Introduction
Epithelioid hemangioendothelioma is a rare vascular
tumour that can occur in any part of the body, including the bones,
lungs and liver. The liver is the most common site (1). Hepatic epithelioid
hemangioendothelioma (HEHE) is a rare, low-grade malignant tumour
(2). Its global prevalence is <1
in 10 million and the average age of onset is 41.7 years (3). Women are more likely to experience
HEHE, with a male-to-female ratio of 3:2. It affects the right lobe
of the liver more than the left lobe (4). Compared with other forms of liver
cancer, HEHE progresses at a relatively slow rate and generally has
a favourable prognosis (5,6). Since HEHE is usually multifocal at the
time of initial diagnosis, with indolent or even aggressive
progression of the disease and a tendency to recur and metastasise,
therapeutic intervention is typically required (7). There is currently no standard
treatment for this condition. Surgical resection is a crucial
treatment modality that facilitates long-term survival (5). This study presents the case of a young
patient with multiple intrahepatic HEHE lesions with the aim of
enhancing the current understanding of the diagnosis and management
of multiple HEHE foci. The importance of liver resection in the
treatment of this condition was emphasised. In addition, the study
recommends the integration of preoperative three-dimensional
reconstruction with intraoperative ultrasonography (IOUS) during
radical resection. This combination may enhance the accuracy of
localising multifocal lesions and improve the thoroughness of the
procedure. Ultimately, the objective of this approach was to
provide a more effective surgical strategy for patients with
multifocal HEHE and improve their prognosis.
Case report
Case presentation
In July 2024, a 23-year-old male patient was
admitted to the First Hospital of Lanzhou University (Lanzhou,
China) after having had a liver mass for over a month. Upon
examination and laboratory tests conducted after hospitalisation,
the following results were noted: All vital signs of the patient
were in the normal ranges and physical examination revealed no
obvious abnormalities. Laboratory tests revealed some abnormal
results: Gamma-glutamyl transferase, 96.3 U/l (normal range: 10–60
U/l); direct bilirubin, 4.1 µmol/l (normal range: 0–4 µmol/l) and
abnormal plasminogen, 13.2 mAU/ml (normal range: 13.62–40.38
mAU/ml). Other laboratory results, including total bilirubin,
albumin, prothrombin time, aspartate aminotransferase, alanine
aminotransferase, alkaline phosphatase, alpha-fetoprotein,
carcinoembryonic antigen and glycoconjugate antigen 19-9, were
within normal limits. The patient had a Child-Pugh liver function
score of 5, corresponding to grade A (Table I). Furthermore, the patient had no
medical or family history of hepatitis or alcohol consumption.
 | Table I.The patient had a Child-Pugh liver
function score of 5, corresponding to grade A. |
Table I.
The patient had a Child-Pugh liver
function score of 5, corresponding to grade A.
| Child-Pugh Grading
Indicator | Result | Child-Pugh grading
score |
|---|
| Total bilirubin | <34μmol/L | 1 |
| Albumin | >35g/L | 1 |
| Prothrombin time
prologation | <4 seconds | 1 |
| Hydroperitoneum | No | 1 |
| Hepatic
encephalopathy | No | 1 |
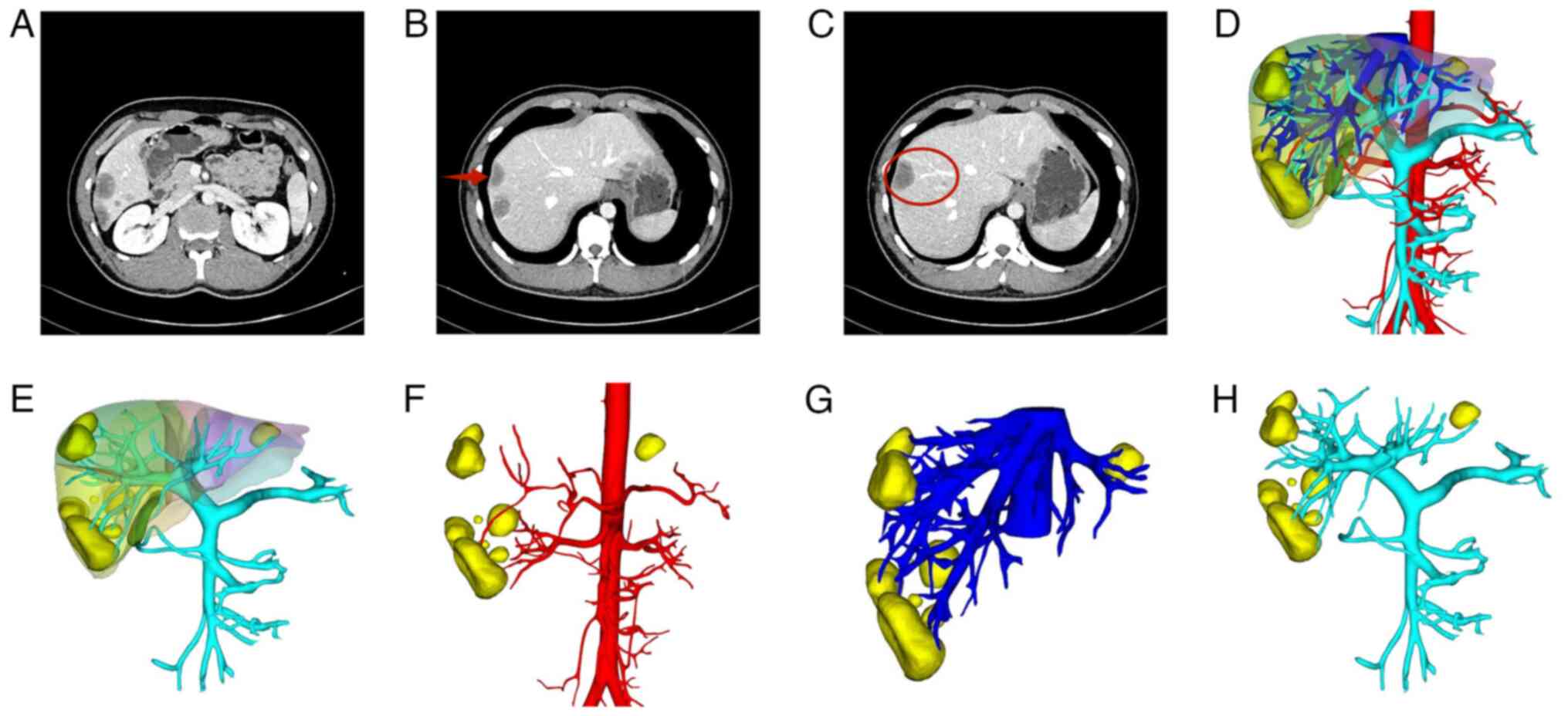
A contrast-enhanced computed tomography (CT) scan of
the upper abdomen, enhanced using the contrast agent iopromide,
along with a three-dimensional reconstruction of the hepatic
vessels, revealed multiple rounded areas of abnormal enhancement
beneath the hepatic capsule (Fig.
1A). There was also evidence of hepatic capsular retraction
(Fig. 1B) and a partial
manifestation of the ‘lollipop’ (Fig.
1C) and ‘fried egg’ signs. Three-dimensional reconstruction of
the liver was performed based on CT scans to clarify the
relationship between the lesions and the surrounding tissues
(Fig. 1D-H). Furthermore,
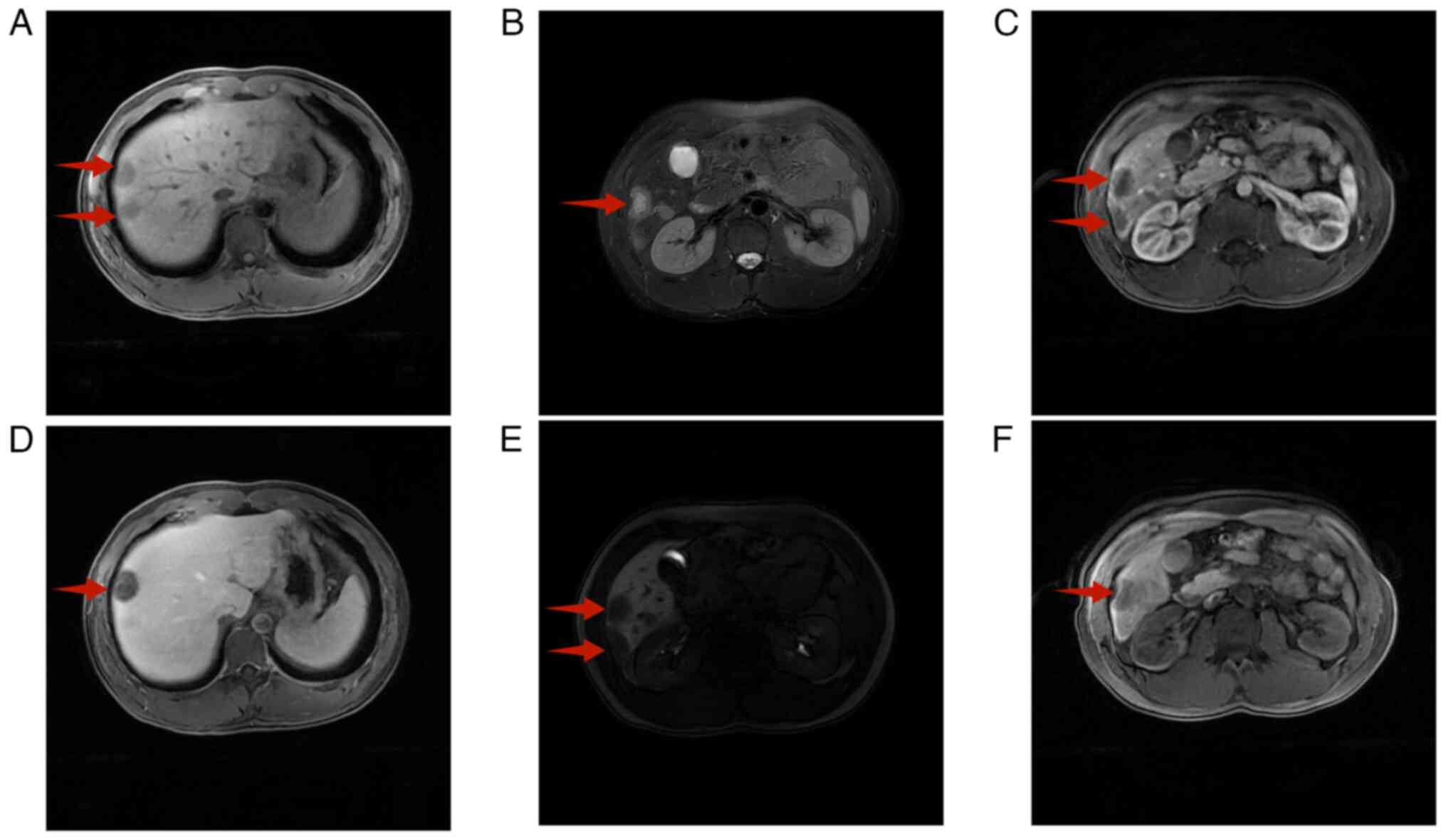
liver-specific magnetic resonance imaging (MRI), enhanced with the
contrast agent gadoxetic acid disodium, identified multiple round
shadows with long T1 and T2 signals at the periphery of the liver
adjacent to the capsule. A heightened T2 signal was evident at the
centre, accompanied by halo signs at the periphery (Fig. 2A and B). Enhancement revealed an
increase in the arterial phase at the periphery (Fig. 2C), whereas progressive enhancement
was observed in the portal vein phase (Fig. 2D).
After a discussion with a multidisciplinary team
(MDT), a diagnosis of HEHE with multiple foci was established. At
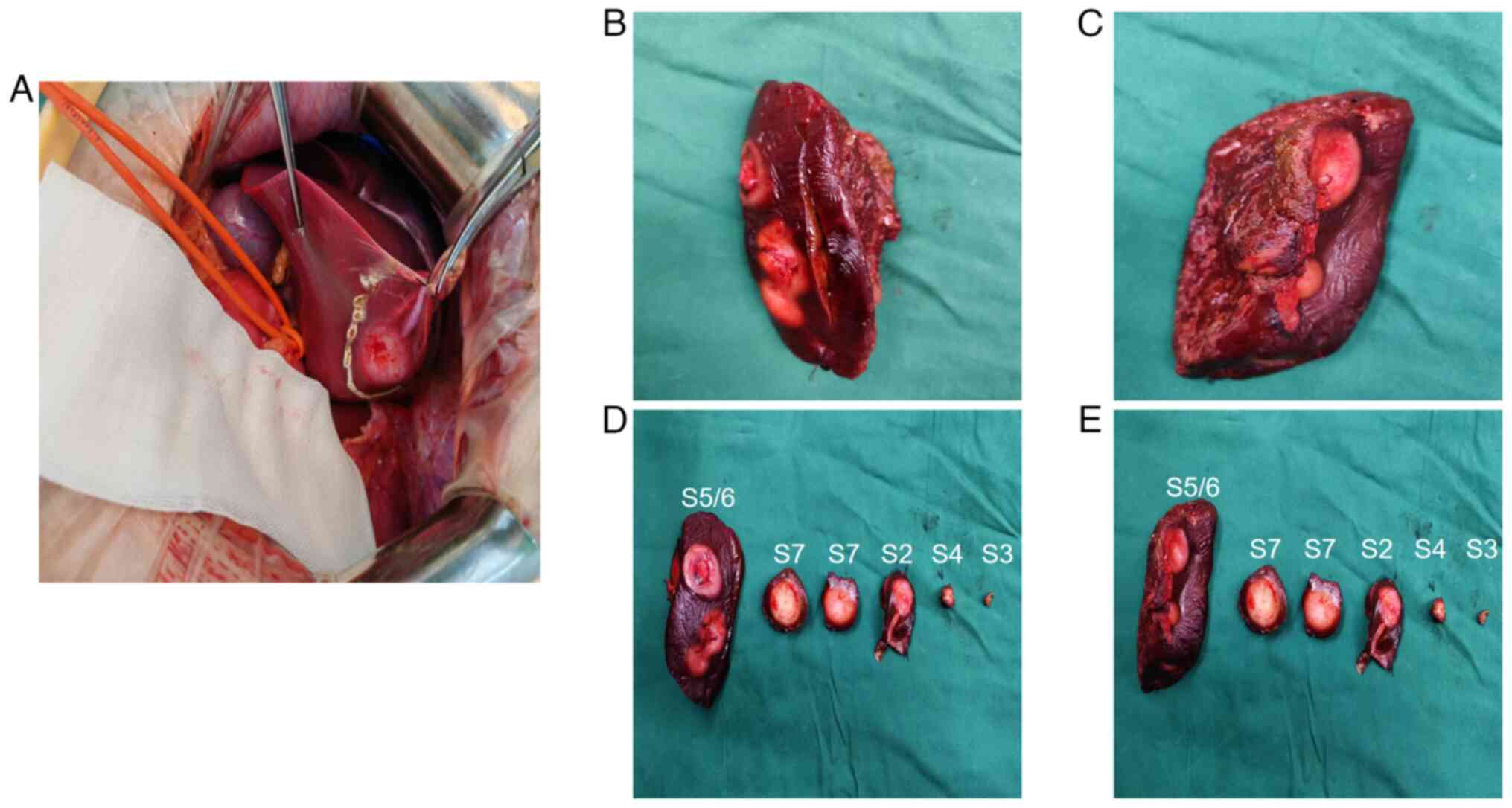
eight days after admission, open hepatic resection was performed
under general anaesthesia to excise masses located in segments S2,
S3, S4, S5, S6 and S7 (Fig. 3A-E).
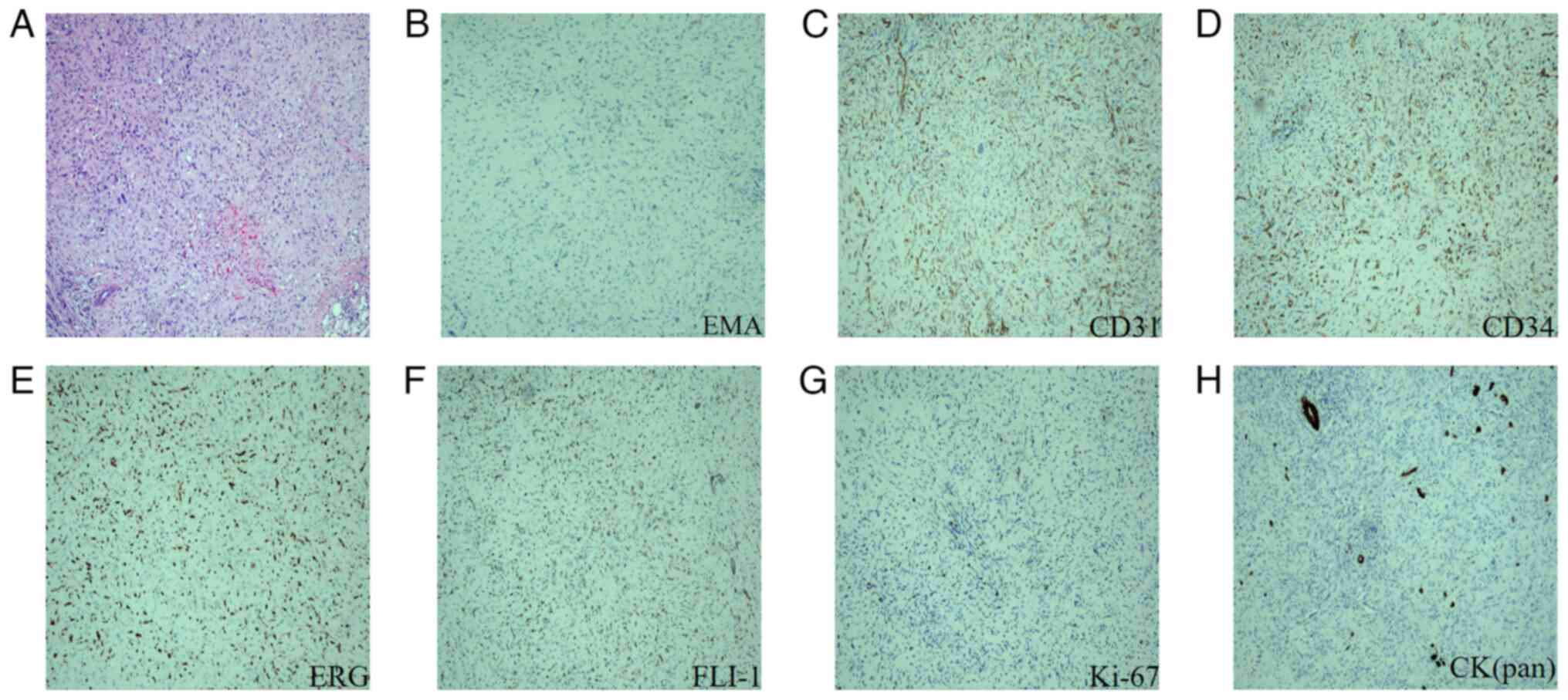
The postoperative pathological diagnosis confirmed the presence of
HEHE (Fig. 4A). The results of the
immunohistochemical analysis were as follows: Epithelial membrane
antigen (EMA) (Fig. 4B) (−),
platelet endothelial cell adhesion molecule-1 (CD31) (+) (Fig. 4C), hematopoietic progenitor cell
antigen (CD34) (+) (Fig. 4D), Ets
related gene (ERG) (+) (Fig. 4E),
friend leukemia virus integration-1 (FLI-1) (+) (Fig. 4F), Kiel-67 antigen (Ki-67) (10%)
(Fig. 4G), cytokeratin pan [CK
(Pan)] (focal+) (Fig. 4H),
cytokeratin 8&18 (CK8&18) (partially+) and transcription
factor E3 (TFE3) (±). The patient was discharged on postoperative
day 6 with satisfactory recovery.
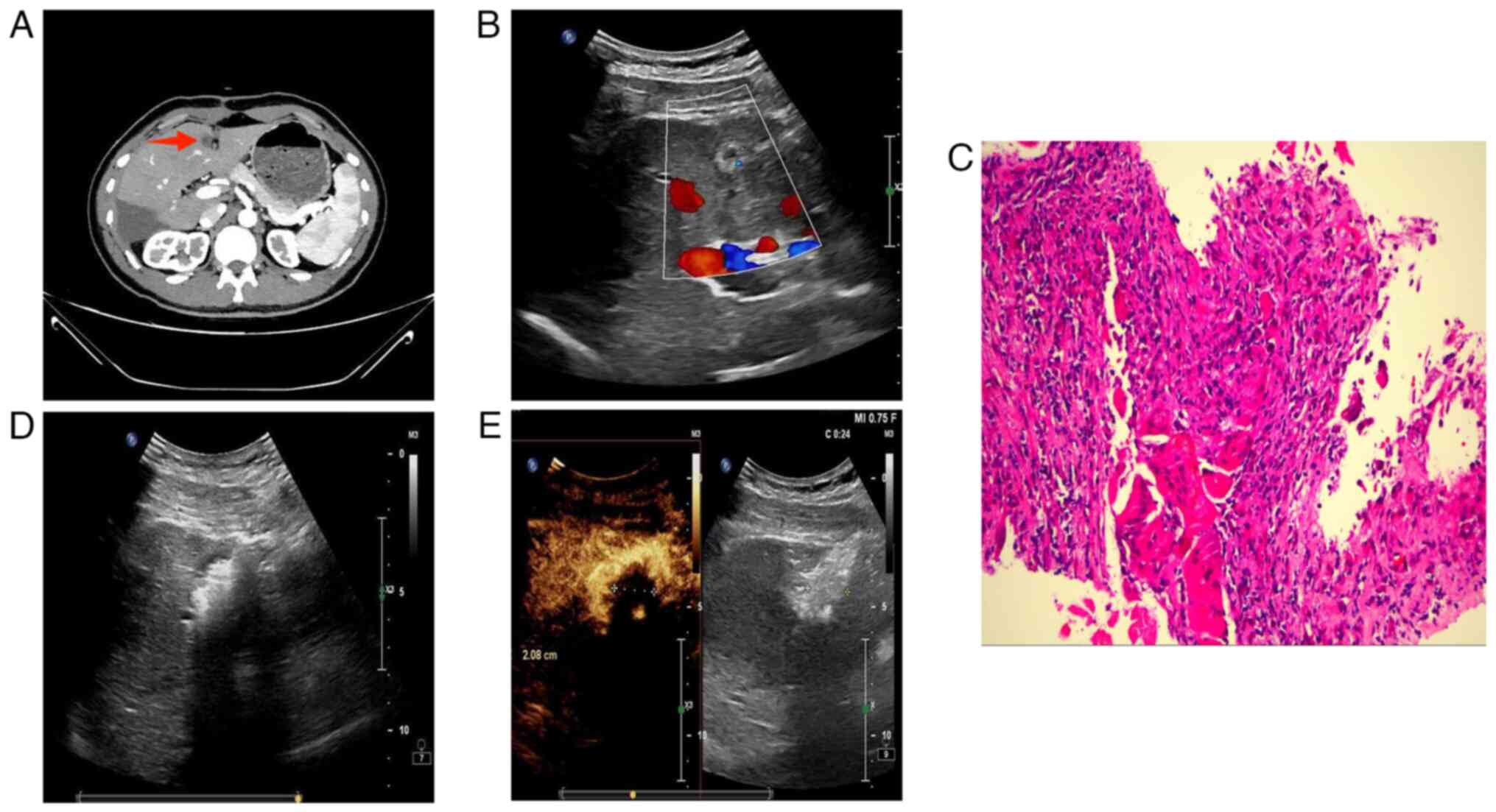
The patient returned to the medical facility for a
follow-up examination three months post-surgery. An
iodixanol-enhanced CT scan of the upper abdomen revealed a rounded,
slightly hypodense shadow in the S4 segment of the liver. This
shadow exhibited mild circular enhancement and was ~11 mm in
diameter (Fig. 5A). Following an
MDT discussion, this was identified as a new HEHE lesion.
Percutaneous hepatic mass aspiration biopsy and microwave ablation
(MWA) were performed under ultrasound guidance (Fig. 5B and D). The procedure was completed
without complications and postoperative ultrasonography revealed
complete tumour inactivation (Fig.
5E). Biopsy results indicated the absence of HEHE tumour cells
(Fig. 5C). The patient recovered
after surgery and was discharged from the hospital. Regular
follow-up visits will be scheduled to assess the prognosis.
Haematoxylin-eosin staining and
immunohistochemistry
After tissue sampling, the specimens were fixed in a
10% neutral formalin solution at room temperature for 6 to 24 h.
This was followed by dehydration using alcohol solutions and
clearing using xylene at room temperature. The samples were then
placed in melted paraffin wax at a temperature of 60 to 65°C,
dipped 2 to 3 times for 1 to 2 h each time. After embedding, the
wax block was cut into 3-µm sections, which were then subjected to
xylene dewaxing and alcohol hydration at room temperature. Finally,
staining was performed using hematoxylin and eosin at room
temperature. The hematoxylin staining lasted for 5 to 10 min,
followed by eosin staining for 1 to 3 min.
Standard procedures were followed for
immunohistochemistry. The primary antibodies used were as follows:
EMA (ready-to-use; cat. no. Kit-0011; Fuzhou Maixin Biotechnology
Development Co., Ltd), CK(Pan) (ready-to-use; cat. no. MAB-0671;
Fuzhou Maixin Biotechnology Development Co., Ltd), CK8&18
(ready-to-use; cat. no. CCM-1012; CELNOVTE), CD31 (ready-to-use;
cat. no. PA007; ABCARTA), CD34 (ready-to-use; catalog no. Kit-0004;
Fuzhou Maixin Biotechnology Development Co., Ltd), ERG
(ready-to-use; cat. no. RMA-0748; Fuzhou Maixin Biotechnology
Development Co., Ltd), FLI-1 (ready-to-use; cat. no. MAB-0649;
Fuzhou Maixin Biotechnology Development Co., Ltd), TFE3
(ready-to-use; cat. no. RMA-0663; Fuzhou Maixin Biotechnology
Development Co., Ltd), Ki-67 (ready-to-use; cat. no. 790-4286;
Roche Diagnostics). The secondary antibody was a horseradish
peroxidase-labeled antibody multimer (ready-to-use; cat. no.
760-500; Roche Diagnostics). All images above were obtained using
an upright microscope (BX43; Olympus Corp.).
Discussion
The aetiology of HEHE, a rare malignant tumour
derived from endothelial cells (8),
remains poorly understood. However, factors such as viral
hepatitis, liver trauma and exposure to certain chemicals
(including oral contraceptives, polyvinyl chloride, asbestos and
contrast agents) have been implicated as possible contributors to
the development of HEHE (9). A
common genetic abnormality associated with HEHE is a reciprocal
translocation involving chromosome t(1;3)(p36.3;q25), which leads
to the fusion of the WW domain-containing transcription coactivator
1 (WWTR1) gene with the calmodulin-binding transcription activator
1 (CAMTA1) gene (10). A small
proportion of patients may also exhibit t(11;X)(q13;p11)
translocation, resulting in the fusion of the Yes-associated
protein 1 (YAP1) gene with the TFE3 (11). YAP1 and Transcriptional co-activator
with PDZ-binding motif (TAZ) (the product of WWTR1) act as
co-transcription factors and are key components of the Hippo
signalling pathway, suggesting that this pathway plays a
significant role in the development of HEHE (12,13).
Furthermore, mutations in the KMT2A, SMARCA4, BAP1, MTOR and
NOTCH1 genes have been identified in HEHE, which could serve
as potential therapeutic targets (14). HEHE is frequently characterised by
the lack of conventional clinical symptoms and specific diagnostic
indicators, and ~68% of patients are asymptomatic at diagnosis
(15). Certain patients may present
with Budd-Chiari syndrome and exhibit clinical signs related to
portal hypertension, such as epigastric pain, abdominal distension,
loss of appetite, fatigue, splenomegaly, jaundice and ascites.
These symptoms are typically associated with tumour invasion of the
hepatic vascular system (16,17).
Enhanced CT scans may reveal the distinctive
‘lollipop’ sign, which is characterised by low-density lesions
(resembling the candy of a lollipop) and occluded blood vessels
(comparable to the lollipop stick). This distinctive finding is
uncommon in other benign and malignant liver tumours (4). MRI revealed that HEHE exhibits
low-signal intensity on T1-weighted images and high signal
intensity on T2-weighted images (9). Additionally, peripheral enhancement
was observed in the hepatic artery phase, with progressive
enhancement noted in the portal vein phase (3). Reportedly, the ‘core pattern’ of
low-signal centres in the hepatocellular phase can also serve as a
distinctive imaging marker of HEHE on MRI (18).
The definitive diagnosis of HEHE relies on
histopathological results. Tumour cells exhibit invasive growth
patterns and comprise epithelioid, dendritic and intermediate
cells, as evidenced by haematoxylin-eosin staining (6). Immunohistochemistry indicates that
most patients are positive for CAMTA1, factor VIII related antigen,
vimentin, CD31, CD34 and D2-40, with a minority showing TFE3
positivity (3,17). CD31 has high specificity for HEHE
diagnosis, while CD34 has high sensitivity. Notably, CAMTA1 is a
critical marker for HEHE diagnosis, exhibiting both high
specificity and sensitivity (6). By
contrast, hepatocellular carcinomas, metastatic carcinomas and
intrahepatic cholangiocarcinomas commonly express cytokeratin,
whereas CD31 and CD34 are less frequently detected (19). Distinguishing HEHE from
haemangiosarcomas, both of which may express CD31 and CD34, poses
additional challenges (20).
Haemangiosarcoma, a high-grade malignant tumour, typically exhibits
considerable nuclear pleomorphism, heterogeneity and substantial
mitotic activity (15). CAMTA1 was
present in the cytosolic nucleus in ~85% of HEHE cases, providing
high sensitivity and specificity for effectively differentiating
HEHE from angiosarcoma (6,20). Unfortunately, CAMTA1
immunohistochemistry could not be performed for the patient in this
study due to unavailability of the CAMTA1 antibody at our hospital.
However, other positive indicators in the immunohistochemistry
programme, such as CD31, CD34, ERG and FLI-1, and the imaging and
pathological findings, fully supported the diagnosis of HEHE.
Classical HEHE is frequently associated with a WWTR1-CAMTA1 fusion,
accounting for ~90% of cases, whereas subtypes with a YAP1-TFE3
fusion are uncommon. In such cases, immunohistochemistry is usually
positive for TFE3 (11,21). Therefore, TFE3 immunostaining has
been suggested as an effective method to differentiate classical
HEHE from its subtypes (22).
Although positive TFE3 immunostaining does not directly imply a
YAP1-TFE3 fusion phenotype, it highly correlates with the phenotype
(23).
The scarcity of literature and the rarity of HEHE
have restricted our understanding of this disease. Currently, the
most commonly used therapeutic modality for HEHE encompasses
hepatectomy, liver transplantation, chemotherapy and follow-up
monitoring. These modalities have been associated with 5-year
survival rates of 75, 54.5, 30.0 and 4.5%, respectively (16). Furthermore, patients who received
therapeutic interventions experienced prolonged survival compared
with those who did not (17).
Surgical intervention, primarily involving partial
hepatectomy and liver transplantation, is currently the only
effective treatment for HEHE. For single or multiple lesions that
can be excised, liver resection is the preferred treatment for
HEHE, given that it not only achieves radical tumour resection but
is also strongly associated with optimal prognosis (6). Conversely, liver transplantation is an
appropriate treatment option for diffuse multifocal disease that
cannot be effectively managed surgically (3). Specifically, hepatectomy should be
preferred in patients with tumour diameter ≤10 cm and ≤10 lesions,
whereas liver transplantation is a more appropriate treatment
strategy in the presence of >10 lesions (24). Zhao and Yin (17) reported that radical hepatectomy with
negative margins was the optimal treatment option for HEHE. The
average survival time of patients who underwent hepatectomy was
158.6 months, which was markedly longer than that of patients who
underwent liver transplantation (147.3 months). However, owing to
the insidious nature of HEHE, its clinical diagnosis is typically
established in the mid-to-late stages. Furthermore, 66.6–87% of
cases exhibit a multinodular or diffuse distribution, making it
difficult to achieve radical hepatic resection (6). Therefore, liver transplantation is
considered the treatment of choice for patients with inoperable or
diffuse HEHE (17). In clinical
practice, a minimum standard of 50% survival after liver
transplantation is universally achievable (15). Even with extrahepatic metastases,
patients have been shown to achieve survival rates of 80 and 70% at
three and five years after liver transplantation, respectively
(25). However, there are numerous
limitations to the application of liver transplantation, including
high cost, donor shortage, unpredictable biological behaviour of
tumours and risk of postoperative recurrence (5,9). Liver
transplantation was found to be associated with a longer operative
time and hospital stay, more intraoperative blood loss, a higher
risk of postoperative infection and substantially higher mortality
rates of 1–5% in the early stage (≤3 months) and 22% in the late
stage (>3 months) than after hepatectomy (0–3 %) (26). Considering the clinical features of
the current patient, including normal liver function, <10
lesions and the largest lesion diameter <10 cm, all lesions were
completely resected, and the decision to perform a hepatectomy was
reached. Patients with HEHE who are non-candidates for surgery can
be treated with chemotherapeutic drugs such as adriamycin,
5-fluorouracil, platinum and cyclophosphamide (17). However, no specific chemotherapeutic
agents have been identified to treat HEHE. Furthermore, combining
anti-vascular endothelial growth factor drugs with cell cycle
inhibitors, such as bevacizumab, capecitabine, cyclophosphamide and
doxorubicin, was found to be an effective treatment for HEHE
(9).
Image-guided MWA and radiofrequency ablation (RFA)
are safe and effective in treating HEHE, achieving a technical
success rate of 93.5%. The overall survival rates for patients
treated with ablation at 1, 3 and 5 years were 87.6, 75.5 and
75.5%, respectively, comparable to the prognosis following liver
resection or liver transplantation (7). While both RFA and MWA induce tumour
necrosis through thermal effects, their heat-generating mechanisms
differ. RFA primarily relies on ion excitation and energetic
collisions, whereas MWA utilises dielectric heating to convert
electromagnetic energy into thermal energy (27). MWA operates at higher frequencies
and shorter wavelengths than RFA, which allows it to reduce
ablation time and increase the ablation area. Additionally, the
ablation effect in MWA is not influenced by tissue impedance,
making it suitable for solid organs and dry tissues while
minimising temperature drops at the margins that could occur due to
the heat sink effect (28). The
main complications associated with MWA stem from insufficient
control of the microwave radiation field and may include vascular,
biliary, mechanical and infectious complications. However,
developing new technologies, such as temperature-controlled
microwave irradiation systems, is anticipated to reduce these
risks. Therefore, MWA remains a safe and effective method for liver
tumour ablation, particularly for lesions smaller than 3 cm in
diameter. It has a high complete ablation rate and a low incidence
of complications (29). In the
current case, ultrasound-guided MWA was used to successfully treat
a suspected neoplastic lesion in the S4 segment, resulting in
complete tumour ablation without any complications.
Despite multiple lesions, preoperative CT, MRI and
three-dimensional reconstruction may indicate that radical
resection of HEHE with multiple lesions could be achieved.
Preoperative three-dimensional reconstruction can accurately assess
the number and location of lesions while considering their spatial
relationship with the hepatic vasculature, thereby facilitating the
development of an optimal resection plan. This approach also helps
preserve postoperative arterial blood supply and venous drainage
(30). In addition, IOUS enables
precise lesion localisation during excision. IOUS uses a
high-frequency ultrasound probe positioned directly on the liver
surface to localise lesions and evaluate surgical margins (31). Imaging plays a pivotal role in HEHE
management. Enhanced CT and MRI techniques serve as the foundation
for accurate diagnosis and evaluation of surgical feasibility.
Combining preoperative three-dimensional reconstruction and IOUS
allows for complete and accurate resection of all multiple HEHE
lesions during the intraoperative period.
Although a tumour number ≥4 was unrelated to tumour
recurrence, a tumour diameter ≥4 cm was associated with a higher
risk of tumour recurrence (1).
Given the unpredictable biological behaviour of tumours, regular
postoperative follow-up is essential for the timely detection of
recurrent lesions. Na et al (1) suggested that patients with junctional
tumours who have undergone hepatectomy should be followed up every
3–4 months for the first year after surgery and every 4–8 months
thereafter. Furthermore, monitoring patients with HEHE every 3
months for the first 2 years after hepatectomy has been
recommended, followed by every 6 months thereafter (32). Based on these recommendations, a
follow-up schedule was established: Every 3 months for the first
year post-surgery and every 6 months thereafter. A review three
months after surgery revealed an abnormal enhancement in the S4
segment of the liver. The lesion was identified as a new HEHE owing
to its considerable distance from the initial surgical resection
site. However, the biopsy did not provide conclusive evidence of
HEHE tumour cells. Nevertheless, owing to the inherent limitations
of puncture biopsy and the inability to completely rule out tumour
recurrence, the team elected to treat the patient of the present
study with MWA to avoid delays. Patients aged ≥65 years, those with
a tumour diameter exceeding 10 cm, those with hepatic dysfunction
and those with intrahepatic metastases (diffuse) were associated
with a poor prognosis (6,17). In the present study, the patient was
a 23-year-old male with normal liver function but with multiple
intrahepatic lesions involving six liver segments, including
tumours with a maximum diameter of ≥4 cm. The patient's prognosis
warrants further follow-up.
Although HEHE is a rare, low-grade malignant tumour
of the liver, CT and MRI examinations reveal distinctive features
that distinguish it from other liver tumours. These imaging
techniques are crucial for preoperative diagnosis and help
determine the appropriate diagnostic and treatment options. When
considering surgical resection for multifocal HEHE,
three-dimensional reconstruction and IOUS can enhance the accuracy
of resection of all lesions. Patients with HEHE who undergo
hepatectomy should be regularly monitored to detect recurrent or
new lesions. MWA is a safe and effective treatment option that
yields favourable results for solitary HEHE lesions <3 cm in
diameter.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation
of Gansu Province (grant no. 22JR11RA023).
Availability of data and materials
The data generated in the present study are included
in the figures and table of this article.
Authors' contributions
JWM performed literature review and drafted and
edited the manuscript. KXZ and ZLZ advised on patient treatment and
performed the surgery. BP and SW collected and analyzed medical
images (e.g. ultrasound, CT, MRI and three-dimensional
reconstruction). YZ collected and analyzed pathological images
(e.g. haematoxylin-eosin staining and Immunohistochemistry). JWM,
ZLZ and KXZ contributed to data analysis and interpretation. All
authors have read and approved the final version of the manuscript.
JWM and KXZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Institutional
Ethics Committee of the First Hospital of Lanzhou University
(approval no. LDYYLL-2025-21; Lanzhou, China).
Patient consent for publication
The patient provided written informed consent for
publication, authorizing the use of their imaging, pathological and
clinical data for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Na BG, Hwang S, Ahn CS, Kim KH, Moon DB,
Ha TY, Song GW, Jung DH, Hong SM and Lee SG: Post-resection
prognosis of patients with hepatic epithelioid
hemangioendothelioma. Ann Surg Treat Res. 100:137–143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawka M, Mak S, Qiu S, Gall TMH and Jiao
LR: Hepatic epithelioid hemangioendothelioma (HEHE)-rare vascular
malignancy mimicking cholangiocarcinoma: A case report. Transl
Gastroenterol Hepatol. 7:422022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Zhang R, Liu Y, Min Q, Zeng Q and
Liu J: Hepatic epithelioid hemangioendothelioma a case report and
literature review. Int J Surg Case Rep. 104:1079262023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Virarkar M, Saleh M, Diab R, Taggart M,
Bhargava P and Bhosale P: Hepatic hemangioendothelioma: An update.
World J Gastrointest Oncol. 12:248–266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Zhou R, Si S, Liu L, Yang S, Han D
and Tan H: Sirolimus combined with interferon-alpha 2b therapy for
giant hepatic epithelioid hemangioendothelioma: A case report.
Front Oncol. 12:9723062022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng L, Li M, Huang Z and Xu M: Hepatic
epithelioid hemangioendothelioma-a single-institution experience
with 51 cases. Front Oncol. 13:12361342023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Q, Luo Y, Yu J, Li X, Jiang TA, Xie
X, Dong G and Liang P: Image-Guided thermal ablation for hepatic
epithelioid hemangioendothelioma: A multicenter experience. J Vasc
Interv Radiol. 35:1004–1011. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gurung S, Fu H, Zhang WW and Gu YH:
Hepatic epithelioid hemangioendothelioma metastasized to the
peritoneum, omentum and mesentery: A case report. Int J Clin Exp
Pathol. 8:5883–5889. 2015.PubMed/NCBI
|
|
9
|
Mo WF and Tong YL: Hepatic epithelioid
hemangioendothelioma after thirteen years' follow-up: A case report
and review of literature. World J Clin Cases. 10:6119–6127. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mundada AD, Deodhar K, Ramadwar M, Bal M
and Kumar R: Hepatic epithelioid hemangioendothelioma: A
clinocopathological correlation. Indian J Pathol Microbiol.
65:133–136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng K, Yang X, Guo S and Tao J: Hepatic
epithelioid hemangioendothelioma with TFE3 rearrangement: A case
report and literature review. Front Oncol. 15:14422332025.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyriazoglou A, Koutsoukos K, Zagouri F,
Liontos M, Dimitriadis E, Tiniakos D and Dimopoulos MA: Metastatic
hepatic epithelioid hemangioendothelioma treated with olaratumab: A
falling star rising? Ther Clin Risk Manag. 16:141–146. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamar JM, Nehru VM and Weinberg G:
Epithelioid hemangioendothelioma as a model of YAP/TAZ-driven
cancer: Insights from a rare fusion sarcoma. Cancers (Basel).
10:2292018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mogler C, Koschny R, Heilig CE, Frohling
S, Schirmacher P, Weichert W and Pfarr N: Molecular
characterization of hepatic epithelioid hemangioendothelioma
reveals alterations in various genes involved in DNA repair,
epigenetic regulation, signaling pathways, and cell cycle control.
Genes Chromosomes Cancer. 59:106–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanduzzi-Zamparelli M, Rimola J, Montironi
C, Nunes V, Alves VAF, Sapena V, da Fonseca LG, Forner A, Carrilho
FJ, Díaz A, et al: Hepatic epithelioid hemangioendothelioma: An
international multicenter study. Dig Liver Dis. 52:1041–1046. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Studer LL and Selby DM: Hepatic
epithelioid hemangioendothelioma. Arch Pathol Lab Med. 142:263–267.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M and Yin F: Hepatic epithelioid
hemangioendothelioma: Clinical characteristics, diagnosis,
treatment, and prognosis. World J Clin Cases. 10:5606–5619. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Zhou Y and Zhang J: A rare
hepatic epithelioid hemangioendothelioma in a cirrhotic liver. Balk
Med J. 38:394–396. 2021. View Article : Google Scholar
|
|
19
|
Lieu DQ, Anh TN, Luan DT, Quynh MT and Duc
NM: A rare case of hepatic epitheliod hemangioendothelioma. Radiol
Case Rep. 18:1695–1699. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolleri JJ, Khaliq A, Ladumor SB,
Habtezghi AB, Koshy SM and Petkar M: Primary hepatic epithelioid
hemangioendothelioma masquerading as a hepatic abscess with
infective picture: A case report. Cureus. 14:e228592022.PubMed/NCBI
|
|
21
|
Lotfalla MM, Folpe AL, Fritchie KJ, Greipp
PT, Galliano GG, Halling KC, Mounajjed T, Torres-Mora J and Graham
RP: Hepatic YAP1-TFE3 rearranged epithelioid hemangioendothelioma.
Case Rep Gastrointest Med. 2019:75308452019.PubMed/NCBI
|
|
22
|
Kou K, Chen YG, Zhou JP, Sun XD, Sun DW,
Li SX and Lv GY: Hepatic epithelioid hemangioendothelioma: Update
on diagnosis and therapy. World J Clin Cases. 8:3978–3987. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shishimoto T, Oura S, Motozato K, Tanaka
H, Takamatsu S and Ono W: Epithelioid hemangioendothelioma of the
liver showing spontaneous complete regression after the cessation
of methotrexate intake. Case Rep Oncol. 16:628–633. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grotz TE, Nagorney D, Donohue J, Que F,
Kendrick M, Farnell M, Harmsen S, Mulligan D, Nguyen J, Rosen C and
Reid-Lombardo KM: Hepatic epithelioid haemangioendothelioma: Is
transplantation the only treatment option? HPB (Oxford).
12:546–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao XY, Rakhda MIA, Habib S, Bihi A,
Muhammad A, Wang TL and Jia JD: Hepatic epithelioid
hemangioendothelioma: A comparison of Western and Chinese methods
with respect to diagnosis, treatment and outcome. Oncol Lett.
7:977–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giovanardi F, Laureiro ZL, Meo GA, Hassan
R and Lai Q: The challenging surgical management of hepatic
epithelioid hemangioendothelioma: A narrative review. Chin Clin
Oncol. 11:272022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Izzo F, Granata V, Grassi R, Fusco R,
Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A and Curley
S: Radiofrequency ablation and microwave ablation in liver tumors:
An update. Oncologist. 24:e990–e1005. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barrow B and Martin RCG: Microwave
ablation for hepatic malignancies: A systematic review of the
technology and differences in devices. Surg Endosc. 37:817–834.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Afaghi P, Lapolla MA and Ghandi K:
Percutaneous microwave ablation applications for liver tumors:
Recommendations for COVID-19 patients. Heliyon. 7:e064542021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang C, An J, Bruno A, Cai X, Fan J,
Fujimoto J, Golfieri R, Hao X, Jiang H, Jiao LR, et al: Consensus
recommendations of three-dimensional visualization for diagnosis
and management of liver diseases. Hepatol Int. 14:437–453. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lubner MG, Gettle LM, Kim DH, Ziemlewicz
TJ, Dahiya N and Pickhardt P: Diagnostic and procedural
intraoperative ultrasound: Technique, tips and tricks for
optimizing results. Br J Radiol. 94:202014062021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu J, Hu S, Li S, Wang W, Zhou X, Wu Y, Su
Z, Cheng X, Gao Y and Zheng Q: Laparoscopic resection of hepatic
epithelioid hemangioendothelioma: Report of eleven rare cases and
literature review. World J Surg Oncol. 18:2822020. View Article : Google Scholar : PubMed/NCBI
|