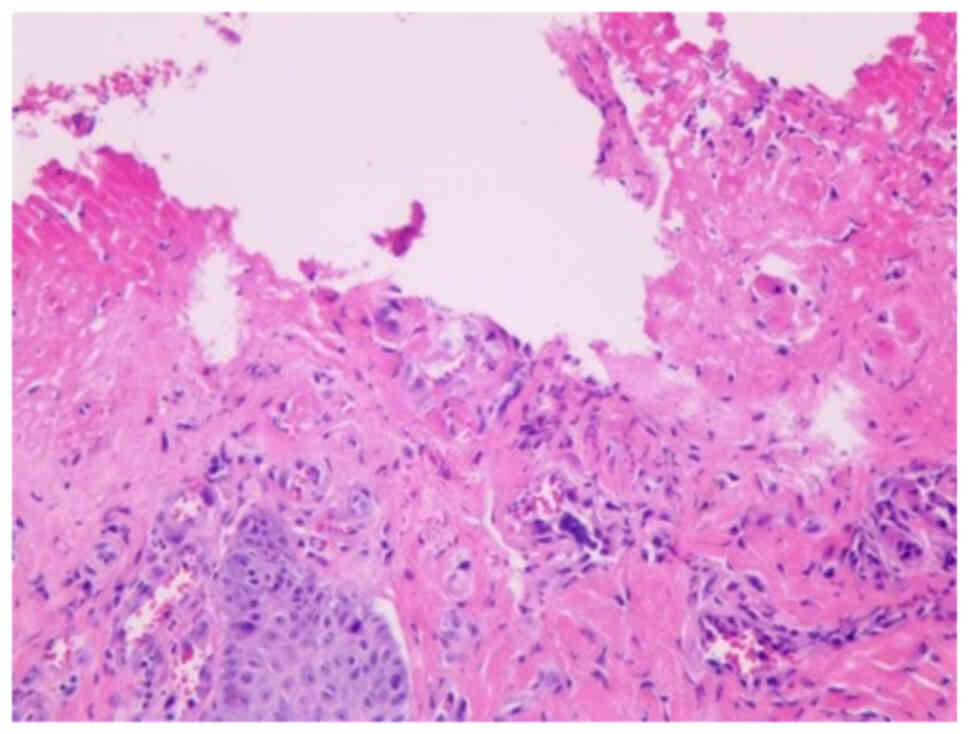
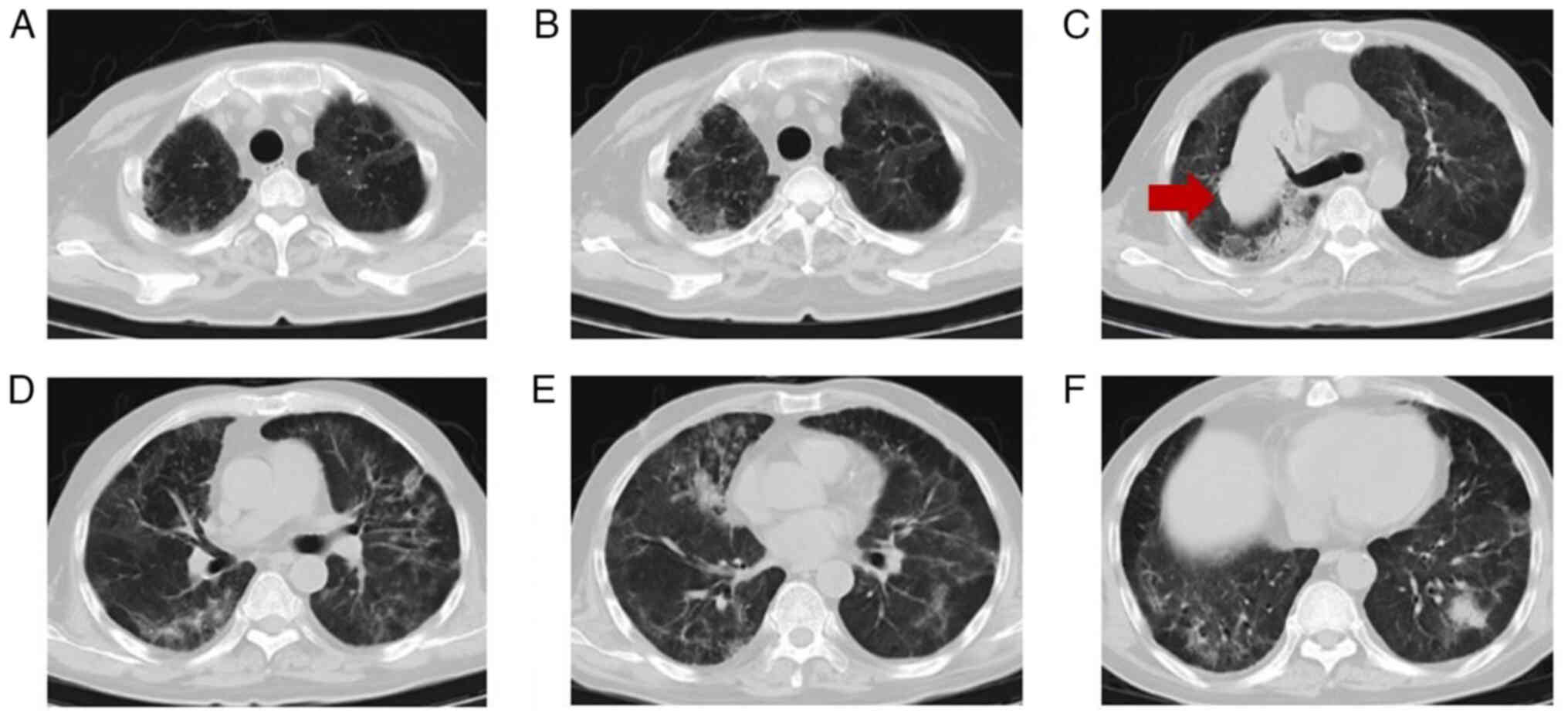
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Passiglia F, Galvano A, Rizzo S, Incorvaia
L, Listì A, Bazan V and Russo A: Looking for the best
immune-checkpoint inhibitor in pre-treated NSCLC patients: An
indirect comparison between nivolumab, pembrolizumab and
atezolizumab. Int J Cancer. 142:1277–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wakamatsu E, Mathis D and Benoist C:
Convergent and divergent effects of co-stimulatory molecules in
conventional and regulatory CD4+ T cells. Proc Natl Acad Sci USA.
110:1023–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simpson TR, Li F, Montalvo-Ortiz W,
Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H
and Wolchok JD: Fc-dependent depletion of tumor-infiltrating
regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy
against melanoma. J Exp Med. 210:1695–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Tang L, Zhou Y, He W and Li W:
Immune checkpoint Inhibitor-associated pneumonitis in Non-small
cell lung cancer: Current understanding in characteristics,
diagnosis, and management. Front Immunol. 12:6639862021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sibaud V: Dermatologic reactions to immune
checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin
Dermatol. 19:345–361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huerth KA, Hassan S and Callender VD:
Therapeutic insights in melasma and hyperpigmentation management. J
Drugs Dermatol. 18:718–729. 2019.PubMed/NCBI
|
|
11
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE and Ha L: Fatal toxic
effects associated with immune checkpoint inhibitors: A systematic
review and Meta-analysis. JAMA Oncol. 4:1721–1728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
Meta-analysis. Front Pharmacol. 9:14302018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishino M, Giobbie-Hurder A, Hatabu H,
Ramaiya NH and Hodi FS: Incidence of programmed cell death 1
inhibitor-related pneumonitis in patients with advanced cancer: A
Systematic review and Meta-analysis. JAMA Oncol. 2:1607–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Zhang X, Yao T and Zhang Y and Zhang
Y: Fatal adverse events associated with immune checkpoint
inhibitors in Non-small cell lung cancer: A systematic review and
Meta-analysis. Front Med (Lausanne). 8:6270892021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong L, Ding N, Tong XL, Li J, Zhang Y,
Wang X, Xu X, Ye M, Li C, Wu X, et al: Tumor-derived DNA from
pleural effusion supernatant as a promising alternative to tumor
tissue in genomic profiling of advanced lung cancer. Theranostics.
9:5532–5541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Yang N, Ou QX, Xiang Y, Jiang T,
Wu X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for Illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Depristo MA, Banks E, Poplin R, Garimella
KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA,
Hanna M, et al: A framework for variation discovery and genotyping
using next-generation DNA sequencing data. Nature Genet.
43:491–498. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koboldt DC, Zhang Q, Larson DE, Shen D,
McLellan MD, Lin L, Miller CA, Mardis ER, Ding L and Wilson RK:
VarScan 2: Somatic mutation and copy number alteration discovery in
cancer by exome sequencing. Genome Res. 22:568–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newman AM, Bratman SV, Stehr H, Lee LJ,
Liu CL, Diehn M and Alizadeh AA: FACTERA: a practical method for
the discovery of genomic rearrangements at breakpoint resolution.
Bioinformatics. 30:3390–3393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amarasinghe KC, Li J and Halgamuge SK:
CoNVEX: Copy number variation estimation in exome sequencing data
using HMM. BMC Bioinformatics. 14 (Suppl 2):S22013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen R and Seshan VE: FACETS:
Allele-specific copy number and clonal heterogeneity analysis tool
for high-throughput DNA sequencing. Nucleic Acids Res. 44:e1312016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Institute NC: Common terminology criteria
for adverse events (CTCAE) version 5.0. Accessed October.
15:20212017.
|
|
25
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M and Peters S:
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jayathilaka B, Mian F, Franchini F,
Au-Yeung G and IJzerman M: Cancer and treatment specific incidence
rates of immune-related adverse events induced by immune checkpoint
inhibitors: A systematic review. Br J Cancer. 132:51–57. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wan G, Chen W, Khattab S, Roster K, Nguyen
N, Yan B, Rajeh A, Seo J, Rashdan H, Zubiri L, et al: Multi-organ
immune-related adverse events from immune checkpoint inhibitors and
their downstream implications: A retrospective multicohort study.
Lancet Oncol. 25:1053–1069. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of Immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Broen JCA and Van Laar JM: Mycophenolate
mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology.
Nat Rev Rheumatol. 16:167–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roccatello D, Careddu A, Ferro M, Naretto
C, Quattrocchio G, Fenoglio R and Sciascia S: The steroid-sparing
effects of a mycophenolate mofetil-based regimen in the management
of immunoglobulin A nephropathy in patients with histologically
active lesions: A comparison with a control cohort receiving
conventional therapy. J Nephrol. 36:2223–2231. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daetwyler E, Wallrabenstein T, König D,
Cappelli LC, Naidoo J, Zippelius A and Läubli H:
Corticosteroid-resistant immune-related adverse events: A
systematic review. J Immunother Cancer. 12:e0074092024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhat R, Tonutti A, Timilsina S, Selmi C
and Gershwin ME: Perspectives on mycophenolate mofetil in the
management of autoimmunity. Clin Rev Allergy Immunol. 65:86–100.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Snijders R and Stoelinga A: An open-label
randomised-controlled trial of azathioprine vs. mycophenolate
mofetil for the induction of remission in treatment-naive
autoimmune hepatitis. J Hepatol. 80:576–585. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busca A, Locatelli F and Falda M: Safety
profile of mycophenolate mofetil: A response. Bone Marrow
Transplant. 27:8922001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim M, Rostas S and Gabardi S:
Mycophenolate fetal toxicity and risk evaluation and mitigation
strategies. Am J Transplant. 13:1383–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teng YS and Yu S: Molecular mechanisms of
cutaneous Immune-related adverse events (irAEs) induced by immune
checkpoint inhibitors. Curr Oncol. 30:6805–6819. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peter JG, Lehloenya R, Dlamini S, Risma K,
White KD, Konvinse KC and Phillips EJ: Severe delayed cutaneous and
systemic reactions to drugs: A global perspective on the science
and art of current practice. J Allergy Clin Immunol Pract.
5:547–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geisler AN, Phillips GS, Barrios DM, Wu J,
Leung DYM, Moy AP, Kern JA and Lacouture ME: Immune checkpoint
inhibitor-related dermatologic adverse events. J Am Acad Dermatol.
83:1255–1268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu S, Fu Y, Zhu B, Zhang B and Wang J:
Pneumonitis induced by immune checkpoint inhibitors: From clinical
data to translational investigation. Front Oncol. 10:17852020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pillai RN, Behera M, Owonikoko TK,
Kamphorst AO, Pakkala S, Belani CP, Khuri FR, Ahmed R and
Ramalingam SS: Comparison of the toxicity profile of PD-1 versus
PD-L1 inhibitors in non-small cell lung cancer: A systematic
analysis of the literature. Cancer. 124:271–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishino M, Chambers ES, Chong CR, Ramaiya
NH, Gray SW, Marcoux JP, Hatabu H, Jänne PA, Hodi FS and Awad MM:
Anti-PD-1 Inhibitor-related pneumonitis in Non-small cell lung
cancer. Cancer Immunol Res. 4:289–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin L, Liu Y, Chen C, Wei A and Li W:
Association between immune-related adverse events and immunotherapy
efficacy in non-small-cell lung cancer: A meta-analysis. Front
Pharmacol. 14:11900012023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Otsuka H, Kita Y, Ito K, Sano T, Inokuchi
J, Tomida R, Takahashi A, Matsumoto K, Kurahashi R, Ozaki Y, et al:
Immune-related adverse events in urothelial cancer patients:
Adjustment for immortal time bias. Cancer Sci. 113:3912–3921. 2022.
View Article : Google Scholar : PubMed/NCBI
|